Supplemental digital content is available in the text.
Abstract
Background
Invasive fungal infections remain a leading cause of morbidity and mortality among liver transplant recipients (LTRs). In this patient population, invasive Candida infections (ICIs) account for the large majority of cases. To date, only small studies and case-series analysing clinical presentation and risk factors for mortality in LTRs with ICIs are available.
Methods
We performed a retrospective multicenter multinational study in 10 centers in Europe and Brazil. All consecutive LTRs developing ICIs during the period January 2011 to December 2013 were included in the study.
Results
A total of 42 LTRs were included. Median age was 52.5 years, and 78.6% of patients were men. Viral hepatitis was the most common cause for liver transplantation (42.9%). Candidemia represented the majority of cases (24, 57.1%), followed by intra-abdominal candidiasis (18, 42.9%). Overall 30-day mortality was 23.8%, with higher mortality in patients with candidemia compared with intra-abdominal candidiasis (37.5% vs 5.6%, P = 0.02). Multivariate analysis showed candidemia to be a risk factor associated with mortality among LTRs presenting ICIs (odds ratio, 11.86; 95% confidence interval, 1.5-280; P = 0.01). Candida albicans represented the most common isolate (59.5%). High rates of antifungal resistances were found, with 16.7% and 4.8% of isolates displaying resistance to azoles and caspofungin, respectively.
Conclusions
Our study confirms the occurrence of high mortality rates in LTRs developing ICIs. Mortality rates varied according to the type of infection, with candidemia representing a risk factor for mortality. The high rates of antifungal resistance should be considered in the choice of the empiric antifungal regimen.
Invasive fungal infections (IFIs), mainly represented by invasive Candida infections (ICIs) and invasive aspergillosis, represent a frequent complication and a leading cause of morbidity and mortality among liver transplant recipients (LTRs).1-3
The introduction of antifungal prophylaxis after liver transplantation has demonstrated to be effective in reducing both incidence and IFIs-related mortality when 1 or more specific risk factors are present4; however, risk factors are not well defined and a consensus regarding which patients should receive antifungal prophylaxis after liver transplantation does not exist so far, with an overall incidence of IFIs of approximately 5% to 7% still reported, irrespective of antifungal prophylaxis.5,6 Among IFIs occurring after liver transplantation, ICIs account for up to 75% of cases, with prolonged or repeated surgery, choledochojejunostomy, retransplantation, dialysis, prolonged antibiotic treatment and hospitalization, cytomegalovirus disease, and Candida colonization reported as common risk factors.4-6 ICIs usually occur within the first 3 months after liver transplantation and are associated with overall mortality rates up to 70%.1,7,8 Despite these alarming figures, only few studies focusing on ICIs in LTRs have been reported. The aim of our study was to describe characteristics, outcome, and risk factors for mortality in LTRs developing ICIs.
MATERIALS AND METHODS
Patient Population and Study Design
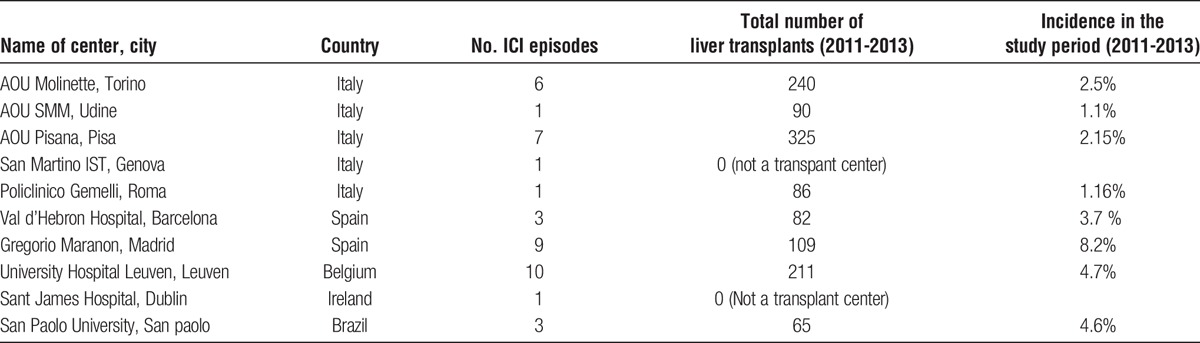
A multicenter, multinational retrospective cohort study was conducted across 10 centers in Europe (Italy, Spain, Ireland, and Belgium) and Brazil over a 3-year period (2011–2013) (Table 1). All consecutive LTRs developing an episode of ICI during the study period were included. No center performed universal antifungal prophylaxis for LTRs.
TABLE 1.
Centers included with local incidence of ICIs
The study was approved by the institutional review board of the coordinating center (Udine University Hospital, Italy) and written patient consent was not required because of the observational nature of this study.
Patients’ baseline characteristics and infection-related variables were collected from the hospital medical records, microbiology databases, and pharmacy databases of the participating centers.
The following data were recorded: age, sex, comorbidities, etiology of liver disease, surgical and medical complications after liver transplant, prior interventions (eg, abdominal surgery, vascular or abdominal device placement), prior antibiotic (more than 7 days in the past 30 days) or azole exposure (in the past 30 days), receipt of antifungal prophylaxis after liver transplantation, parenteral nutrition and/or immunosuppressant administration and recurrence of cirrhosis after liver transplant, including the degree of severity.
Definitions
Candidemia was defined as the isolation of Candida spp. from at least 1 blood culture, according with the current guidelines.9
An episode of intra-abdominal candidiasis (IAC) was defined as follows:
Candida detection by direct microscopy examination or growth in culture from purulent or necrotic intra-abdominal specimens obtained during surgery or by percutaneous aspiration;
Candida growth from bile, intra-biliary ducts devices, and biopsy of intra-abdominal organs;
Candida growth from blood cultures in clinical setting of secondary and tertiary peritonitis;
Candida growth from drainage tubes only if placed less than 24 hours before the cultures10;
Child-Pugh score and model for end-stage liver disease (MELD) score have been calculated for patients with recurrence of liver cirrhosis after transplantation. Infections were classified as community-acquired, health care–associated and hospital-acquired according with Friedman criteria.11 Setting of acquisition and time between transplant and infection onset were recorded. Clinical presentation, in particular the presence of fever (T > 38°C), septic shock and Sequential Organ Failure Assessment (SOFA) score, was recorded. Septic shock was defined according to current guidelines.12 Microbiological data analyzed included Candida species and antifungal susceptibility to fluconazole, voriconazole, echinocandins, and amphotericin B. Treatment-related characteristics examined were timing of adequate antifungal treatment relative to blood or abdominal samples cultures positivity, type of antifungal prescribed as initial treatment and adequate source control. Antifungal therapy was considered adequate if the organism was shown to be susceptible to the prescribed antifungal treatment and the dosage of antifungal was adequate. The following antifungal dosages were considered adequate: fluconazole 800 mg loading dose (for patients with body mass index >30, 1200-1600 mg) followed by a daily dosage of at least 400 mg (600-800 mg for body mass index > 30); liposomal amphotericin B (L-AmB), 3 mg/kg per day; amphotericin B lipid complex, 5 mg/kg per day; caspofungin, 70 mg loading dose (100 mg) followed by 50 mg/d; micafungin, 100 mg/d, anidulafungin 200 mg loading dose followed by 100 mg/d. These dosages refer to patients with normal hepatic and renal function. In case of hepatic or renal impairment dose adjustment have been considered adequate according with the per package indications. Source control was considered adequate in the following cases: (1) devices or foreign bodies removal, (2) drainage of infected fluid collections, (3) debridement of infected solid tissue, (4) definitive measures to correct anatomic derangements resulting in ongoing microbial contamination. The primary outcome variable was all-cause 30-day mortality.
Blood Cultures and Microbiology Analysis
Candida species were isolated using the BACTEC 860 system (Becton-Dickinson, Inc., Sparks, MD) and BacT/Alert 3D (BioMérieux, Marcy-l'Étoile- France). The species were identified using API ID 32C system (BioMérieux, Marcy-l'Étoile- France) or Vitek 2 system (BioMérieux, Marcy-l'Étoile- France). In case of inconclusive results by both systems, isolates were definitively identified using supplemental tests, such as presence or absence of well-formed pseudohyphae on cornmeal-Tween 80 agar and growth at 42°C to 45°C. The last test was also required to differentiate isolates of Candida albicans from those of Candida dubliniensis. Antifungal susceptibility testing to amphotericin B, caspofungin, fluconazole and voriconazole was performed using the Sensititre YeasOne colorimetric plate (Trek Diagnostics Systems, Cleveland, OH) or by agar diffusion using E-test strips (BioMérieux, Marcy-l'Étoile- France) and interpreted by the Clinical Laboratory Standards Institute breakpoints.
Statistical Analysis
Continuous and categorical data were reported as mean and SD or median, 25th and 75th percentile and frequency distributions, respectively. Differences existed between groups for continuous variables were evaluated through the Student t test or, when appropriate, the median test. Categorical variables were evaluated using chi-square or, when appropriate, the 2-tailed Fisher exact test. Multiple logistic regression analysis was performed to identify risk factors that were associated with 30-day hospital mortality (JMP, SAS, NC). Covariates that were significant at 0.10 in the univariate analysis and therapy-related variables were further evaluated for inclusion in multivariable regression models, using a backward stepwise algorithm. Odds ratio and 95% confidence interval were reported. All tests were 2-tailed, and a P value less than 0.05 was determined to represent statistical significance.
RESULTS
Patient's Characteristics
Overall incidence of ICIs during the study period was 3.47% (Table 1). A total of 42 LTRs with ICIs were included in the study. Clinical characteristics of patients included are described in Table 2. Median age was 52.5 years and 78.6% of patients were men. Viral hepatitis was the most common cause for liver transplantation (42.9%), followed by alcohol abuse (26.2%). The median number of days between transplantation and infection onset was 151 days, with an interquartile range of 14 to 1037 days. At the time of diagnosis 21 (50%) patients were hospitalized in intensive care unit (ICU), 8 (19%) in internal medicine ward, 6 (14.3%) in surgical ward and 7 (16.7%) in other wards.
TABLE 2.
Clinical characteristics, treatment, and clinical outcome of liver transplant patients with ICIs and comparison between survivors and nonsurvivors
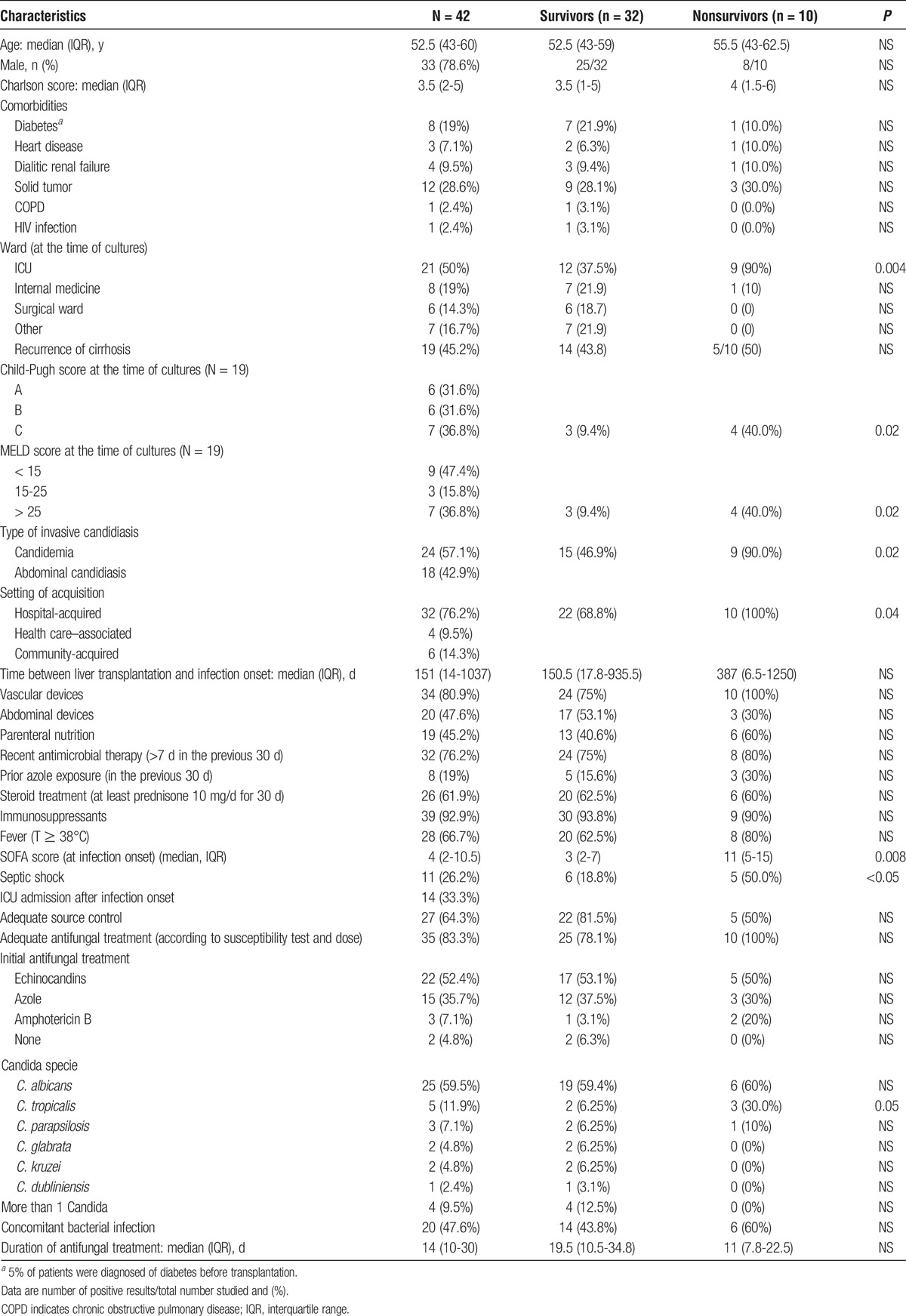
Among the classical risk factors for ICIs occurrence post-OLT, reoperation was the most common (33.3%), followed by choledochojejunostomy (23.8%) and prolonged operation (>8 hours) (14.3%); less common were retransplantation and the presence of an anastomotic leakage. Other factors included the need for renal replacement therapy (26.2%), transfusion of more than 40 units of cellular blood products during the intervention (21.4%), organ rejection (16.7%), and cytomegalovirus reactivation (>100.000 cp/mL) (9.5%). A total of 10 (23.8%) patients received antifungal prophylaxis after liver transplantation. Most patients were receiving an immunosuppressive treatment (92.9%) and/or steroids (61.9%) at the time of cultures. Vascular devices and abdominal drainages were frequent (80.9% and 47.6 % of cases, respectively). The large majority of patients (76.2%) had been recently treated with antimicrobials and 19 (45.2%) were receiving parenteral nutrition. Recurrence of liver cirrhosis after transplantation was diagnosed in 19 (45.2%) patients at the time of cultures; the majority of these patients were classified as Child-Pugh C (36.8%), and MELD score was higher than 15 in 10 (52.6%) cases.
Clinical Presentation and Timing of Infection Onset
Candidemia accounted for the majority of ICIs cases (24, 57.1%), followed by IAC (18, 42.9%). Peritonitis and abdominal abscesses were the most common types of IAC, (38.9% in both cases), followed by biliary tract infections (16.7%). A total of 11 (26.2%) patients presented with septic shock and 14 (33.3%) were admitted to ICU after the onset of the infection. Fever was present in 28 (66.7%) cases. Only 1 case of Candida blood cultures positivity was reported among IAC cases.
In our cohort, about 34.1% and 46.3% cases of ICIs occurred, respectively, during the first month and within 3 months after surgery. Patients with early (<3 months from transplant) ICIs were more likely to be hospitalized in ICU ward (68.4% vs 31.8%, P = 0.02) and to have acute renal failure (50% vs 10%, P = 0.06). Late ICIs in LTRs was more likely to be acquired in community (0% vs 27.3%, P = 0.03) and in internal medicine wards setting (5.3% vs 31.8%, P = 0.07). No si7gnificant differences were detected between early and late IC regarding clinical presentation. Table S1, SDC, http://links.lww.com/TXD/A38 reports the differences between liver transplant patients presenting with early and late ICIs.
Microbiology
Candida albicans was the most common species and was isolated in 25 (59.5%) patients. Among nonalbicans Candida spp., C. tropicalis was the most frequently isolated (11.9%), followed by C. parapsilosis (7.1%), C. glabrata, and C. kruzei (4.8% in both cases), C. dublinensis (2.4%), and only 4 (9.5%) patients had a polyfungal infection. A total of 83.3% of Candida strains were susceptible to fluconazole and voriconazole, whereas 95.2% were susceptible to amphotericin B and to caspofungin. Overall, 7 cases of fluconazole resistance and 2 cases of echinocandin resistance were reported. A total of 20 (47.6%) patients had a concomitant bacterial infection.
Antifungal Therapy
A total of 35 (83.3%) patients received an adequate antifungal treatment according to susceptibility test and dose; among these, antifungal treatment was prescribed within 24, 48, and 72 hours from cultures positivity in 40%, 20%, and 17.1% of cases, respectively. Eight (22.9%) patients received antifungal treatment after 72 hours. Echinocandins were the most common antifungal agent prescribed as initial treatment (52.4%), followed by azoles (35.7%) and amphotericin B (7.1%). An adequate source control was performed in 27 (64.3%) patients within 24, 48, and 72 hours from cultures positivity in 51.9%, 22.2%, and 11.1% of cases, respectively.
Outcome and Risk Factors for Mortality
Overall 30-day mortality was 23.8%. Table 2 summarizes the significant differences between patients who died within 30 days from the diagnosis of ICI compared with survivors by univariate analysis. Patients who died were more frequently admitted to ICU compared with survivors (90% vs 37.5%, P = 0.004), were more likely to present with septic shock (50.0% vs 18.8%, P = <0.05), and had higher values of SOFA score (11 vs 3, P = 0.008). Hospital-acquired infections were more common among nonsurvivor compared with survivors (100% vs 68.8%, P = 0.04). Child-Pugh C and MELD score greater than 25 were more frequent among patients who died (40% vs 9.4%, P = 0.02 in both cases). Candidemia was more common among nonsurvivors (90% vs 46.9%, P = 0.02). The isolation of C. tropicalis was more common among nonsurvivors compared with survivors (30% vs 6.25%, P = 0.05). No significant differences were detected between survivors and nonsurvivors regarding timing of adequate antifungal treatment and source control. Moreover, a concomitant bacterial infection was not significantly associated with increased mortality. Multivariate analysis demonstrated that candidemia (odds ratio, 11.86; 95% confidence interval, 1.5-280; P = 0.01) was a risk factor associated with 30-day mortality in LTRs presenting with ICIs. An increased risk for mortality associated with clinical presentation with septic shock and Child-Pugh C liver failure was reported but did not reach statistical significance.
Comparison Between Liver Transplant Patients Presenting With Candidemia or IAC
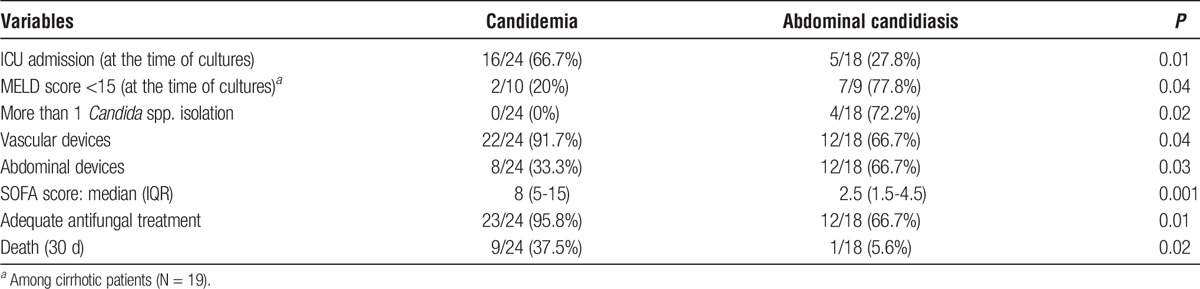
Table 3 reports the differences between liver transplant patients presenting with candidemia or IAC. Specifically, ICU admission at the time of cultures and vascular device placement were more common among patients with candidemia; conversely, patients with IAC were more likely to have abdominal devices placement, polyfungal infections and MELD score < 15. No differences were detected in clinical presentation with septic shock, but SOFA score was significantly higher in patients with candidemia. Of note, in patients with IAC lower rates of adequate antifungal treatment prescription were found (66.7% vs 95.8%, P = 0.01), with no significant differences regarding both adequacy and timing of source control. However, significantly higher 30-day mortality rates were reported in patients with candidemia compared with IAC (37.5% vs 5.6%, P = 0.02).
TABLE 3.
Characteristics of LTRs presenting with candidemia (N = 24) compared with patients presenting with abdominal candidiasis (N = 18)
DISCUSSION
ICIs have been associated with mortality rates ranging between 30% and 50% in the general population and up to 80% among critically ill patients.13,14 Data including LTRs, however, are limited, showing large variability in mortality.7,15,16 We reported overall mortality of 23.8% with higher rates in candidemia compared with IAC. To the best of our knowledge, no study had previously investigated the relationship between type of ICIs and clinical outcomes among LTRs. These data warrant, especially among patients with candidemia, prompt antifungal treatment along with a timely source control (eg, central venous catheter removal) to improve the patients’ outcome.
Our study showed overall mortality rates slightly lower than those reported in the general population with IAC (26.8%).17 Possible reasons include a limited number of septic shock at presentation and significantly, lower mean age, that represented independent risk factors for mortality.17 Moreover, cardiovascular, pulmonary diseases, and diabetes, that may be contraindications to perform a liver transplant, were less common among LTRs compared with the general population developing ICIs. Septic shock, in particular, has been associated with mortality in up to 50% of patients with candidemia,18 and is an independent risk factor for mortality among patients with IAC.17 In our study, an increased risk associated with septic shock at presentation was reported, but statistical significance was not achieved probably because of the small number of patients included in this group.
Decompensated liver cirrhosis represents a risk factor for the development of both bacterial and fungal infections18-20 and was present in 45.2% of patients in our study. Other reports did not show these results, probably due to limited follow-up times. In our study, median time between transplantation and Candida infections was 5 months, and nearly half of patients developed ICIs after 6 months from liver transplant. Recurrence of decompensated cirrhosis after liver transplant may therefore represent a risk factor for late occurrence of ICIs.
Our data confirmed C. albicans as the most common isolate.13,17 Isolation of nonalbicans species has been associated with significantly higher mortality.21,22 We showed higher mortality rates in C. tropicalis compared with other species, with no differences between candidemia and IAC.
In patients with IC, the synergistic effect of timely treatment adequacy and source control has shown to be crucial and has impacted in the management of invasive candidiasis. In our study, early (<24 hours) source control (51.9%) and adequate antifungal therapy (83.3%) were achieved in a high proportion of the patients. However, we did not observe a clear effect adequate antifungal treatment (based on susceptibility pattern and dosage of antifungals) and source control on mortality rates. These results are probably related to the relatively small sample size of our cohort, which may have introduced a type II error in the statistical analysis.
Significant rates of both azole and echinocandin resistance were reported in our study (16.7% and 4.8%, respectively). Antifungal resistance in Candida spp. represents a rising problem, even if a wide variability is present among different countries and species, with overall resistance rates of fluconazole resistance of approximately 5% in C. albicans. Studies in LTR reported overall rates of fluconazole resistance up to 57%, attributed to the high rates of nonalbicans strains and previous antifungal prophylaxis.21,22 Echinocandin resistance, instead, is reported in less than 2% of Candida isolates worldwide.23 Interestingly, only 23.8% of patients received antifungal prophylaxis after liver transplantation, and recent treatment with azoles was documented in 19% of cases in our report without association with antifungal resistance. The majority of cases of fluconazole-resistance and both cases of echinocandin resistance were detected in IAC, confirming recently reported data.24 IAC has been investigated as a hidden reservoir for echinocandin-resistant strains, particularly in patients with prolonged echinocandin exposure and breakthrough infections.25 Overall, our results suggest that LTRs are at risk for Candida infections, including nonalbicans strains, with higher probability of fluconazole and, less frequently, echinocandin resistance compared to the general population. These factors, together with the knowledge of local epidemiology, should be taken into account in the management of ICIs in LTRs. In particular, risk factors for fluconazole resistance should be considered when treating empirically candidemia in LTR.
Echinocandins were the most commonly prescribed antifungals in candidemia and IAC, similarly to other European studies and according to recently published guidelines.17,18,26,27 In LTRs, in particular, echinocandins have demonstrated low liver toxicity and limited drug-drug interactions compared to azoles. These characteristics, together with a low probability of antifungal resistance and the enhanced antibiofilm activity, make echinocandins an effective empiric treatment in this population.
Interestingly, in our study, an adequate antifungal treatment was prescribed in only 66.7% of IAC compared with 95.8% of cases of candidemia. This percentage was similar to the 1 reported in general population with IAC17 probably due to the increased rates of fluconazole resistance reported.
No correlation was found between mortality and adequate antifungal treatment or source control, which has been found to be independently correlated with clinical outcome both in candidemia and in IAC.17,18
In conclusion, our study highlights high mortality rates associated with candida infections, especially candidemia, among liver transplant patients. In this setting, the choice of an adequate therapy is paramount, and risk factors for fluconazole resistance should be taken into consideration to optimize patients’ management. The limitations of our study include the retrospective observational nature although a strength is represented by its international design. However, these data highlight that a multicenter randomized controlled trial is warranted to assess the efficacy of antifungal prophylaxis in LTRs.
Supplementary Material
Footnotes
Published online 18 April, 2017.
Authorship: Study conception and design: M.B., M.P., C.V., A.C., E.R., F.A., C.T., C.A. Acquisition of data: M.M., A.S., J.W., K.L., F.M., A.M., F.D.R., L.L., J.R., A.L.C., A.V., P.M. Analysis and interpretation of data: M.T., E.R., M.B., C.V. Drafting of manuscript: M.B., E.R., M.P., A.C., C.T. Critical revision: C.V., M.T., I.M.L.
The authors declare no conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 2.Yang CH, He XS, Chen J, et al. Fungal infection in patients after liver transplantation in years 2003 to 2012. Ann Transplant. 2012;17:59–63. [DOI] [PubMed] [Google Scholar]
- 3.Rabkin JM, Oroloff SL, Corless CL, et al. Association of fungal infection and increased mortality in liver transplant recipients. Am J Surg. 2000;179:426–430. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Ling Z, Li L, et al. Invasive fungal infections in liver transplantation. Int J Infect Dis. 2011;15:e298–e304. [DOI] [PubMed] [Google Scholar]
- 5.Winston DJ, Limaye AP, Pelletier S, et al. Randomized, double-blind trial of anidulafungin versus fluconazole for prophylaxis of invasive fungal infections in high-risk liver transplant recipients. Am J Transplant. 2014;14:2758–2764. [DOI] [PubMed] [Google Scholar]
- 6.Eschenauer GA, Kwak EJ, Humar A, et al. Targeted versus universal antifungal prophylaxis among liver transplant recipients. Am J Transplant. 2015;15:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto-Rodriguez JA, Kusne S, Mañez R, et al. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surg. 1996;223:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Wen TF, Mi K, et al. Analysis of infections in the first 3-month after living donor liver transplantation. World J Gastroenterol. 2012;18:1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. [DOI] [PubMed] [Google Scholar]
- 10.Bassetti M, Marchetti M, Chakrabarti A, et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med. 2013;39:2092–2106. [DOI] [PubMed] [Google Scholar]
- 11.Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassetti M, Merelli M, Righi E, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013;51:4167–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng K, Schorr C, Reboli AC, et al. Incidence and mortality of sepsis, severe sepsis, and septic shock in intensive care unit patients with candidemia. Infect Dis (Lond). 2015;47:584–587. [DOI] [PubMed] [Google Scholar]
- 15.van Hal SJ, Marriott DJ, Chen SC, et al. Candidemia following solid organ transplantation in the era of antifungal prophylaxis: the Australian experience. Transpl Infect Dis. 2009;11:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzaban R, Salah M, Mukhtar AM, et al. Fungal infections in liver transplant patients admitted to the intensive care unit. Ann Transplant. 2014;19:667–673. [DOI] [PubMed] [Google Scholar]
- 17.Bassetti M, Righi E, Ansaldi F, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41:1601–1610. [DOI] [PubMed] [Google Scholar]
- 18.Bassetti M, Righi E, Ansaldi F, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med. 2014;40:839–845. [DOI] [PubMed] [Google Scholar]
- 19.Bartoletti M, Giannella M, Caraceni P, et al. Epidemiology and outcomes of bloodstream infection in patients with cirrhosis. J Hepatol. 2014;61:51–58. [DOI] [PubMed] [Google Scholar]
- 20.Hwang SY, Yu SJ, Lee JH, et al. Spontaneous fungal peritonitis: a severe complication in patients with advanced liver cirrhosis. Eur J Clin Microbiol Infect Dis. 2014;33:259–264. [DOI] [PubMed] [Google Scholar]
- 21.Krashin E, Lishner M, Chowers M, et al. Candida albicans in peritoneal fluid in a patient with hepatic encephalopathy. Isr Med Assoc J. 2015;17:193–194. [PubMed] [Google Scholar]
- 22.Raghuram A, Restrepo A, Safadjou S, et al. Invasive fungal infections following liver transplantation: incidence, risk factors, survival, and impact of fluconazole-resistant Candida parapsilosis (2003-2007). Liver Transpl. 2012;18:1100–1109. [DOI] [PubMed] [Google Scholar]
- 23.Husain S, Tollemar J, Dominguez EA, et al. Changes in the spectrum and risk factors for invasive candidiasis in liver transplant recipients: prospective, multicenter, case-controlled study. Transplantation. 2003;75:2023–2029. [DOI] [PubMed] [Google Scholar]
- 24.Giacobino J, Montelli AC, Barretti P, et al. Fungal peritonitis in patients undergoing peritoneal dialysis (PD) in Brazil: molecular identification, biofilm production and antifungal susceptibility of the agents. Med Mycol. 2016;54:725–32. [DOI] [PubMed] [Google Scholar]
- 25.Castanheira M, Messer SA, Rhomberg PR, et al. Antifungal susceptibility patterns of a global collection of fungal isolates: results of the SENTRY antifungal surveillance program (2013). Diagn Microbiol Infect Dis. 2016;85:200–204. [DOI] [PubMed] [Google Scholar]
- 26.Shields RK, Nguyen MH, Press EG, et al. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014;58:7601–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;62:409–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


