Abstract
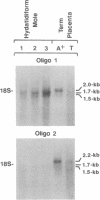
A genomic clone containing two linked human pregnancy-specific beta 1-glycoprotein (PS beta G) genes has been isolated and characterized. The two genes are arranged in the same 5'----3' orientation; the 3' region (including the A2 and B-C exons) of the upstream gene, PSGGA, is linked to the 5' region (including the 5'/L and L/N exons) of PSGGB, the downstream gene. Depending upon the domains compared, PSGGA and PSGGB share 92-98% nucleotide and 86-95% amino acid sequence identity with PSG93, the most abundant PS beta G transcript. The 3' exon (B-C) of PSGGA contains four alternative splice sites and three polyadenylylation sites, which account for the 3' heterogeneity previously reported in the PS beta G family. Each of the predicted PSGGA-encoded proteins would have a different carboxyl terminus. PSGGB corresponds to the previously identified cDNA PSG6, which encodes proteins containing a 34-amino acid leader peptide and a 108-amino acid N domain, which is one amino acid shorter than the majority of PS beta G N domains. Additionally, the PSGGB-encoded proteins contain the cell-surface recognition tripeptide Arg-Gly-Asp, shared by several previously reported PS beta Gs as deduced from cDNA sequences. Northern blot hybridization performed with a PSGGB-specific oligonucleotide probe to the N domain revealed that PSGGB or a PSGGB-like gene encodes a major 1.7-kilobase mRNA in hydatidiform mole tissues and a major 2.0-kilobase mRNA in term placenta tissues. Moreover, the PSGGB-specific probe hybridized most strongly with mRNA from molar trophoblastic tissue, suggesting that the PSGGB-like species may be the gene preferentially expressed in gestational trophoblastic disease. Additionally, the sequence of a 2315-base-pair PS beta G cDNA (PSG95) that contains an N-A1-A2-B2-C domain arrangement is reported. The coding region of PSG95 is identical to the previously reported cDNA clones PSG1d and FL-NCA, but PSG95 contains an additional 518 and 523 base pairs in the 3' end as compared with PSG1d and FL-NCA, respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett T. R., Pickle W., 2nd, Rae P. M., Hart J., Kamarck M., Elting J. Human pregnancy-specific beta 1-glycoproteins are coded within chromosome 19. Am J Hum Genet. 1989 Jun;44(6):890–893. [PMC free article] [PubMed] [Google Scholar]
- Beauchemin N., Benchimol S., Cournoyer D., Fuks A., Stanners C. P. Isolation and characterization of full-length functional cDNA clones for human carcinoembryonic antigen. Mol Cell Biol. 1987 Sep;7(9):3221–3230. doi: 10.1128/mcb.7.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. Y., Borjigin J., Zheng Q. X., Shupert W. L. Characterization of cDNA encoding human pregnancy-specific beta 1-glycoprotein from placenta and extraplacental tissues and their comparison with carcinoembryonic antigen. DNA. 1988 Oct;7(8):545–555. doi: 10.1089/dna.1.1988.7.545. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Grudzinskas J. G., Gordon Y. B., Menabawey M., Lee J. N., Wadsworth J., Chard T. Identification of high-risk pregnancy by the routine measurement of pregnancy-specific beta 1-glycoprotein. Am J Obstet Gynecol. 1983 Sep 1;147(1):10–12. doi: 10.1016/0002-9378(83)90075-3. [DOI] [PubMed] [Google Scholar]
- Hertz J. B., Schultz-Larsen P. Human placental lactogen, pregnancy-specific beta-1-glycoprotein and alpha-fetoprotein in serum in threatened abortion. Int J Gynaecol Obstet. 1983 Apr;21(2):111–117. doi: 10.1016/0020-7292(83)90047-4. [DOI] [PubMed] [Google Scholar]
- Khan W. N., Hammarström S. Carcinoembryonic antigen gene family: molecular cloning of cDNA for a PS beta G/FL-NCA glycoprotein with a novel domain arrangement. Biochem Biophys Res Commun. 1989 Jun 15;161(2):525–535. doi: 10.1016/0006-291x(89)92631-4. [DOI] [PubMed] [Google Scholar]
- Khan W. N., Osterman A., Hammarström S. Molecular cloning and expression of cDNA for a carcinoembryonic antigen-related fetal liver glycoprotein. Proc Natl Acad Sci U S A. 1989 May;86(9):3332–3336. doi: 10.1073/pnas.86.9.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lin T. M., Halbert S. P., Spellacy W. N. Measurement of pregnancy-associated plasma proteins during human gestation. J Clin Invest. 1974 Sep;54(3):576–582. doi: 10.1172/JCI107794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D. J., Scott J. M., Gemmell R. S., Mack D. S. A prospective study of three biochemical fetoplacental tests: serum human placental lactogen, pregnancy-specific beta 1-glycoprotein, and urinary estrogens, and their relationship to placental insufficiency. Am J Obstet Gynecol. 1983 Oct 15;147(4):430–436. doi: 10.1016/s0002-9378(16)32239-6. [DOI] [PubMed] [Google Scholar]
- Masson G. M., Anthony F., Wilson M. S. Value of Schwangerschaftsprotein 1 (SP1) and pregnancy-associated plasma protein-A (PAPP-A) in the clinical management of threatened abortion. Br J Obstet Gynaecol. 1983 Feb;90(2):146–149. doi: 10.1111/j.1471-0528.1983.tb08899.x. [DOI] [PubMed] [Google Scholar]
- McLenachan T., Mansfield B. Expression of CEA-related genes in the first trimester human placenta. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1486–1493. doi: 10.1016/0006-291x(89)90842-5. [DOI] [PubMed] [Google Scholar]
- Niemann S. C., Flake A., Bohn H., Bartels I. Pregnancy-specific beta 1-glycoprotein: cDNA cloning, tissue expression, and species specificity of one member of the PS beta G family. Hum Genet. 1989 Jun;82(3):239–243. doi: 10.1007/BF00291162. [DOI] [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kosaki G., Nakazato H. Exon-intron organization of a gene for pregnancy-specific beta 1-glycoprotein, a subfamily member of CEA family: implications for its characteristic repetitive domains and C-terminal sequences. Biochem Biophys Res Commun. 1988 Oct 14;156(1):68–77. doi: 10.1016/s0006-291x(88)80806-4. [DOI] [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Matsuoka Y., Kosaki G., Nakazato H. A pregnancy-specific beta 1-glycoprotein, a CEA gene family member, expressed in a human promyelocytic leukemia cell line, HL-60: structures of protein, mRNA and gene. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1021–1031. doi: 10.1016/0006-291x(89)92324-3. [DOI] [PubMed] [Google Scholar]
- Oikawa S., Nakazato H., Kosaki G. Primary structure of human carcinoembryonic antigen (CEA) deduced from cDNA sequence. Biochem Biophys Res Commun. 1987 Jan 30;142(2):511–518. doi: 10.1016/0006-291x(87)90304-4. [DOI] [PubMed] [Google Scholar]
- Rooney B. C., Horne C. H., Hardman N. Molecular cloning of a cDNA for human pregnancy-specific beta 1-glycoprotein:homology with human carcinoembryonic antigen and related proteins. Gene. 1988 Nov 30;71(2):439–449. doi: 10.1016/0378-1119(88)90061-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streydio C., Lacka K., Swillens S., Vassart G. The human pregnancy-specific beta 1-glycoprotein (PS beta G) and the carcinoembryonic antigen (CEA)-related proteins are members of the same multigene family. Biochem Biophys Res Commun. 1988 Jul 15;154(1):130–137. doi: 10.1016/0006-291x(88)90660-2. [DOI] [PubMed] [Google Scholar]
- Sørensen S. Pregnancy-"specific" beta 1-glycoprotein (SP1): purification, characterization, quantification and clinical application in malignancies (a review). Tumour Biol. 1984;5(6):275–302. [PubMed] [Google Scholar]
- Tamsen L., Johansson S. G., Axelsson O. Pregnancy-specific beta 1-glycoprotein (SP1) in serum from women with pregnancies complicated by intrauterine growth retardation. J Perinat Med. 1983;11(1):19–25. doi: 10.1515/jpme.1983.11.1.19. [DOI] [PubMed] [Google Scholar]
- Tatarinov Iu S., Masiukevich V. N. Immunokhimicheskaia identifikatsiia novogo beta-1-globulina v syvorotke krovi beremennykh zhenshchin. Biull Eksp Biol Med. 1970 Jun;69(6):66–68. [PubMed] [Google Scholar]
- Tatarinov Y. S. Trophoblast-specific beta1-glycoprotein as a marker for pregnancy and malignancies. Gynecol Obstet Invest. 1978;9(2-3):65–97. doi: 10.1159/000300972. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Chou J. Y. Human pregnancy-specific beta 1-glycoprotein: a new member of the carcinoembryonic antigen gene family. Biochem Biophys Res Commun. 1988 Apr 29;152(2):762–768. doi: 10.1016/s0006-291x(88)80103-7. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Chou J. Y. Isolation and characterization of complementary DNAs encoding human pregnancy-specific beta 1-glycoprotein. J Biol Chem. 1988 Feb 5;263(4):2049–2054. [PubMed] [Google Scholar]
- Würz H., Geiger W., Künzig H. J., Jabs-Lehmann A., Bohn H., Lüben G. Radioimmunoassay of SP1 (pregnancy-specific beta1-glycoprotein) in maternal blood and in amniotic fluid normal and pathologic pregnancies. J Perinat Med. 1981;9(2):67–78. doi: 10.1515/jpme.1981.9.2.67. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Weiss M., Thompson J. A. cDNA cloning demonstrates the expression of pregnancy-specific glycoprotein genes, a subgroup of the carcinoembryonic antigen gene family, in fetal liver. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1197–1209. doi: 10.1016/0006-291x(89)91105-4. [DOI] [PubMed] [Google Scholar]