SUMMARY
Since the discovery of the CD40-CD154 costimulatory pathway and its critical role in the adaptive immune response, there has been considerable interest in therapeutically targeting this interaction with monoclonal antibodies in transplantation. Unfortunately, initial promise in animal models gave way to disappointment in clinical trials following a number of thromboembolic complications. However, recent mechanistic studies have identified the mechanism of these adverse events, as well as detailed a myriad of interactions between CD40 and CD154 on a wide variety of immune cell types and the critical role of this pathway in generating both humoral and cell-mediated alloreactive responses. This has led to resurgence in interest and the potential resurrection of anti-CD154 and anti-CD40 antibodies as clinically viable therapeutic options.
Keywords: Transplantation, CD40, CD40L, thromboembolism, costimulation, Fc receptor
A myriad of studies over the past two decades have revealed the importance of CD40-CD154 interactions in the generation of alloreactive responses, and as such it represents an extremely attractive target for therapeutic intervention in transplantation. This pathway requires the interaction of the CD40 molecule with its ligand, CD40L (CD154). These molecules belong to the tumor necrosis factor (TNF) superfamily and are expressed on a wide range of tissues and cell types, with CD40 constitutively expressed on antigen-presenting cells (APC), including B cells, macrophages and dendritic cells (DC), and CD154 inducibly expressed mainly on CD4+ T cells and endothelial cells following activation [1]. The first descriptions of this pathway focused on the importance of CD40-CD154 interactions in promoting T cell-dependent humoral responses [2], but it quickly became apparent that these interactions were also essential for generating cell-mediated immunity. Seminal studies showed that the crosslinking of CD40 on dendritic cells by CD154 on activated CD4+ helper T cells led to “licensing” of the APC, leading to the upregulation of the B7 family of costimulatory molecules and the elaboration of proinflammatory cytokines [3, 4]. More recent studies have also suggested that the interaction of CD154 on activated CD4+ T cells and CD40 on CD8+ T cells also plays a critical role in generating effective cytotoxic T cell responses [5].
The potent immunostimulatory effects of CD40-CD154 interactions make it one of the most attractive targets for immunomodulatory therapy. A number of preclinical rodent and non-human primate studies in islet and solid organ transplantation showed the dramatic efficacy of targeting this pathway with anti-CD154 monoclonal antibodies [6–8], and thus several clinical trials were initiated to test their efficacy in autoimmune disease and transplantation. Three separate antibodies – ruplizumab, toralizumab, and ABI793 – were developed and tested, but in a number of these trials patients experienced thromboembolic complications (reviewed in [9]). Several groups attempted to confirm these results in non-human primate studies, with some reports confirming the thrombogenic properties of anti-CD154 antibodies [10], while other studies saw no evidence of thromboembolism [11]. Other reports suggested that agents co-administered with anti-CD154, such as calcium chloride, may have been responsible for adverse events [12]. However, given the recurrence of thromboembolism during multiple trials with anti-CD154 antibodies that bound distinct epitopes of CD154, many groups sought to elucidate the mechanism by which anti-CD154 therapy led to these adverse events.
CD40 and CD154 are expressed on endothelial cells and activated platelets, respectively, and while CD40-CD154 interactions have been shown to play a role in thrombus stabilization and platelet activation [13], the exact nature of the required interactions is still somewhat unclear. Indeed, unequivocal evidence of the mechanism linking anti-CD154 therapy-related thromboembolism to expression of CD154 on platelets was still lacking. Several studies have investigated the potential of CD154 to interact to molecules distinct from its classical interaction with CD40, and the possible role of these interactions in the etiology of observed clinical thromboembolisms. One study in particular by Andre et al suggested that CD154 is able to bind an αIIbβ3 integrin, and that the inhibition of this interaction by anti-CD154 therapy may destabilize platelet plugs [14]. Another set of reports demonstrated the ability of CD154 to bind Mac-1 (CD11b) on monocytes and macrophages [15, 16]. The expression of CD154 on activated endothelium allowed for the recruitment and migration of Mac-1-expressing leukocytes into atherosclerotic lesions, which could be inhibited by the systemic injection of a peptide that blocked the specific binding motif used by CD154 to interact with Mac-1 [15]. These results highlight the myriad roles that CD154 plays in both immune and inflammatory conditions and the potential consequences of global inhibition, as well as demonstrate the potential for the development of strategies that prevent specific CD154 interactions while preserving others. However, several in vitro studies pointed to the low affinity activating Fc receptor FcγRIIa as the major cause of anti-CD154 antibody-mediated platelet activation seen during clinical trials [17–19]. These studies argued that immune complexes of anti-CD154 antibodies and soluble CD154 (sCD154), which is released from CD4+ T cells following activation, could crosslink FcγRIIa on human platelets, leading to further platelet activation and thromboembolism. Furthermore, this Fc receptor is not expressed on murine platelets, which would explain the lack of evidence for thromboembolism in mouse studies. In fact, in a study using a humanized mouse model in which the human FcγRIIa was expressed by murine platelets, Robles-Carrillo and colleagues observed platelet activation and thrombus formation in mice following the administration of preformed immune complexes of sCD154 and anti-CD154 antibodies [20]. Further support for this hypothesis can be found in a study demonstrating that anti-thymocyte globulin, a preparation of polyclonal antibodies against human thymocytes, can also activate platelets via an Fc receptor-dependent mechanism [17, 21]. Therefore, if anti-CD154 antibodies could be designed to avoid this Fc receptor interaction, that may prevent clotting events from occurring, thus providing a safer alternative to the pro-thrombotic anti-CD154 therapeutics. In addition, several studies have attempted to alternatively disrupt CD40-CD154 interactions by targeting the CD40 molecule, which will be discussed later in the review.
NEW MECHANISTIC INSIGHTS
As mentioned above, CD40-CD154 ligation leads to a number of profoundly immunostimulatory events, including the licensing of APC, class switching of B cells, and T cell activation. Initial studies determined that targeting CD40-CD154 interactions with anti-CD154 antibodies inhibited the expansion and effector function of alloreactive CD4+ and CD8+ effector T cells, and led to an expansion of regulatory CD4+ T cell populations [22]. However, a number of recent studies have furthered our understanding of the mechanisms underlying these phenomena, and the interactions required for efficacy of these therapies.
Antigen-presenting cells and cytokine production
Historically, the main interaction targeted by anti-CD154 antibodies has been that of CD154 on activated CD4+ T cells and the CD40 molecules constitutively expressed on APC. APC licensing through the ligation of CD40 leads to the upregulation of costimulatory molecules such as CD80 and CD86, as well as major histocompatibility (MHC) class I and II molecules, and the elaboration of pro-inflammatory cytokines such as IL-6, IL-12, and TNF [23]. Our group recently demonstrated that the use of anti-CD154 antibodies prevents the production of these cytokines by APC, but does not inhibit their upregulation of costimulatory or MHC molecules [24]. While these results are surprising given the accepted model of CD40 ligation leading to DC licensing, the power of these proinflammatory cytokines to promote allograft rejection has been demonstrated by a number of recent studies. APC-derived TNF and IL-6 were shown to synergize to promote alloreactive T cell proliferation and allograft rejection [25], and IL-6 deficiency or neutralization can prolong graft survival in the presence of CD28 costimulation blockade [26]. IL-6 is of particular interest in the field of CD40-CD154 antagonism, given that this cytokine acts as a rheostat to control the development of CD4+ T cells into either regulatory T cells or Th17 cells [27], and the conversion of naïve CD4+ T cells into Foxp3+ regulatory T cells is one of the hallmarks of CD40-CD154 blockade [28]. Given these results, it may be that one of the main mechanisms of action of CD154 blockade is the prevention of pro-inflammatory cytokine production by APC, and may explain why this therapy synergizes with other agents, such as the B7-CD28 blocker CTLA4-Ig, to more potently prevent alloreactivity and promote graft tolerance.
Additionally, differential intracellular signaling pathways downstream of CD40 have been shown to be important for the maturation and cytokine elaboration of dendritic cells. Mackey et al showed that by mutating the intracellular portion of CD40 at the binding sites for different TNF receptor-associated factors (TRAF), they could affect distinct DC maturation programs [29]. Specifically, mutating the binding site for TRAF2, 3 and 5 as well as the site for TRAF6 prevented the upregulation of costimulatory molecules on DC, whereas mutating the site for TRAF6 alone prevented DC from producing IL-12, an important component of CD40-dependent DC licensing. This knowledge could have dramatic implications for the generation of small molecule inhibitors targeting specific intracellular signaling interactions for immunotherapy or the induction of tolerance following transplantation.
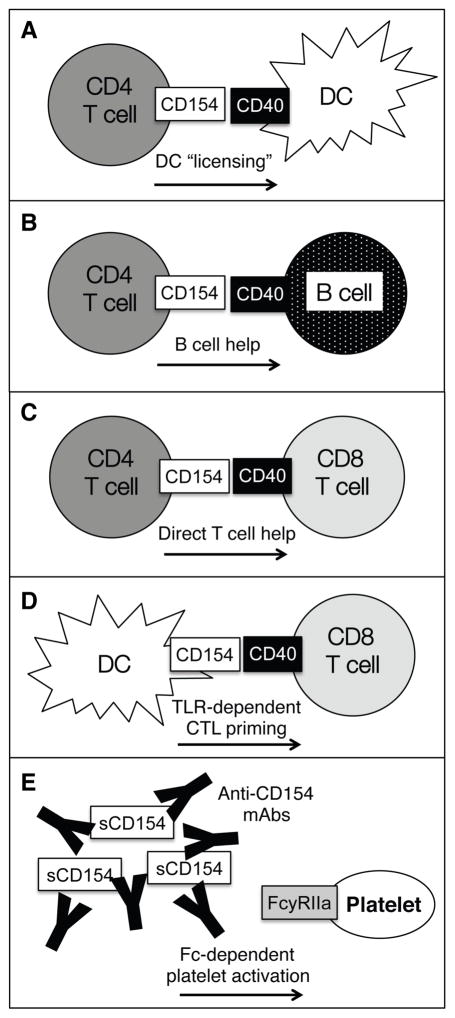
Another surprising recent finding describes the expression of CD154 on dendritic cells and its role in generating cell-mediated immune responses. This study used models of influenza and immunization to show that CD154 can be inducibly expressed on DC following activation of toll-like receptors by their microbial ligands, and this DC-expressed CD154 can provide help to CD8+ T cells during priming, even in the absence of CD4+ T cells [30]. These results highlight the complexity of CD40-CD154 signaling in immune responses and underscore the need for further studies detailing all of the interactions that are affected by blockade of these molecules, as shown in Figure 1.
Figure 1. Complexities of CD40-CD154 Interactions in the Immune System.
The TNF-family member CD40 and its ligand, CD40L (CD154), are expressed by a wide variety of cell types and the signaling induced by this binding leads to a range of potent immunostimulatory events. (A) Activated CD4+ T cells upregulate CD154, which binds to the CD40 expressed by dendritic cells. This leads to DC activation, which includes the production of proinflammatory cytokines such as IL-12. (B) CD4+ T cells and B cells undergo linked recognition, wherein T cells recognize their cognate peptide:MHC Class II complex expressed by B cells. T cells then provide costimulatory signals through B cell-expressed CD40, and this “T cell help” is critical for generating effective humoral responses. (C) CD4+ T cell-expressed CD154 can also provide direct help to CD8+ T cells through CD40, which has been suggested to play a role in the formation of CD8+ T cell memory. (D) CD154 is also inducibly expressed on DC following toll-like receptor ligation, and the interaction with CD40 expressed on CD8+ T cells can help generate potent pathogen-reactive CD8+ T cell responses. (E) A hypothesis for anti-CD154-associated thromboembolic complications centers on the expression of the activating Fc receptor FcγRIIa on human platelets, which can be crosslinked by immune complexes of soluble CD154 and anti-CD154 antibodies, leading to further platelet activation and undesired clotting events.
Induction of CD4+ regulatory T cells and the immune microenvironment
One of the most potent regulators of the adaptive immune response is the population of CD4+ CD25+ Foxp3+ regulatory T cells (Treg). These cells are critical for the control of self-reactive lymphocytes and have been shown in a number of models to be able to inhibit both acute and chronic allograft rejection [31]. While the expansion of Treg cell populations following CD154 antagonism has been noted for decades [32], their origin and antigen specificity remained unknown for some time. Recently, two distinct lineages of Treg have been described – thymically-derived natural Treg (nTreg), and peripherally induced Treg (iTreg) [33]. While some debate exists over the specific contributions of these populations to overall immunological tolerance, as well as the exact lineage markers that define them, it is clear that iTreg play an important, non-redundant role in maintaining peripheral tolerance, as the specific depletion of these cells following adoptive transfer to Foxp3-deficient mice led to profound autoimmunity [34]. However, in regards to CD40-CD154 blockade, it was unclear if the regulatory population seen following CD154 antagonism was a result of the expansion of pre-existing nTreg populations, or the peripheral induction of de novo iTreg. Using a transgenic ovalbumin model in which alloantigen-specific CD4+ and CD8+ T cells could be identified and tracked, our group demonstrated that a population of alloantigen-specific Treg expand following transplantation and anti-CD154 treatment, and that these cells are present in both secondary lymphoid organs and the allograft itself [28]. In addition, by crossing these antigen-specific CD4+ T cells to a Rag−/− background, we confirmed that naïve CD4+ Foxp3− T cells were induced to become Foxp3+ iTreg de novo following transplantation [28]. More recent data from another group showed that anti-CD154 therapy could enhance the ratio of intragraft Treg to effector CD4+ and CD8+ T cells in a model of orthotopic lung transplantation [35], again demonstrating the potency of CD154 antagonism to shift the balance of an alloreactive response toward a regulatory phenotype.
Another perhaps underappreciated aspect of anti-CD154-induced regulatory T cells involves the modulation of T cell immunity as a result of distinct localization patterns of Treg and alloreactive effector T cells in secondary lymphoid organs. One of the first studies to address this phenomenon showed that during tolerance induction with a regimen of anti-CD154 antibodies and donor-specific transfusion, tolerogenic plasmacytoid dendritic cells (pDC) were responsible for the conversion of alloantigen-specific iTreg [36]. Importantly, the migration of pDC to secondary lymphoid organs and their interaction with naïve effector cells in the location was required to convert these CD4+ T cells to iTreg, as blocking pDC lymph node homing with antibodies against CD62L prevented tolerance induction. While Treg have been known to be present in both secondary lymphoid organs as well as allograft tissue [31], this work highlights the importance of understanding the stepwise interactions of DC, regulatory cells and effector cells necessary for tolerance induction. Adding another layer of complexity, it appears that nTreg and iTreg have distinct trafficking patterns, as nTreg were shown to migrate to the inflamed tissue prior to returning to lymphoid organs in an islet transplant model [37], whereas iTreg are generated within the lymphoid organs themselves [36]. The most recent report also implicates laminins, a family of glycoproteins involved in cell adhesion and trafficking, as defining the fate of alloreactive T cells. Specifically, higher ratios of expression of laminin α4 to laminin α5 within high endothelial venules is required for colocalization of tolerogenic pDC, existing Treg, and naïve allospecific T cells within the cortical ridge of the lymph node, resulting in Treg induction and tolerance [38]. These basic findings clearly illustrate the complex nature of cell-cell interactions required for tolerance induction and the need to tailor costimulation blockade strategies accordingly.
Taken together, it would appear that the antigen-specific, induced Treg arise following CD154 blockade and play a critical role in the efficacy of this therapy in promoting long term graft survival and tolerance. Recently, the importance of antigen specificity in the ability of regulatory T cells to suppress alloreactive responses was highlighted in a study that showed that alloantigen-induced Treg could prolong graft survival of the MHC type used to induce the Treg, but not grafts expressing a third-party MHC [39]. Further studies will determine if the antigen-specific Treg induced specifically by CD154 blockade are necessary or sufficient to allow for long-term graft survival.
Direct CD4:CD8 T cell help
Another CD40-CD154 interaction that may play a role in modulating graft-reactive responses is the interaction of CD154 on activated CD4+ T cells and CD40 expressed by activated CD8+ T cells. While some studies have suggested that this arm of CD40 signaling is dispensable for mounting effective cytotoxic responses, the contribution of this form of “T cell help” to the generation and maintenance of CD8+ T cell populations remained somewhat controversial, and the potential requirement for these interactions in responses to transplant were unknown.
While resting CD8+ T cells do not express CD40, a percentage of CD8+ T cells upregulate this molecule following activation [5, 40]. An initial report from Bourgeois et al showed that in an adoptive transfer model in which CD8+ T cells were responding to the male histocompatibility antigen H-Y, CD40 was not required on CD8+ T cells for initial response but was necessary to generate populations capable of secondary recall responses [41]. Conversely, other studies indicated that CD40 expressed by CD8+ T cells is not required for optimal cytotoxic responses or memory formation in response to either a bacterial or viral infection [42, 43]. However, a critical difference in these studies is the nature of the immune challenge presented. CD40 on CD8+ T cells appeared to be required for an effective response to the tissue antigen H-Y, whereas in infectious models, microbial components may induce potent inflammatory responses that bypass the requirement for CD40-CD154 signaling.
Our lab recently investigated the role of this interaction in the generation of graft-reactive CD8+ T cell responses by again taking advantage of the transgenic ovalbumin system [5]. By using defined populations of alloantigen-specific CD4+ and CD8+ T cells, this system allowed for the specific genetic ablation of CD40 on a number of different cell subsets in isolation, including alloreactive CD8+ T cells, donor-derived antigen-presenting cells, or recipient-derived APC. CD40 deficiency on only CD8+ T cells was sufficient to prolong graft rejection and significantly reduce the expansion and cytokine production of this population. However, the origin of the CD154 signals that help generate these CD8+ responses is still unclear, as they may be provided by CD4+ or APC populations. Nonetheless, we confirmed that CD8+ T cell-intrinsic CD40 signals are not required in an infectious model, as CD40−/− OT-I T cells responded as well as WT OT-Is to a bacterial challenge [5]. Therefore, it would appear that the requirement for CD40 signaling on CD8+ T cells depends on the inflammatory microenvironment during T cell priming. While the source of CD154 signals cannot be determined from these studies, the ovalbumin model may provide a template for future studies to tease apart the exact interactions required for these effects.
Humoral immunity
The detrimental effect of alloreactive antibody responses on graft survival has also been recognized for decades, and recent work has focused on identifying cases of antibody-mediated rejection (AMR) and preventing them [44]. The CD40-CD154 pathway was first described as a critical interaction in the initiation of de novo T cell-dependent antibody responses [1], and thus should be an attractive target for preventing AMR. Using a novel tetramer binding strategy to identify alloreactive B cells, Chen and colleagues described the kinetics and magnitude of anti-donor antibody responses, and showed that treatment with anti-CD154 was able to dissolve ongoing germinal center reactions and abrogate graft rejection [45].
However, as in T cell responses, B cell and antibody memory generated by previous exposure to HLA antigens or by heterologous immunity may be less reliant on costimulatory signals, thus circumventing attempts to prevent these responses with costimulation blockade. To this end, a recent report demonstrated the ability of memory B cell responses to overcome the effects of anti-CD154 treatment and mediate heart allograft rejection in a CD28-dependent manner [46], indicating that a strategy to combine different types of costimulatory blockade with CD40-CD154 antagonism may be necessary to curtail memory responses.
Recent strides have also been made in developing reliable and more clinically relevant non-human primate models of AMR and the effect of CD40-CD154 blockade in these models [47]. Notably, a study from Kim et al showed that treatment with an anti-CD40 antibody was able to effectively disrupt germinal center formation and alloantibody production following renal transplantation in rhesus macaques [48], again emphasizing the potential of CD40-CD154 blockade to ameliorate clinical AMR.
AVOIDING THROMBOEMBOLISM AND TRANSLATION TO CLINIC
Interest in CD40-CD154 as a clinically-viable therapeutic target has been revived in the past few years, and much of the work has involved either targeting the CD154 molecule with safer antibodies, or using anti-CD40 antibodies to avoid targeting platelets.
Dependence of Anti-CD154 Therapy on Fc-Receptor Interactions
As detailed previously, the exact mechanism by which anti-CD154 antibodies modulate donor-reactive T cell responses in animal models remains somewhat controversial. Several studies have proposed that Fc-mediated effect of anti-CD154 treatment, specifically the depletion of CD154-expressing alloreactive T cells, is actually required for the efficacy of these drugs. One such report by Monk et al suggested that the tolerogenic effects of anti-CD154 antibodies were Fc- and complement-dependent, as anti-CD154 therapy in FcγR- or complement-deficient animals, or the treatment of mice with F(ab′)2 fragments of anti-CD154, was ineffective in prolonging graft survival [49]. However, there are some caveats to these findings, namely that the knockout animals may have been poor models to demonstrate the dependence of anti-CD154 treatment on Fc-mediated depletion of alloreactive cells. Complement, specifically the iC3b fragment, is required in a model of intraocular tolerance induction [50], and FcγR are involved in antigen uptake and processing by dendritic cells and macrophages [51]. A recent study also suggested that Fc receptors may play an intrinsic inhibitory role on T cells [52], and thus cells lacking these receptors may be hyper-immune and impervious to tolerizing regimens. Therefore, while Fc-dependent interactions may play some role in the efficacy of anti-CD154 therapy, this role may depend on the nature and strength of antigenic challenge. To this end, one study demonstrated that aglycosylated anti-CD154 antibodies were effective in preventing a non-human primate model of systemic lupus erythematosis, but lacked efficacy in islet and renal transplantation [53]. However, Daley and colleagues demonstrated that an aglycosylated form of anti-CD154, which exhibited reduced ability to bind Fc receptors and activate complement, was able to prolong bone marrow chimerism and skin graft survival as effectively as the glycosylated form [54].
Furthermore, encouraging mechanistic results have recently been reported in engineering safer, but still efficacious, Fc-modified anti-CD154 antibodies. Researchers developed a novel domain antibody by selecting variable regions that bind to CD154 in a phage display library and fusing them to a mutated Fc tail [55]. This murine IgG1 tail contained a D265A mutation that has been shown to abrogate Fc binding and complement activation [56–58]. Importantly, this study demonstrated that 5C8, the antibody used in previous clinical trials, led to platelet activation in vitro, whereas the novel Fc-modified dAb did not [55]. A study from our group demonstrated in both a fully allogeneic model of murine model of murine skin transplantation, the Fc-modified dAb resulted in abrogation of alloreactive effector responses and conversion of Foxp3+ Treg comparable to levels achieved with Fc-intact anti-CD154 MR-1 clone [59].
These findings suggest that Fc-dependent interactions are not an absolute requirement for anti-CD154 therapy to be effective. Perhaps Fc-mediated depletion of alloreactive cells may be useful in certain models, but the involvement of these interactions in thromboembolism precludes their use clinically, and thus the demonstration of equally efficacious therapy that avoids this mechanism augers well for the clinical translation of this modality.
Anti-CD40 Antibodies
While most strategies to block CD40-CD154 interactions have targeted the inducibly-expressed CD154 ligand, more recently many groups have attempted to instead target the CD40 molecule expressed on APC. Results in preclinical models have been encouraging, and clinical trials are currently underway to test the efficacy of this strategy in humans.
One of the first anti-CD40 clones tested in non-human primate models of transplantation was Chi220, a chimeric mouse anti-human CD40 antibody. In a preliminary study, transient therapy with Chi220 alone was able to prolong renal allograft survival, and prevented the production of donor-specific antibodies when given in combination with CTLA4-Ig [60]. However, prolonged Chi220 exposure impaired primary anti-viral responses to CMV infection, and this treatment also led to the peripheral depletion of CD20+ B cells [60]. Therefore, it was unclear if the blockade of CD40-CD154 interactions or simply the general depletion of APC was more important for the mechanism of action of Chi220, or if both actions contributed to the overall efficacy. Later studies of Chi220 showed that this antibody was partially agonistic, and that a short course of Chi220 combined with LEA29Y (belatacept), a CTLA4-Ig derivative, significantly prolonged islet allograft survival without impacting pre-existing anti-viral immunity [61]. Chi220 was also effective in promoting the engraftment of neonatal porcine islets in non-human primates, despite the high barrier posed by the potent xenogeneic response in recipient animals [62].
A number of other anti-CD40 antibodies have been developed since the introduction of Chi220. 3A8 is another partially agonistic antibody, but does not deplete B cells in vivo. Despite its inability to block the binding of soluble CD154 to CD40 and the fact that it induces CD80 and CD86 expression on APC in vitro, treatment with 3A8 was able to significantly prolong islet allograft survival and prevent alloantibody formation in rhesus macaques [63, 64]. 2C10, a fully human antibody to CD40, binds to a distinct epitope of the CD40 molecule from 3A8 and Chi220, does not activate or deplete B cells, and prolonged allogeneic islet survival in a non-human primate model [65]. However, the anti-CD40 therapeutic that is furthest along in the clinical translation pipeline is 4D11, also known as ASKP1240. Initially described in both renal and islet transplantation models in cynomolgus monkeys, 4D11 showed minimal efficacy when given as induction therapy, and also caused significant depletion of peripheral B cells. However, maintenance treatment led to long-term kidney allograft survival, as well as islet allograft survival as a monotherapy, and also prevented donor-specific antibody formation [66–68]. These exciting results led to the initiation of clinical trials, and phase I studies in humans indicated that this therapy was well-tolerated and effective at achieving CD40 receptor occupancy at doses similar to those used in non-human primates, suggesting that this is a promising avenue for use in human autoimmune disease and transplantation [69].
Molecular Silencing of CD40
Another potential avenue for abrogating CD40-CD154 interactions involves the molecular silencing of CD40 through the use of small interfering RNA (siRNA). These short double-stranded RNA sequences, approximately 19–21 base pairs in length, integrate into the endogenous RNA interference machinery within cells and effectively quench expression of their target genes [70]. Initial in vitro studies demonstrated that siRNA targeting CD40 expression in human endothelial cell lines could prevent activation of these target cells by CD154-expressing Jurkat T cells [71]. This approach abrogated the upregulation of adhesion molecules on endothelial cells, a key step in the inflammatory and leukocyte migratory process. siRNA silencing of CD40 has also shown promise in vivo. In a model of collagen-induced arthritis, the infusion of CD40-siRNA dampened collagen-specific T cell responses and ameliorated disease severity [72]. In a separate study, in vivo silencing of CD40 moderately prolonged renal allograft survival in rats, with marked improvement over controls in preventing humoral alloresponses [73].
While these therapies may offer some benefits compared to antibody therapy, such as the lack of potential Fc-mediated side effects, there are several barriers to delivery of siRNA, including the loss of RNA through kidney filtration and the potential immunogenicity of the RNA itself [70]. A number of non-CD40 RNA-based therapeutics are currently undergoing clinical trials [74], demonstrating the potential and excitement surrounding this treatment modality, but additional research ensuring the safety and efficacy of CD40-directed therapies must be performed to confirm the feasibility of translation to clinic.
Combination therapy and synergistic costimulation blockade
While CD40-CD154 blockade can be remarkably effective in inhibiting alloreactive responses, it is well appreciated that a number of different costimulatory interactions are responsible for T cell activation, and blocking multiple interactions simultaneously may be advantageous for promoting long-term survival and tolerance. This was made abundantly clear in early studies of costimulation blockade that showed while anti-CD154 therapy alone was able to prolong cardiac graft survival in a stringent BALB/c to B6 murine model to a mean survival time of 70 days, the combination of anti-CD154 with CTLA4-Ig led to indefinite graft survival [6]. Since that time, a number of studies have shown synergistic results using dual costimulation blockade in both murine and non-human primate models [61, 75, 76]. During the recent clinical trials of belatacept, a CTLA4-Ig derivative, belatacept-treated patients experienced higher rates of acute cellular rejection (ACR) compared to those treated with calcineurin inhibitors [77]. Therefore, there is a growing need for additional targeted therapies that could potentially pair with belatacept and ameliorate acute rejection, while preserving the functional benefit associated with CNI avoidance. This need is underscored by the fact that even patients that had episodes of ACR while on belatacept exhibited better renal function at 3 years post-transplant than those patients who did not experience ACR but were treated with cyclosporine. Patients who received more or less intensive belatacept regimens and experienced ACR in the first three years had a mean calculated glomerular filtration rate (cGFR) of 66.8 mL/min/1.73m2 and 57.8% mL/min/1.73m2, respectively, compared to a mean cGFR of 44.4 mL/min/1.73m2 for patients receiving cyclosporine who did not experience ACR [78]. Belatacept-treated patients also had significantly lower mean blood pressure and less of an increase in non-high density lipoproteins, indicating that despite the increased incidence of ACR, belatacept still provides a dramatic benefit in overall health and kidney function compared to calcineurin inhibitors.
One potential downside of CD28 blockade and a hypothesis for the increased ACR incidence with belatacept is its negative effect on regulatory T cell populations [79–82]. While this therapy may negatively impact Treg, the discovery that CD154 antagonism leads to the generation and expansion of antigen-specific Treg offers hope that these treatments can be used together to avoid the early rejection that has characterized the clinical experience with belatacept. Importantly, our group recently demonstrated that while endogenous populations of Treg were diminished in the presence of CTLA4-Ig, the alloantigen-specific iTreg populations that were generated in the presence of CD154 antagonism with an Fc-silent domain antibody were maintained in the presence of CTLA4-Ig [59], suggesting that these allospecific Treg populations can survive and maintain suppressive function even in the presence of CD28 blockade.
CONCLUSION AND FUTURE PERSPECTIVE
In conclusion, recent mechanistic insight into the role of the CD40-CD154 pathway in alloreactivity has resurrected the idea of targeting these molecules therapeutically in clinical transplantation. This costimulatory pathway is one of the central means by which alloreactive responses are generated, and thus the roadblocks presented by the failure of initial clinical trials should spur further investigation to uncover the mechanisms underlying these failures and work around them. Furthermore, recent work has shown that therapeutic CD40-CD154 antagonism does not simply target the interaction between CD154 on CD4+ T cells and CD40 on APC. Rather, these molecules are expressed on a wide variety of immune cell types, and often both are expressed on the same cell.
Further studies should aim to elucidate each interaction and its relative impact on both alloimmune responses and thrombosis. Work suggesting that Fc receptors play a critical role in the thromboembolic side effects experienced by patients receiving anti-CD154 antibodies has led to the creation of safer therapeutics with potent efficacy. Given the recent resurgence in studies detailing additional mechanisms of CD40-CD154 interactions and the development of several promising candidates for clinical translation, it can be speculated that the next few years will see an increase in clinical trials aiming to bring viable CD40-CD154 therapies to the bedside. Belatacept is a first in class costimulation blocker that can suppress alloreactive responses without the inherent toxicity of calcineurin inhibitors, but even this therapy still requires lifelong immunosuppression to prevent rejection. CD40-CD154 antagonism has the potential to transform the paradigm of systemic long-term immunosuppression following transplantation, as a number of studies have shown the potential of blockade of this pathway to promote long-term graft tolerance even after the cessation of treatment. By pursuing safer strategies in the lab and testing optimal CD40-CD154 targeting regimens in the clinic, there is once again promise that this pathway can serve as critical target for improving clinical transplantation.
EXECUTIVE SUMMARY.
CD40-CD154 Interactions and Adaptive Immune Responses
CD40 and its binding partner CD154 are expressed on a wide range of immune cell types, and this pathway is a potent costimulator of adaptive immune responses
Treatment with anti-CD154 monoclonal antibodies showed remarkable promise in animal models of transplantation
Clinical trials were halted in the early 2000s due to thromboembolic complications, stemming from immune complex crosslinking of FcγRIIa on human platelets
Antigen-Presenting Cells and Proinflammatory Cytokine Production
Ligation of CD40 expressed on DC by CD154 on CD4+ T cells leads to DC activation
This interaction is responsible for the elaboration of proinflammatory cytokines such as IL-6 and IL-12, but not the upregulation of MHC and costimulatory molecules
Signaling nodes downstream of different TRAF adaptor molecules associated with CD40 in dendritic cells leads to distinct DC differentiation programs
TLR-induced expression of CD154 by DC can provide help to CD8+ T cells
Induced Regulatory T Cells and the Immune Microenvironment
Treatment with anti-CD154 following transplantation leads to the generation of alloantigen-induced Treg from naïve CD4+ Foxp3− precursors
The location and timing of the interactions between regulatory T cells, antigen-presenting cells and effector T cells has a dramatic impact on the generation of Treg and the ability to induce transplant tolerance with anti-CD154 antibodies
Direct CD4:CD8 T cell help
The contribution of direct interactions between CD154 expressed by activated CD4+ T cells and CD40 expressed by activated CD8+ T cells to allospecific and pathogen-specific immunity is somewhat controversial
CD8+ T cell-intrinsic CD40 signals are required for effective cytotoxic responses to alloreactive, but not necessarily pathogen-reactive, responses
The source of CD154 signals for this interaction is still unclear
Humoral Immunity
CD4+ T cell help provided to B cells through the CD40-CD154 pathway leads to potent humoral responses which can result in antibody-mediated rejection
CD40-CD154 interactions are critical for the maintenance of germinal center reactions
B cell memory responses are independent of CD40-CD154 costimulation
Dependence on Fc Interactions
The contribution of Fc-mediated deletion of CD154-expressing cells following anti-CD154 therapy is still controversial
Fc-modified antibodies have shown similar efficacy to Fc-intact anti-CD154 in numerous murine models of transplantation
Whether Fc-intact antibodies are required for anti-CD154 efficacy may depend on the model and strength of antigenic challenge
Anti-CD40 Therapy
A variety of anti-CD40 therapeutics targeting CD40 expressed on antigen-presenting cells have been developed
Clones 3A8, 2C10, and Chi220 showed a range of efficacies in non-human primate models, with varying impact on peripheral B cell levels and protective immunity
Phase I clinical trials of the anti-CD40 antibody ASKP1240 showed good receptor coverage and was well tolerated
Molecular Silencing of CD40
Several studies have attempted to use siRNA to knock down expression of CD40 in vivo to prevent CD40-CD154 costimulation without the use of monoclonal antibodies
RNAi is moderately successful in autoimmune and transplant models in vivo, suggesting that this may be an effective treatment modality
Combination Therapy
A combination of CD40-CD154 and CD28-B7 blockade can synergize to promote graft tolerance
Belatacept, a CTLA4-Ig derivative, provides functional benefits in clinical transplantation, but concerns exist over increased early acute cellular rejection and the negative impact of CD28 blockade on Treg
The pro-Treg effects of CD154 blockade may be effective in alleviating the early rejection seen with belatacept treatment
Conclusions
Mechanistic studies have elucidated the etiology of anti-CD154-induced thrombosis Recent work has resurrected the CD40-CD154 pathway as a viable target in clinical transplantation
Footnotes
FINANCIAL AND COMPETING INTERESTS DISCLOSURE
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or a financial conflict with the subject matter or materials discussed in the manuscript.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
David F. Pinelli, Email: dpinell@emory.edu.
Mandy L. Ford, Email: mandy.ford@emory.edu.
References
- 1.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Ann Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180(4):1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184(2):747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D, Ferrer IR, Konomos M, Ford ML. Inhibition of CD8+ T cell-derived CD40 signals is necessary but not sufficient for Foxp3+ induced regulatory T cell generation in vivo. J Immunol. 2013;191(4):1957–1964. doi: 10.4049/jimmunol.1300267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 7.Parker DC, Greiner DL, Phillips NE, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci U S A. 1995;92(21):9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5(6):686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 9.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 11.Buhler L, Alwayn IP, Appel JZ, 3rd, Robson SC, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism. Transplantation. 2001;71(3):491. doi: 10.1097/00007890-200102150-00028. [DOI] [PubMed] [Google Scholar]
- 12.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416–417. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 13.Henn V, Slupsky JR, Grafe M, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 14.Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002;8(3):247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 15.Zirlik A, Maier C, Gerdes N, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115(12):1571–1580. doi: 10.1161/CIRCULATIONAHA.106.683201. [DOI] [PubMed] [Google Scholar]
- 16.Wolf D, Hohmann JD, Wiedemann A, et al. Binding of CD40L to Mac-1’s I-domain involves the EQLKKSKTL motif and mediates leukocyte recruitment and atherosclerosis--but does not affect immunity and thrombosis in mice. Circ Res. 2011;109(11):1269–1279. doi: 10.1161/CIRCRESAHA.111.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ankersmit HJ, Roth GA, Moser B, et al. CD32-mediated platelet aggregation in vitro by anti-thymocyte globulin: implication of therapy-induced in vivo thrombocytopenia. Am J Transplant. 2003;3(6):754–759. doi: 10.1034/j.1600-6143.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 18.Newman PM, Chong BH. Heparin-induced thrombocytopenia: new evidence for the dynamic binding of purified anti-PF4-heparin antibodies to platelets and the resultant platelet activation. Blood. 2000;96(1):182–187. [PubMed] [Google Scholar]
- 19.Langer F, Ingersoll SB, Amirkhosravi A, et al. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb Haemost. 2005;93(6):1137–1146. doi: 10.1160/TH04-12-0774. [DOI] [PubMed] [Google Scholar]
- 20.Robles-Carrillo L, Meyer T, Hatfield M, et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185(3):1577–1583. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 21.Roth GA, Zuckermann A, Klepetko W, et al. Thrombophilia associated with anti-CD154 monoclonal antibody treatment and its prophylaxis in nonhuman primates. Transplantation. 2004;78(8):1238–1239. doi: 10.1097/01.tp.0000135457.69220.5b. author reply 1239. [DOI] [PubMed] [Google Scholar]
- 22.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005;175(2):771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 23.Van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Ferrer IR, Liu D, Pinelli DF, Koehn BH, Stempora LL, Ford ML. CD40/CD154 blockade inhibits dendritic cell expression of inflammatory cytokines but not costimulatory molecules. J Immunol. 2012;189(9):4387–4395. doi: 10.4049/jimmunol.1201757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. J Am Soc Nephrol. 2009;20(5):1032–1040. doi: 10.1681/ASN.2008070778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Boenisch O, Yeung M, et al. Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. Am J Transplant. 2012;12(1):90–101. doi: 10.1111/j.1600-6143.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 27.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. European J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer IR, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade. Proc Natl Acad Sci U S A. 2011;108(51):20701–20706. doi: 10.1073/pnas.1105500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mackey MF, Wang Z, Eichelberg K, Germain RN. Distinct contributions of different CD40 TRAF binding sites to CD154-induced dendritic cell maturation and IL-12 secretion. European J Immunol. 2003;33(3):779–789. doi: 10.1002/eji.200323729. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S, Zhan Y, Sutherland RM, et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity. 2009;30(2):218–227. doi: 10.1016/j.immuni.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood KJ, Bushell A, Jones ND. Immunologic unresponsiveness to alloantigen in vivo: a role for regulatory T cells. Immunol Rev. 2011;241(1):119–132. doi: 10.1111/j.1600-065X.2011.01013.x. [DOI] [PubMed] [Google Scholar]
- 32.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilate AM, Lafaille JJ. Induced CD4(+)Foxp3(+) Regulatory T Cells in Immune Tolerance. Ann Rev Immunol. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 34.Haribhai D, Williams JB, Jia S, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35(1):109–122. doi: 10.1016/j.immuni.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dodd-O JM, Lendermon EA, Miller HL, et al. CD154 blockade abrogates allospecific responses and enhances CD4(+) regulatory T-cells in mouse orthotopic lung transplant. Am J Transplant. 2011;11(9):1815–1824. doi: 10.1111/j.1600-6143.2011.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7(6):652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 37.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren KJ, Iwami D, Harris DG, Bromberg JS, Burrell BE. Laminins affect T cell trafficking and allograft fate. J Clin Invest. 2014;124(5):2204–2218. doi: 10.1172/JCI73683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, Bushell A. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. European J Immunol. 2011;41(3):726–738. doi: 10.1002/eji.201040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munroe ME, Bishop GA. A costimulatory function for T cell CD40. J Immunol. 2007;178(2):671–682. doi: 10.4049/jimmunol.178.2.671. [DOI] [PubMed] [Google Scholar]
- 41.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297(5589):2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 42.Sun JC, Bevan MJ. Cutting edge: long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172(6):3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 43.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient environment. J Exp Med. 2003;198(11):1759–1764. doi: 10.1084/jem.20031440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13(9):2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burns AM, Ma L, Li Y, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182(3):1314–1324. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 47.Page EK, Page AJ, Kwun J, et al. Enhanced de novo alloantibody and antibody-mediated injury in rhesus macaques. Am J Transplant. 2012;12(9):2395–2405. doi: 10.1111/j.1600-6143.2012.04074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant. 2014;14(1):59–69. doi: 10.1111/ajt.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monk NJ, Hargreaves RE, Marsh JE, et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med. 2003;9(10):1275–1280. doi: 10.1038/nm931. [DOI] [PubMed] [Google Scholar]
- 50.Sohn JH, Bora PS, Suk HJ, Molina H, Kaplan HJ, Bora NS. Tolerance is dependent on complement C3 fragment iC3b binding to antigen-presenting cells. Nat Med. 2003;9(2):206–212. doi: 10.1038/nm814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woelbing F, Kostka SL, Moelle K, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203(1):177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starbeck-Miller GR, Badovinac VP, Barber DL, Harty JT. Cutting edge: Expression of FcgammaRIIB tempers memory CD8 T cell function in vivo. J Immunol. 2014;192(1):35–39. doi: 10.4049/jimmunol.1302232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrant JL, Benjamin CD, Cutler AH, et al. The contribution of Fc effector mechanisms in the efficacy of anti-CD154 immunotherapy depends on the nature of the immune challenge. Int Immunol. 2004;16(11):1583–1594. doi: 10.1093/intimm/dxh162. [DOI] [PubMed] [Google Scholar]
- 54.Daley SR, Cobbold SP, Waldmann H. Fc-disabled anti-mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am J Transplant. 2008;8(11):2265–2271. doi: 10.1111/j.1600-6143.2008.02382.x. [DOI] [PubMed] [Google Scholar]
- 55.Xie JH, Yamniuk AP, Borowski V, et al. Engineering of a novel anti-CD40L domain antibody for treatment of autoimmune diseases. J Immunol. 2014;192(9):4083–4092. doi: 10.4049/jimmunol.1303239. [DOI] [PubMed] [Google Scholar]
- 56.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23(1):41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 58.Baudino L, Shinohara Y, Nimmerjahn F, et al. Crucial role of aspartic acid at position 265 in the CH2 domain for murine IgG2a and IgG2b Fc-associated effector functions. J Immunol. 2008;181(9):6664–6669. doi: 10.4049/jimmunol.181.9.6664. [DOI] [PubMed] [Google Scholar]
- 59.Pinelli DF, Wagener ME, Liu D, et al. An Anti-CD154 Domain Antibody Prolongs Graft Survival and Induces Foxp3 iTreg in the Absence and Presence of CTLA-4 Ig. Am J Transplant. 2013 doi: 10.1111/ajt.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson TC, Trambley J, Odom K, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 61.Adams AB, Shirasugi N, Jones TR, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174(1):542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 62.Thompson P, Cardona K, Russell M, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11(5):947–957. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badell IR, Thompson PW, Turner AP, et al. Nondepleting anti-CD40-based therapy prolongs allograft survival in nonhuman primates. Am J Transplant. 2012;12(1):126–135. doi: 10.1111/j.1600-6143.2011.03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badell IR, Russell MC, Cardona K, et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. Am J Transplant. 2012;12(7):1918–1923. doi: 10.1111/j.1600-6143.2012.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowe M, Badell IR, Thompson P, et al. A novel monoclonal antibody to CD40 prolongs islet allograft survival. Am J Transplant. 2012;12(8):2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13(8):1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 67.Aoyagi T, Yamashita K, Suzuki T, et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am J Transplant. 2009;9(8):1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 68.Okimura K, Maeta K, Kobayashi N, et al. Characterization of ASKP1240, a Fully Human Antibody Targeting Human CD40 With Potent Immunosuppressive Effects. Am J Transplant. 2014;14(6):1290–1299. doi: 10.1111/ajt.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am J Transplant. 2013;13(4):1040–1046. doi: 10.1111/ajt.12082. [DOI] [PubMed] [Google Scholar]
- 70.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature reviews Genetics. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 71.Pluvinet R, Petriz J, Torras J, et al. RNAi-mediated silencing of CD40 prevents leukocyte adhesion on CD154-activated endothelial cells. Blood. 2004;104(12):3642–3646. doi: 10.1182/blood-2004-03-0817. [DOI] [PubMed] [Google Scholar]
- 72.Zheng X, Suzuki M, Zhang X, et al. RNAi-mediated CD40-CD154 interruption promotes tolerance in autoimmune arthritis. Arthritis Res Ther. 2010;12(1):R13. doi: 10.1186/ar2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ripoll E, Pluvinet R, Torras J, et al. In vivo therapeutic efficacy of intra-renal CD40 silencing in a model of humoral acute rejection. Gene Ther. 2011;18(10):945–952. doi: 10.1038/gt.2011.39. [DOI] [PubMed] [Google Scholar]
- 74.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 75.Kirk AD, Harlan DM, Armstrong NN, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haanstra KG, Ringers J, Sick EA, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75(5):637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 77.Pestana JO, Grinyo JM, Vanrenterghem Y, et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12(3):630–639. doi: 10.1111/j.1600-6143.2011.03914.x. [DOI] [PubMed] [Google Scholar]
- 78.Vincenti F, Larsen CP, Alberu J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12(1):210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 79.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171(7):3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 80.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123(2):580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riella LV, Liu T, Yang J, et al. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 2012;12(4):846–855. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 82.Wojciechowski D, Vincenti F. Belatacept in kidney transplantation. Curr Opin Organ Transpl. 2012;17(6):640–647. doi: 10.1097/MOT.0b013e32835a4c0d. [DOI] [PubMed] [Google Scholar]