Abstract
Background
Exposure to pollutants through inhalation is a risk factor for lung diseases including cancer, asthma, and lung transplant rejection, but knowledge of the effects of inhaled pollutants on pathologies outside of the lung is limited.
Methods
Using the minor-mismatched model of male C57BL/6J (B6) to female B6 skin grafts, recipient mice were treated with an inhaled urban dust particle sample every 3 days before and after grafting. Graft survival time was determined, and analysis of the resulting immune response was performed at time before rejection.
Results
Significant prolongation of male skin grafts occurred in recipient female mice treated with urban dust particles compared with controls and was found to be dependent on aryl hydrocarbon receptor (AHR) expression in the recipient mouse. T cell responses to the male histocompatibility antigen (H-Y) Dby were not altered by exposure to pollutants. A reduction in the frequency of IFNγ-producing CD4 T cells infiltrating the graft on day 7 posttransplant was observed. Flow cytometry analysis revealed that AHR expression is upregulated in IFNγ-producing CD4 T cells during immune responses in vitro and in vivo.
Conclusions
Surprisingly, inhalation of a pollutant standard was found to prolong graft survival in a minor-mismatched skin graft model in an AHR-dependent manner. One possible mechanism may be an effect on IFNγ-producing CD4 T cells responding to donor antigen. The increased expression of AHR in this CD4 T cell subset suggests that AHR ligands within the particulate matter may be directly affecting the type 1 T helper cell response in this model.
Best known as the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the aryl hydrocarbon receptor (AHR) is associated with the effects of environmental toxins including hepatocellular damage, thymic involution, cancer, and immunosuppression.1 Although previous studies of the AHR focused on its role in toxicology, more recent research has documented its importance in the immune system.2-4 In addition to a well-defined role in responding to endogenous ligands and its importance in embryologic development, the data support the hypothesis that the AHR serves as a sensor to environmental triggers, alerting the immune system to pathologic exposures and modulating the immune response.5 Initial studies have focused on the effects on T cell differentiation, with some AHR ligands enhancing the development of either regulatory T (Treg) cells and other ligands promoting the proinflammatory Th17 cells both in vitro and in vivo.3,4,6,7 Recent work has brought this into question, because the outcome in T cell differentiation and autoimmune response may be dependent as much on the inflammatory milieu and route of exposure as the ligand itself.8
There is ample evidence that particulate matter (PM) exposure can increase inflammatory response in vitro and in the airway.9-11 Recent work from our laboratory has demonstrated the effects of pollutants, including single polycyclic aromatic hydrocarbons (PAHs) and atmospheric PM, on the enhancement of Th17 differentiation in an AHR-dependent manner.12 Exposure to PM worsens various forms of lung disease13,14 including clinical lung transplantation15-17 although the mechanisms remain unclear. Lung transplant rejection, unlike other forms of organ rejection, has a well-established role for interleukin 17A (IL-17A) and Th17 cells.18-20 The effects of PM on lung transplantation via a mechanism involving AHR activation by ligands contained in PM and subsequent enhancement of Th17 responses is intriguing.21,22
The effect of PM on immune-related pathologies outside of the lung has been suggested for cardiovascular, hepatic, and digestive disease.23-25 However, outside of the lung, the effects of PM on the outcome of solid organ rejection is not clear. We previously have shown that TCDD given intraperitoneally to mice prolonged skin graft survival, whereas 6-formylindolo(3,2-b)carbazole (FICZ), an AHR ligand derived from tryptophan, given intraperitoneally accelerated skin graft rejection.26 To explore the effects of pollutant in transplantation, female C57BL/6 J (B6) mice were treated with PM intranasally before and after engraftment with male B6 skin. To our surprise, we found that mice treated with inhaled pollutants before receiving minor antigen mismatched skin transplants were found to have delayed rejection. By using AHR null (AHR−/−) mice, we were able to demonstrate that this delay in rejection is AHR-dependent. During the ensuing immune response after skin graft placement, AHR expression is upregulated in a number of T cell subsets including type 1 T helper cell (Th1) cells. Although the exact mechanisms responsible for prolonged survival remain unclear, this study shows that inhalation of environmental PM can have an effect on peripheral immune responses.
MATERIALS AND METHODS
Animals
Six- to 12-week-old male and female B6 (H-2b) and male BALB/c (H-2d) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). AHR−/− mice on a B6 background27 were bred and maintained under specific pathogen-free conditions. Animal experiments were carried out according to institutional guidelines.
Intranasal Administration of PM
Standard Reference Material (SRM) 1649b Urban Dust was obtained from the National Institute of Standards and Technology (Gaithersburg, MD) to serve as our model PM. The certificate of analysis for SRM1649b used is available online. For intranasal (i.n.) administration, an SRM1649b suspension was created by sonication in sterile phosphate-buffered saline (PBS) at a concentration of 40 mg/mL for 15 minutes in a cooling water bath. Determination of endotoxin contamination was performed using the LAL Chromogenic Endotoxin Quantitation kit from Pierce (Rockford, IL). Endotoxin levels of a stock suspension of 40 mg/mL SRM1649b was below the level of detection (0.1 EU/mL). For i.n. administration, either PBS (20 μL) or SRM1649b (20 μL) was given every 3 days for a total of 5 to 9 doses while mice were anesthetized using isoflurane.
Skin Transplantation
Full-thickness skin procured from BALB/c or B6 donor mice was transplanted on the dorsal area of B6 recipients. Necrosis of 80% or more of the transplanted skin surface was considered rejection, and all assessments were performed by blinded observers. All mice were anesthetized for bandage removal on postoperative day 6. Recipients received SRM1649b intranasally starting 12 days before transplant.
Delayed-Type Hypersensitivity Assay of HY-Peptide Response
Briefly, 10 μg of HY peptide antigen (Dby; Anaspec, Fremont, CA) was injected into footpads of naïve CB17-SCID mice in 20 μL of PBS. A dual thickness gauge (Mitutoyo, Kawasaki, Japan) was used to measure footpad thickness at 0 hour and 24 hours after injections. Footpad swelling was defined as thickness at 24 hours minus thickness at 0 hours with units of 10−4 in.
Real-Time Quantitative PCR
Total RNA was extracted using the Fibrous RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and used as template for complementary cDNA synthesis using SuperScript VILO cDNA synthesis kit (Life Technologies, Carlsbad, CA). The RT-PCR was performed on the Applied Biosystems 7900HT Fast Real-Time PCR System using TaqMan Gene Expression Assays and TaqMan Universal Master Mix II, no UNG (Life Technologies). Assays included actin, beta (Actb; Mm00607939_s1), cytochrome P450, family 1, subfamily a, polypeptide 1 (Cyp1a1; Mm00487218_m1), IL-17A (Mm00444241_m1), IFNγ (Mm01168134_m1), FoxP3 (m00475162_m1), and Ido1 (Mm00492586_m1). The data were analyzed using the Δthreshold cycle method, with actin serving as the endogenous reference.
In Vitro Cytokine Production
Spleen and draining lymph nodes (LNs) were harvested and prepared for cell culture by processing through a 100-μm cell strainer with RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 100-μg/mL streptomycin, 100 U/mL penicillin, 50-μM 2-mercaptoethanol, 25-mM HEPES, and 2-mM l-glutamine, 1-mM sodium pyruvate, and 1× nonessential amino acids (Mediatech, Manassas, VA). Red blood cells within splenocyte preparation were lysed using RBC Lysis Buffer Solution (eBioscience, San Diego, CA). Cells were plated at a concentration of 2 × 105 cells per 200 μL final volume in round-bottom 96-well culture plates. Cells were stimulated with either 1-μg/mL anti-CD3ε (2C11; R&D, Minneapolis, MN) or 2-μg/mL Dby peptide (AnaSpec, Fremont, CA). Culture supernatants were harvested on day 4 of culture, and IL-17A and IFNγ concentrations were measured by ELISA (R&D, Minneapolis, MN) according to manufacturer's instructions.
Flow Cytometry
Spleen and draining LNs were harvested and prepared for flow cytometry as was described for cell culture previously. Skin grafts were harvested, minced into approximately 1 mm2 pieces, and placed into 2 mL of Hank's buffered salt solution with Liberase (Roche Diagnostics, Indianapolis, IN). After incubation at 37°C for 30 minutes, the digested tissue was passed through a 100-μm cell strainer and washed twice with supplemented RPMI. Splenocytes (SPLs), LN cells, and skin graft cells were stimulated with phorbal 12-myristate 13-acetate (PMA) and ionomycin in the presence of Brefeldin A for 4 hours at 37°C. Cells were stained for 30 minutes at room temperature first with LIVE/DEAD Fixable Blue Dead Cell Stain (Life Technologies) and then with surface stain antibodies for 20 minutes as indicated in Table S1, SDC, http://links.lww.com/TXD/A37. The cells were then processed for intracellular staining using Intracellular Fixation & Permeabilization Buffer Set (eBioscience) as described by the manufacturer. Cells were then stained with intracellular antibodies (Table S1, SDC, http://links.lww.com/TXD/A37) overnight at 4°C and analyzed using a BD LSR Fortessa (BD Biosciences, San Jose, CA).
Statistics
Statistical differences of skin graft survival data were determined by using the Kaplan-Meier analysis. Student t test was used to compare ELISA data. Repeated measures 1-way or 2-way analysis of variance (ANOVA) with Bonferroni post-ANOVA testing was used for all other statistical analysis. Statistical significance was defined as a P of less than 0.05. The data are showed as mean ± SD. All statistical analyses were performed using Prism 5 software (GraphPad, San Diego, CA).
RESULTS
Intranasal PM Affects Peripheral Immunity by Enhancing IL-17A Expression
We have previously reported that PM given intranasally results in elevated IL-17A expression in the lungs of SRM1649b-treated mice compared with controlled mice.12 To examine the effects of i.n. PM on peripheral immune responses, B6 mice were treated with SRM1649b every 3 days for a total of 5 doses and sacrificed 1 day after the last dose. The final SRM1649b dose corresponds to the day of transplant in the skin graft experiments to follow. Spleen and popliteal, axillary, and mandibular LNs were harvested. SPLs and pooled LN cells were stimulated with anti-CD3 antibody concentration, and the culture supernatant was harvested after 4 days. Treatment with SRM1649b i.n. significantly increased IL-17A expression in SPLs ex vivo with a trend toward an increase in LN cells (Figure 1A), but had no effect on IFNγ expression in either type of cells (Figure 1B).
FIGURE 1.
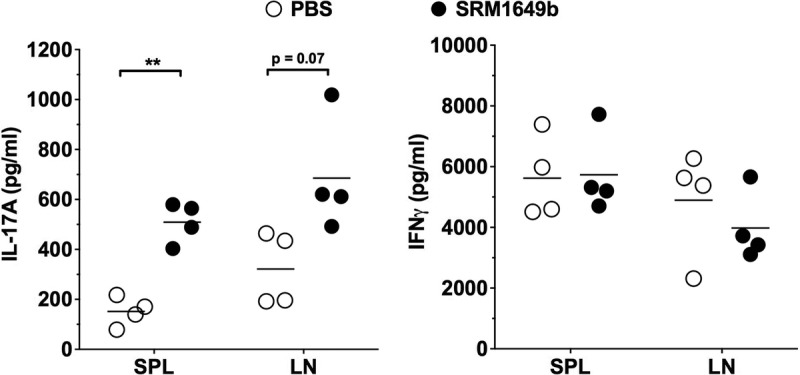
Intranasal instillation of PM enhances IL-17A expression in lymphoid tissue. Male B6 mice were exposed to either PBS or SRM1649b, a standard PM sample of urban dust particles, by i.n. instillation every 3 days for a total of 5 doses. Twenty-four hours after the last dose, mice were killed, and SPLs and axillary LN cells were isolated. Cells were stimulated in vitro for 3 days with 1 μg/mL anti-CD3ε. Supernatants were harvested and IL-17A, and IFNγ concentration was measured by ELISA. ELISA data were analyzed by paired Student t test. **P < 0.01.
Inhalation of PM Delays Skin Graft Rejection in a Minor-Mismatched Model in an AHR-Dependent Manner
To determine whether inhalation of pollutants could cause accelerated rejection of skin grafts, B6 mice were treated with PBS or SRM1949b every 3 days intranasally (Figure 2A). On the day of the fifth dose, the mice were grafted with BALB/c skin, and i.n. treatments were continued every 3 days for a total of 4 additional treatments. In this fully allogeneic mismatched skin graft model, i.n. PM did not significantly alter graft survival time (GST) compared with control (Figure 2B).
FIGURE 2.
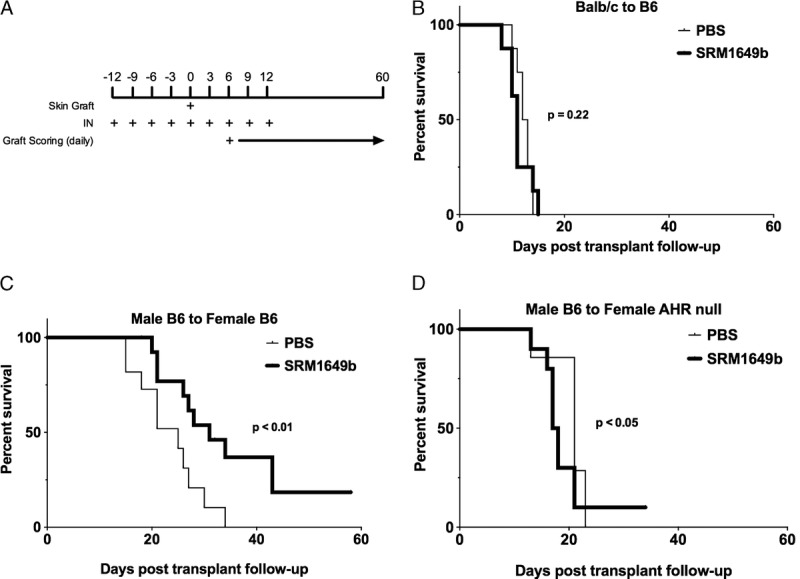
Intranasal instillation of PM prolongs allograft survival of minor but not major antigen mismatched skin grafts in an AHR-dependent manner. A, Diagram of the timing of i.n. instillation relative to skin graft. B, Male B6 mice received BALB/c skin grafts and were exposed to i.n. instillation of either PBS (n = 8; median survival time [MST] = 12.5) or SRM1649b suspended in PBS (n = 8, MST = 11), a standard PM sample of urban dust particles. Exposure of PBS or PM started 12 days before transplant and continued every 3 days for a total of 9 doses. Skin graft was performed on the day of the fifth dose. C, Female B6 mice received male B6 skin grafts and were exposed to i.n. instillation of either PBS (n = 11, MST = 25) or SRM1649b suspended in PBS (n = 16, MST = 31). D, Female AHR null mice with a B6 background mice received male B6 skin grafts and were exposed to i.n. instillation of either PBS (n = 7, MST = 21) or SRM1649b suspended in PBS (n = 10, MST = 17.5). Kaplan-Meier analysis was used to compare treatment groups.
Given the lack of effect on a fully mismatched skin graft model, which is mediated primarily by a Th1/IFNγ immune response, a second model of male B6 skin to female B6 recipient was used. This model has been shown to have graft rejection mediated via a Th17/IL-17A–biased immune response28 Unexpectedly, the mice that received SRM1649b intranasally had significantly prolonged GST (P < 0.01) compared with controlled mice that received PBS (Figure 2C). SRM1649b is a complicated mixture that includes PAHs, polychlorinated biphenyl congeners, chlorinated pesticides, nitro-substituted PAHs, decabromodiphenyl ether, toxaphene congeners, polychlorinated dibenzo-p-dioxin, and dibenzofuran congeners, hopanes, steranes, ketones, and alkanes. Of these compounds, the PAHs are known AHR ligands found at the highest concentrations in SRM1649b. Using AHR−/− female recipient mice with a B6 background, male skin grafts were found to reject more quickly when recipient mice were treated with SRM1649b i.n. compared with control (Figure 2). These data support that PM causes prolongation of skin GST through an AHR-dependent mechanism but may also contain components that accelerate graft loss by an AHR-independent mechanism. Histological analysis did not show any dramatic differences in pathology, including damage to keratinocytes or inflammatory infiltrates.
Intranasal PM has Minimal Effect on Either Protolerogenic or Proinflammatory Immune Responses to Male Antigens in the B6 Male-to-Female Skin Graft Model
To examine the effect of i.n. PM on the immune response of female B6 mice to the male skin graft, female mice were exposed to i.n. SRM1649b or PBS and transplanted with male B6 skin as mentioned previously. Recipient mice were killed on day 18 before rejection. Skin grafts were harvested for evaluation. RT-PCR analysis showed no significant difference in mRNA expression as measured by RT-PCR of protolerogenic markers FoxP3 and indoleamine 2,3-dioxygenase or proinflammatory markers IFNγ or IL-17A (Figure 3A). In contrast to our previously reported findings in the lungs of PM-treated mice, there was no significant relative increase in Cyp1A1 mRNA, a common marker of AHR activation, in the skin grafts of PM-treated mice (Figure 3A). Furthermore, a delayed-type hypersensitivity assay performed before euthanasia demonstrated that treatment with PM did not increase or decrease the immune response to HY antigen, because there was no difference in the net swelling response seen in PBS- versus PM-treated mice (Figure 3B). Similarly, HY peptide stimulation of day 18–harvested SPLs in vitro resulted in comparable increases in both IL-17A and IFNγ levels in culture supernatant (Figure 3C, D). Based on these data, we did not demonstrate in this model that the prolongation of skin graft survival seen in PM-treated female B6 mice engrafted with male B6 skin was due to suppression or regulation of the anti–male immune response.
FIGURE 3.
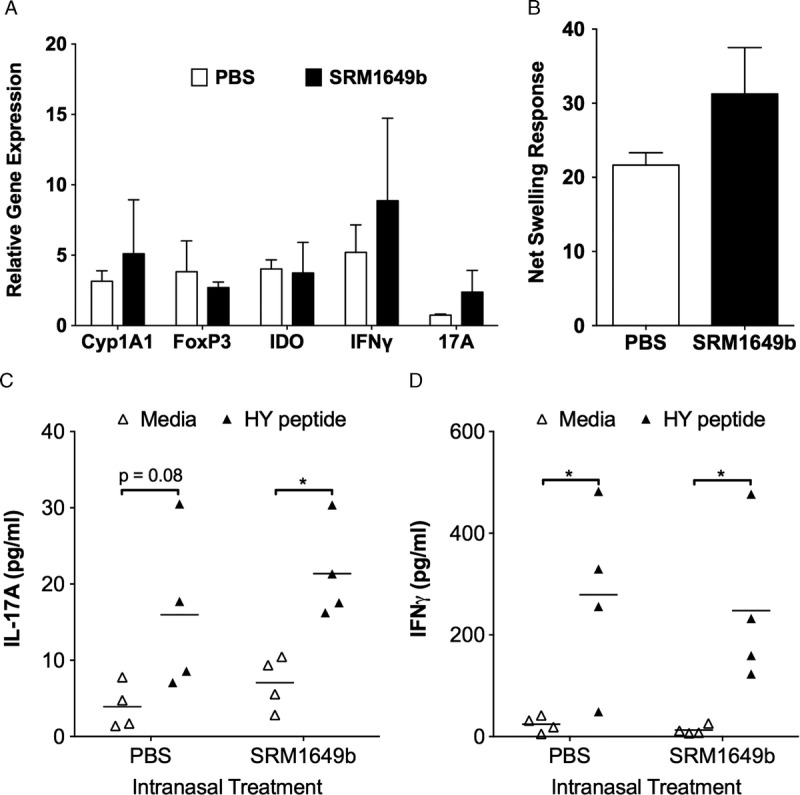
Intranasal instillation of PM does not suppress immune responses to male antigen after engraftment of male B6 skin on female B6 recipient mice. Male B6 skin grafts were harvested from PBS- and SRM1649b-treated mice on day 18 posttransplant and processed for A, RT-PCR analysis of mRNA expression for markers of AHR activation (Cyp1A1), immune tolerance (FoxP3, indoleamine 2,3-dioxygenase), or inflammation (IFNγ, IL-17A). PCR data were normalized to actin-b expression. B, Female B6 mice received male B6 skin grafts and were exposed either PBS (n = 3) or SRM1649b suspended in PBS (n = 3) and were tested for responsiveness to the male HY antigen in a direct delayed-type hypersensitivity assay on day 18 posttransplant. Net swelling was determined by subtracting the footpad thickness at the time of injection from the footpad thickness 24 hours later. C and D, SPLs were isolated from female B6 mice treated with either PBS (n = 4) or SRM1649b (n = 4) 18 days posttransplant and stimulated in vitro for 3 days +/− Dby peptide. Culture supernatants were harvested, and IL-17A (C) and IFNγ (D) concentration were measured by ELISA. ELISA data were analyzed by paired Student t test. *P < 0.05.
Infiltration of IFNγ-Producing CD4 T Cells Into the Male Skin Graft of Female Recipient B6 Mice is Delayed by Intranasal PM Treatment
Given the lack of evidence for a suppressive effect of PM exposure on the anti-HY immune response, PM-mediated alterations in cell trafficking were examined using flow cytometry. Female B6 mice were treated with PBS or SRM1649b as previously described and engrafted with male B6 skin. SPLs, axillary LN cells, and graft-infiltrating lymphocytes (GILs) were harvested on either post-operative day (POD)7 or POD13 and subjected to intracellular cytokine staining for expression of both IL-17A and IFNγ in CD4+ FoxP3− (CD4 effector T [Teff] cell), CD4+ FoxP3+ (CD4 Treg cells), CD8β+ (CD8), and CD4− CD8β− double negative (DN) T cell subsets. Unlike SPLs and LNs where CD4 Teff and CD8 T cells dominated, GILs were comprised of equal frequencies to CD4 Teff cells, CD4 Treg cells, and DN T cells and a small percentage of CD8 T cells (Figure 4A; Figure S1, SDC, http://links.lww.com/TXD/A37) on POD7. At this point, mice receiving SRM1649b had a significantly higher frequency of CD4 Teff cells but lower frequency of CD4 Treg cells in the GILs compared with controlled mice (Figure 4A). Although there was no difference between control and treatment in the frequency of IL-17A–producing cells in any of the T cell subsets examined (Figure 4B), SRM1649b exposure did reduce the frequency of IFNγ-producing CD4 Teff cells in the GIL on POD7 (Figure 4C). No significant differences were found on POD13 between PBS and SRM1649b in any cell type because the composition of T cells subsets with the GILs shifted toward a CD8 T cell–dominant response (Figure S2, SDC, http://links.lww.com/TXD/A37). Preliminary data suggest that the reduction of Th1 cells from the graft in PM-treated mice is AHR-dependent (Figure S3, SDC, http://links.lww.com/TXD/A37).
FIGURE 4.
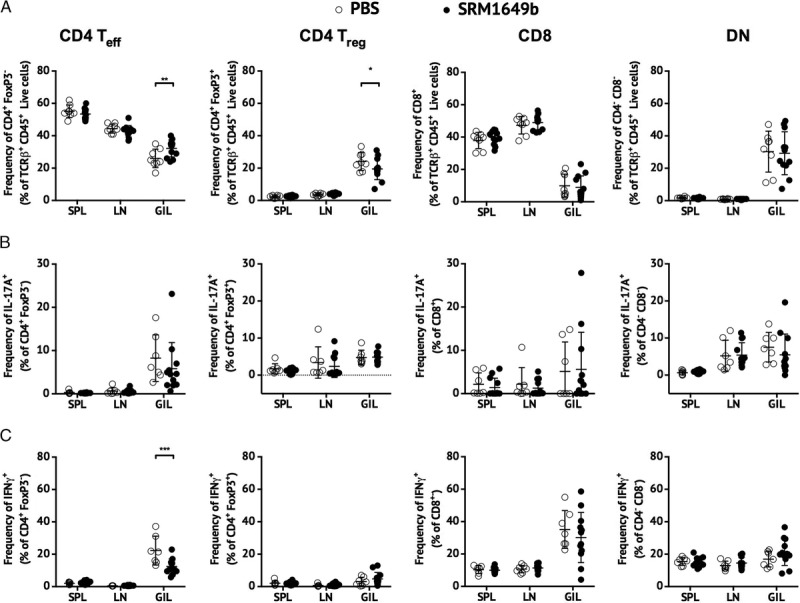
Intranasal instillation of PM delays graft infiltration of IFNγ-expressing CD4 T cells after the male B6 skin to female B6 recipient skin graft model. SPLs, LNs, and GILs were harvested from PBS-treated (n = 8) and SRM1649b-treated (n = 12) female mice on day 7 posttransplant and stimulated with PMA/ionomycin in the presence of Brefeldin A for flow cytometric analysis of cytokine-expressing cells. A, percentage of TCRβ T cells that are Teff (CD4+FoxP3−), Treg (CD4+FoxP3+), CD8 (CD8β+), and DN (CD4−CD8β−) subsets. B, percentage of IL-17A-expressing within the Teff, Treg, CD8, and DN T cell subsets. C, percentage of IFN-expressing within the Teff, Treg, CD8, and DN T cell subsets. The data were analyzed using a repeated measures 2-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
AHR Expression Is Upregulated in Th1 Cells During Immune Responses In Vivo
The adverse effect by PM on the accumulation of Th1 cells within the skin graft was surprising to us given that RT-PCR experiments in vitro suggest that Th1 cells express minimal levels of AHR compared with either Treg cells or Th17 cells. To examine whether the AHR expression pattern was similar in vivo, AHR was measured within graft-infiltrating cells by flow cytometry using a monoclonal antibody specific to mouse AHR. Specificity of this antibody was determined by comparing the fluorescent signal in splenic CD4 T cells from B6 or AHR null mice cultured overnight in the presence of anti-CD3 antibody, anti-CD3 antibody with hTGFβ, or anti-CD3 antibody with hTGFβ/mIL-6. Cells from both strains had similar staining with the AHR antibody suggesting that AHR expression is below detection when stimulated with anti-CD3 antibody only (Figure 5A). However, when cultured with hTGFβ +/− mIL-6, only CD4 T cells from B6 mice demonstrated increased AHR expression. In addition to adding TGFβ +/− IL-6 to the cultures, upregulation of the AHR required T-cell receptor (TCR) stimulation (Figure S4, SDC, http://links.lww.com/TXD/A37). A similar experiment was performed using AHR null or littermate AHR+/− SPLs cultured overnight anti-CD3 antibody + hTGFβ/mIL-6 to examine IFNγ- and IL-17A-producing cells as well as FoxP3+ cells. Only cells from the AHR+/− mouse were positive for AHR expression, including a large percentage of Th1 (IFNγ+) and Treg (FoxP3+) cells that are AHRhigh (Figure 5B).
FIGURE 5.
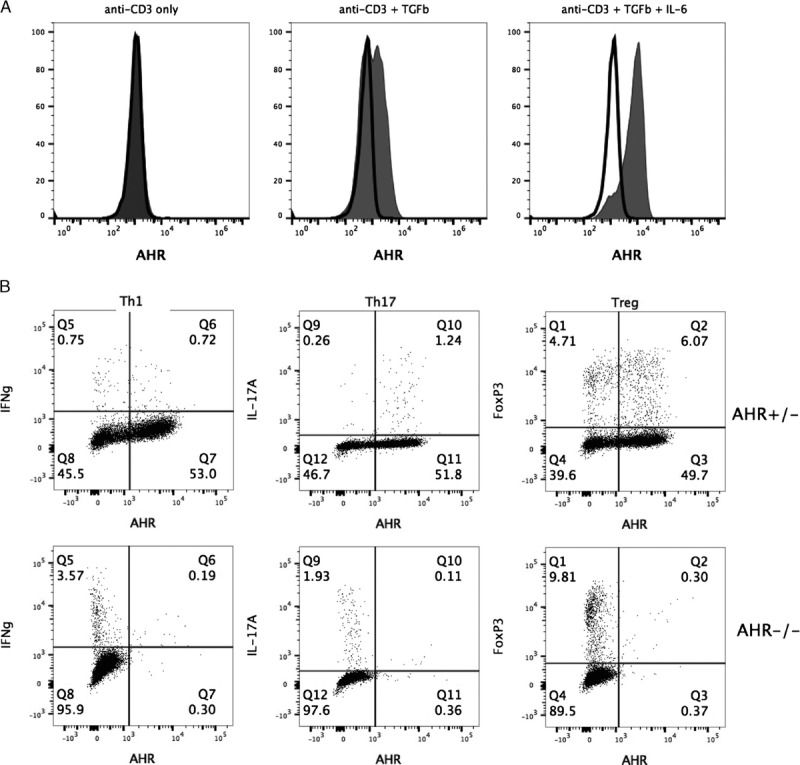
Upregulation in vitro of AHR expression in CD4 T cells is not limited to the Th17 subset. A, SPLs from B6 mice (gray fill) or AHR−/− mice (black line) were stimulated with anti-CD3/CD28 +/− hTGFβ +/− mIL-6. Sixteen hours later, AHR expression within the CD4 T cell population was measured by flow cytometry. B, SPLs from AHR+/− or AHR−/− mice were stimulated with anti-CD3/CD28 +/− hTGFβ/mIL-6. Sixteen hours later, cells were stimulated for intracellular cytokine staining and, 4 hours later, stained to measure AHR expression within Th1 (IFNγ+), Th17 (IL-17A+), or Treg (FoxP3+) CD4 T cells.
To examine the expression of AHR in T cells after skin engraftment, an experiment was performed using female B6, male B6, and BALB/c skin graft donors transplanted into untreated female B6 recipient mice. Ten days after engraftment, SPLs, axillary LN cells, and GILs were analyzed for AHR expression in both FoxP3− and FoxP3+ CD4 T cell subsets. In all cases, there was enhanced expression of the AHR found in both FoxP3+ and FoxP3− GIL CD4 T cells (Figure 6A). Additional experiments were performed in the male B6 to female B6 skin graft model to further identify which CD4 T cell subsets demonstrated AHR upregulation after transplantation. Compared with other subsets, Th17 cells generally had the highest level of AHR median fluorescent intensity (MFI) at all 3 locations, and there was no significant difference in the MFI between any of the 3 cell sources (Figure 6B). In contrast, both TH1 and Treg cells demonstrated higher AHR expression within the graft compared with analogous cells from spleen or LN. This suggests that, during inflammatory responses, the AHR is upregulated in responder T cells located at the site of the immune response.
FIGURE 6.
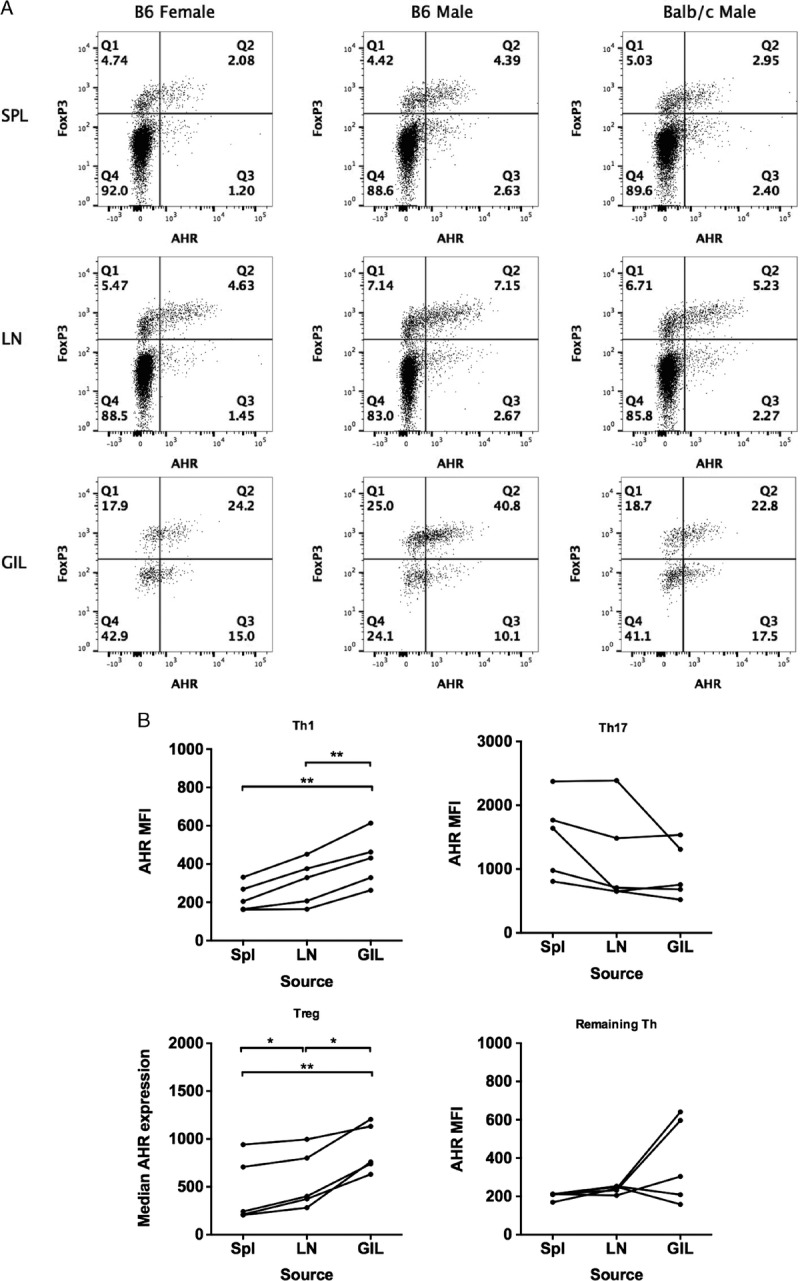
AHR expression is upregulated within skin graft-infiltrating Th1 and Treg CD4 T cells. A, Female B6 mice were engrafted with female B6, male B6, or BALB/c skin without immunosuppression. Seven days later, SPLs, LN, and GIL were isolated. Cells were stained for flow cytometry to identify CD4 Teff (FoxP3−) and Treg (FoxP3+) cells and to measure AHR expression. The data are representative of 2 to 5 mice per graft source. B, Female B6 mice (n = 5; pooled from 2 experiments) were engrafted with male B6 skin, and 7 days later, SPLs, LNs, and GILs were isolated. Cells were stimulated with PMA/ionomycin in the presence of Brefeldin A for flow cytometric analysis of cytokine-expressing cells. The expression of AHR was measured by median fluorescence intensity in Th1 (FoxP3−, IFNγ+), Th17 (FoxP3−, IL-17A+), Treg (FoxP3+), or the remaining CD4 T cells (FoxP3−, IFNγ−, IL-17A−). To analyze the data accounting for the measurement of AHR expression in T cells from SPL, LN and GIL within the same mouse, a repeated measures 1-way ANOVA was used. *P < 0.05, **P < 0.01.
DISCUSSION
Our laboratory has previously reported that skin GST could be prolonged in a major-mismatched murine skin graft model by administration of the exogenous AHR ligand, TCDD, whereas administrations of an endogenous AHR ligand, FICZ, shortened GST.26 These results were anticipated given the previous data that TCDD could enhance tolerogenic T cell responses in vitro and in vivo4 and that FICZ was associated with proinflammatory Th17 responses in vitro and in vivo.3 SRM1649b, a standard PM sample that contains numerous exogenous AHR ligands, has been shown by our laboratory to enhance Th17 responses in cell culture and when given to mice intranasally.12 The data in the current study demonstrate that exposure to SRM1649b does not affect GST in the BALB/c to B6 skin graft model used in our previous study. Given that most AHR ligands in SRM1649b have less AHR activity than either TCDD or FICZ and that this major mismatch model is not dependent on a Th17 response, this result is not entirely surprising.
Rejection of male B6 to female B6 skin grafts in response minor HY antigens has been previously shown to be driven by Th17 cells and a subsequent neutrophil infiltration.28 We hypothesized that exposure to PM would accelerate rejection in this minor-mismatched model, secondary to an increase in a Th17 response. The results were exactly the opposite, with median GST increasing by 6 days (PBS median GST = 25 days vs SRM1649b median GST = 31 days). This delay in graft rejection was not associated with any convincing evidence of a change in the quality of Th17 immune response to male Dby antigen as measured by IL-17A production by SPLs in vitro or the fraction of GIL that are IL17-producing CD4 T cells. Delayed graft rejection was AHR-dependent, however, as treatment of AHR null mice with SRM1649b resulted in a significantly more rapid graft rejection (median GST = 17.5 days) compared with PBS-treated mice (median GST = 21 days). These results suggest that (1) the AHR may inhibit the rejection response and (2) PM may contain compounds that can promote rejection in an AHR-independent manner. The effects of the inhaled AHR ligands were likely not limited to the lung but were systemic, because inhaled PAHs have been shown to be rapidly absorbed through alveolar epithelium after inhalation and appear in the circulation, LNs, and various organs. This effect is even more robust when PAHs are associated with PM.29-31 Although not done in this study, we have previously seen effects in the lung12 and LNs (data not shown) after inhalation of PAH-containing PM. Given that our finding of graft prolongation is AHR-dependent, the active compounds are likely either the PAHs or products of their metabolism by the cytochrome P450 enzymes that themselves are ligands of the AHR.
Mice with a global AHR deletion have been characterized as having several physiologic phenotypes including patent ductus venosus to the liver, splenomegaly, and altered gut immune-cell composition.27,32-35 The effect of AHR deletion in mouse immune response models has been mixed. In the BALB/c to B6 skin graft model and an alloimmune challenge model with P815 tumor cells, AHR null mice behaved similar to wild type B6 mice.26,36 In contrast, we (unpublished observations) and others have found induction of experimental autoimmune encephalomyelitis in AHR null mice results in delayed onset and reduced clinical score compared with controlled mice.3 This suggests that the AHR activation enhances autoimmunity. Finally, AHR-deficient mice are generally more susceptible to bacterial infections,35,37,38 possibly secondary to the alterations in gut immune cells in these mice, although this difference in response to colitis models seems to depend on which model is tested.39 Taken together, the effect of the AHR on immune responses is complex, and the model described here offers many tools and opportunities for uncovering more on the AHR's role in minor-antigen alloimmune responses.
On day 7 after transplant, a reduction in the fraction of IFNγ-producing CD4 T cells infiltrating the male B6 skin graft of female B6 mice treated with SRM1649b may have played a role in delayed rejection of these grafts. This finding suggests that PM may either hamper the development of alloreactive Th1 cells or may alter trafficking of Th1 cells but not other T cell subsets. However, we would not have predicted that a delay in accumulation of Th1 cells would be the mechanism behind the delay in graft rejection given that graft loss in the male B6 to female B6 skin graft model is more dependent on a Th17 response than a Th1 response. Previous studies show that unlike IL-17A blockade, IFNγ blockade did not affect the survival of male grafts on female recipient mice.28,40 However, Th1 cells may contribute to the rejection of the male skin graft in an IFNγ-independent manner.
Recent studies have focused on the role of the AHR in Th17, Treg, and Tr1 cells rather than Th1 cells.3,4,6,12,41,42 Furthermore, AHR expression has been reported as being low in most T cell subsets other than those just mentioned. To determine whether AHR expression is regulated during immune responses, we used flow cytometry to examine the level of AHR expression in T cell subsets both in vitro and in vivo. Th17 cells were identified as the CD4 subset with the highest AHR expression levels, and they did increase in vitro after TCR stimulation. However, AHR expression was not altered in Th17 cells in vivo when examined in spleen, LN, or the skin graft on day 7 posttransplant. In contrast, AHR expression was found to be significantly increased in Th1 and Treg cells both in vivo within GIL and in vitro. This upregulation of the AHR in Th1 cells suggests that the AHR does indeed have physiological role in Th1-cell biology and that AHR ligands can directly alter the Th1 responses. This novel finding can be investigated more thoroughly with the use of transgenic mouse strains with T cell– or dendritic cell–specific deletion of the AHR to delineate the cell type through which PM-derived AHR ligands are acting. Furthermore, adoptive transfer experiments using either AHR wild-type and AHR null T cells into B6 Rag1−/− female or fluorescently tagged TCR-transgenic Marilyn CD4 T cells43 into AHR null and wild type controlled mice would more accurately identify the specific alterations in T cell biology that may account for our findings.
There are other potential mechanisms to explain the effects of PM on graft survival in this model, and further studies will be needed to test these possibilities. For instance, skin transplantation is a nonvascularized model, and the AHR is expressed in endothelial cells44 and has a role in vascular development and endothelial cell function.45-47 PM exposure may alter revascularization that in turn delays graft rejection. Second, PM-mediated graft prolongation may involve cell trafficking. These grafts did not become tolerant but instead exhibited delayed rejection, and PM inhalation may alter the expression of chemokines and other factors within the lungs that alter cell trafficking. This could potentially explain the reduction of IFNγ-producing CD4 T cells in the treated grafts. Indeed, PM and its components have been shown to increases expression of chemokines, such as MCP-1/CCL2 and MIP-2/CXCL2.48,49 Regardless of the exact mechanism, we have identified a reliable transplant model for examining in detail the role of the AHR in alloimmunity and transplantation.
Supplementary Material
Footnotes
Published online 25 April, 2017.
This work was supported in part by grants from the National Institute of Environmental Health Sciences at the National Institutes of Health (1RO1ESO23842-01 and 1R21ESO25304-01 to J.D.M.) and the National Cancer Institute at the National Institutes of Health (T32CA090217-14 to W.J.). Further funding was received from Department of Surgery, Division of Transplant Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI. Funding for the BD LSR Fortessa used through the University of Wisconsin Carbone Cancer Center (UWCCC) Flow Cytometry Laboratory was provided by National Institutes of Health (1S100OD018202-01).
The authors declare no conflicts of interest.
W.J. and J.H.F. performed experiments, analyzed data, and wrote the article; L.O., C.O., L.Z., J.A.S., L.F., and A.M. performed experiments and analyzed data; and J.D.M. participated in the oversight of the project, provided all the funding, analyzed the data, and wrote and edited the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. [DOI] [PubMed] [Google Scholar]
- 2.Marshall NB, Vorachek WR, Steppan LB, et al. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181:2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. [DOI] [PubMed] [Google Scholar]
- 4.Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. [DOI] [PubMed] [Google Scholar]
- 5.Julliard W, Fechner JH, Mezrich JD. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front Immunol. 2014;5:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezrich JD, Nguyen LP, Kennedy G, et al. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PLoS One. 2012;7:e44547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence BP, Denison MS, Novak H, et al. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte JH, Di Meglio P, Hirota K, et al. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One. 2013;8:e79819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steenhof M, Mudway IS, Gosens I, et al. Acute nasal pro-inflammatory response to air pollution depends on characteristics other than particle mass concentration or oxidative potential: the RAPTES project. Occup Environ Med. 2013;70:341–348. [DOI] [PubMed] [Google Scholar]
- 10.Kido T, Tamagawa E, Bai N, et al. Particulate matter induces translocation of IL-6 from the lung to the systemic circulation. Am J Respir Cell Mol Biol. 2011;44:197–204. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoli-Rocha F, Fernandes S, Einicker-Lamas M, et al. Roles of oxidative stress in signaling and inflammation induced by particulate matter. Cell Biol Toxicol. 2010;26:481–498. [DOI] [PubMed] [Google Scholar]
- 12.van Voorhis M, Knopp S, Julliard W, et al. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazzola M, Matera MG. IL-17 in chronic obstructive pulmonary disease. Expert Rev Respir Med. 2012;6:135–138. [DOI] [PubMed] [Google Scholar]
- 14.Silverpil E, Linden A. IL-17 in human asthma. Expert Rev Respir Med. 2012;6:173–186. [DOI] [PubMed] [Google Scholar]
- 15.Verleden SE, Scheers H, Nawrot TS, et al. Lymphocytic bronchiolitis after lung transplantation is associated with daily changes in air pollution. Am J Transplant. 2012;12:1831–1838. [DOI] [PubMed] [Google Scholar]
- 16.Nawrot TS, Vos R, Jacobs L, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66:748–754. [DOI] [PubMed] [Google Scholar]
- 17.Bhinder S, Chen H, Sato M, et al. Air pollution and the development of posttransplant chronic lung allograft dysfunction. Am J Transplant. 2014;14:2749–2757. [DOI] [PubMed] [Google Scholar]
- 18.Fan L, Benson HL, Vittal R, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basha HI, Ramachandran S, Tiriveedhi V, et al. Critical role for IL-17A/F in the immunopathogenesis of obliterative airway disease induced by Anti-MHC I antibodies. Transplantation. 2013;95:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Voorhis M, Fechner JH, Zhang X, et al. The aryl hydrocarbon receptor: a novel target for immunomodulation in organ transplantation. Transplantation. 2013;95:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julliard W, Owens LA, O'Driscoll CA, et al. Environmental exposures-the missing link in immune responses after transplantation. Am J Transplant. 2016;16:1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: an environmental factor contributing to intestinal disease. J Crohns Colitis. 2011;5:279–286. [DOI] [PubMed] [Google Scholar]
- 24.Bai N, Khazaei M, van Eeden SF, et al. The pharmacology of particulate matter air pollution-induced cardiovascular dysfunction. Pharmacol Ther. 2007;113:16–29. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Park S, Lim CW, et al. The role of air pollutants in initiating liver disease. Toxicol Res. 2014;30:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauly SK, Fechner JH, Zhang X, et al. The aryl hydrocarbon receptor influences transplant outcomes in response to environmental signals. Toxicol Environ Chem. 2012;94:1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt JV, Su GH, Reddy JK, et al. Characterization of a murine AHR null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vokaer B, Van Rompaey N, Lemaitre PH, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 2010;185:3417–3425. [DOI] [PubMed] [Google Scholar]
- 29.Gerde P, Muggenburg BA, Lundborg M, et al. Respiratory epithelial penetration and clearance of particle-borne benzo[a]pyrene. Res Rep Health Eff Inst. 2001:5–25; discussion 27–32. [PubMed] [Google Scholar]
- 30.Uski OJ, Happo MS, Jalava PI, et al. Acute systemic and lung inflammation in C57Bl/6 J mice after intratracheal aspiration of particulate matter from small-scale biomass combustion appliances based on old and modern technologies. Inhal Toxicol. 2012;24:952–965. [DOI] [PubMed] [Google Scholar]
- 31.Aquilina NJ, Delgado-Saborit JM, Meddings C, et al. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ Int. 2010;36:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JS, Cella M, McDonald KG, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. [DOI] [PubMed] [Google Scholar]
- 34.Kiss EA, Vonarbourg C, Kopfmann S, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. [DOI] [PubMed] [Google Scholar]
- 35.Qiu J, Heller JJ, Guo X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vorderstrasse BA, Steppan LB, Silverstone AE, et al. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharmacol. 2001;171:157–164. [DOI] [PubMed] [Google Scholar]
- 37.Kimura A, Abe H, Tsuruta S, et al. Aryl hydrocarbon receptor protects against bacterial infection by promoting macrophage survival and reactive oxygen species production. Int Immunol. 2014;26:209–220. [DOI] [PubMed] [Google Scholar]
- 38.Wagage S, Harms Pritchard G, Dawson L, et al. The group 3 innate lymphoid cell defect in aryl hydrocarbon receptor deficient mice is associated with T cell hyperactivation during intestinal infection. PLoS One. 2015;10:e0128335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Julliard W, De Wolfe TJ, Fechner JH, et al. Amelioration of clostridium difficile infection in mice by dietary supplementation with indole-3-carbinol. Ann Surg. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YH, Lim Y, Park CG. Influence of interferon-γ deficiency in immune tolerance induced by male islet transplantation. Immune Netw. 2011;11:358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mezrich JD, Fechner JH, Zhang X, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mascanfroni ID, Takenaka MC, Yeste A, et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lantz O, Grandjean I, Matzinger P, et al. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. [DOI] [PubMed] [Google Scholar]
- 44.Agbor LN, Elased KM, Walker MK. Endothelial cell-specific aryl hydrocarbon receptor knockout mice exhibit hypotension mediated, in part, by an attenuated angiotensin II responsiveness. Biochem Pharmacol. 2011;82:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasquez A, Atallah-Yunes N, Smith FC, et al. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by AhR knockout mice. Cardiovasc Toxicol. 2003;3:153–163. [DOI] [PubMed] [Google Scholar]
- 46.Lahvis GP, Lindell SL, Thomas RS, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A. 2000;97:10442–10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walisser JA, Bunger MK, Glover E, et al. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279:16326–16331. [DOI] [PubMed] [Google Scholar]
- 48.Gorr MW, Youtz DJ, Eichenseer CM, et al. In vitro particulate matter exposure causes direct and lung-mediated indirect effects on cardiomyocyte function. Am J Physiol Heart Circ Physiol. 2015;309:H53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Natarajan S, Vaickus LJ, et al. Diesel exhaust particulates exacerbate asthma-like inflammation by increasing CXC chemokines. Am J Pathol. 2011;179:2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


