Abstract
The bacteriocin piscicocin CS526 was inactivated by proteolytic enzymes, was stable at 100°C for 30 min, had a pH range of 2 to 8, and was active against Enterococcus, Listeria, Pediococcus, and Leuconostoc. The N-terminal sequence was YGNGL, not the YGNGV consensus motif common in class IIa bacteriocins (alternate residues underlined). The molecular mass of piscicocin CS526, which had a bactericidal mode of action, was ∼4,430 Da.
Bacteriocins are ribosomally synthesized proteins that inhibit bacteria closely related to the producer strain, food-borne pathogens, and spoilage bacteria (12, 18). Carnobacterium piscicola CS526 inhibits Listeria monocytogenes growth in cold-smoked salmon (21). Several bacteriocin-producing carnobacteria have been reported (1, 4, 10, 11, 13, 17). The objectives of this study were to purify and characterize piscicocin CS526.
Preliminary characterization of the bacteriocin.
To determine the effects of enzymes on the bacteriocin, the cell supernatants (180 μl) of the culture neutralized with NaOH were incubated with 20 μl of enzyme solutions (10 mg/ml in 50 mM phosphate buffer [pH 7.0]) at 35°C for 1 h. After boiling for 5 min, the residual activity was determined by the agar well diffusion assay (21). The inhibitory activity was completely destroyed by α-chymotrypsin, papain, proteinase K, and actinase and partly inhibited by trypsin (67% of residual activity). Other enzymes, such as catalase, RNase, and lipase, did not affect the activity.
To determine the thermal stability, the cell supernatant of the neutralized culture was heated for 15 or 30 min at 70, 80, 90, 100, and 121°C and then assayed for activity. The inhibitory activity was completely stable after 30 min at 100°C but was partially inactivated at 121°C for 15 min (44% of residual activity).
To determine the effect of pH on bacteriocin activity, 50 μl of the cell supernatant was added to 950 μl of tryptic soy broth with 0.6% yeast extract (TSBYE) adjusted with NaOH or HCl to different pH values. The samples were assayed for activity after 1 h at 25°C. The activity was completely stable at pH 2 to 8 but reduced at pH values of 9 to 11 (67, 44, and 30% of residual activity at pH 9, 10, and 11, respectively).
The antagonistic effect of cell supernatant of an overnight culture of CS526 in TSBYE against 27 strains of gram-positive bacteria and 6 strains of gram-negative bacteria was determined by the agar well diffusion assay. Enterococcus faecalis JCM8726, Enterococcus faecium JCM8727, Enterococcus durans JCM8725, Enterococcus hirae JCM8729, Pediococcus pentosaceus JCM5890, Tetragenococcus halophilus JCM5888, and Leuconostoc mesenteroides subsp. mesenteroides JCM6124 were inhibited. L. monocytogenes IID578 and IID581, Listeria innocua FTHU8221, and Listeria grayi subsp. murrayi FTHU204 were also inhibited. Gram-positive spore-forming bacteria (Bacillus cereus JCM2152, Bacillus subtilis IFO13719, Bacillus megaterium IAM13418, Bacillus licheniformis IFO12107, Bacillus sphaericus IAM13420, and Paenibacillus polymyxa JCM2507) and gram-negative bacteria (Escherichia coli IFO15034, Salmonella enterica serovar Enteritidis RIMD1933001, Pseudomonas fluorescens JCM5963m and Aeromonas hydrophila IFO3820) were not inhibited. These results demonstrated that the inhibitory activity of the C. piscicola CS526 was due to a proteinaceous molecule exhibiting excellent heat stability. Therefore, the antilisterial substance produced by C. piscicola CS526 was confirmed as a bacteriocin and the bacteriocin has been named piscicocin CS526.
Mode of action.
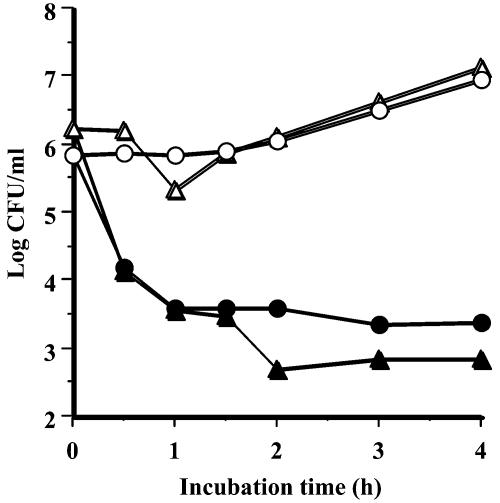
The effect of the bacteriocin on nongrowing cells of L. monocytogenes IID581 and E. hirae JCM8729 was studied in TSBYE. Both strains were grown at 30°C for 16 h in TSBYE, harvested by centrifugation, and resuspended in fresh TSBYE (pH 7.0) to ∼106 cells/ml. The filter-sterilized cell supernatant of the C. piscicola CS526 culture was immediately added to final concentrations of 600 arbitrary units (AU)/ml, and the cells were incubated at 30°C. The viable cell counts of L. monocytogenes and E. hirae decreased quickly from 8.0 × 105 and 1.7 × 106 CFU/ml to 1.5 × 104 and 1.4 × 104 CFU/ml, respectively, after the treatment (30 min) with piscicocin CS526 (Fig. 1), indicating that piscicocin CS526 is bactericidal rather than bacteriostatic.
FIG. 1.
Survivor curve of L. monocytogenes IID581 (circles) and E. hirae JCM8729 (triangles) in the presence of piscicocin CS526. The concentrations of the bacteriocin were 0 (open symbols) and 600 (closed symbols) AU/ml.
Bacteriocin purification and molecular properties.
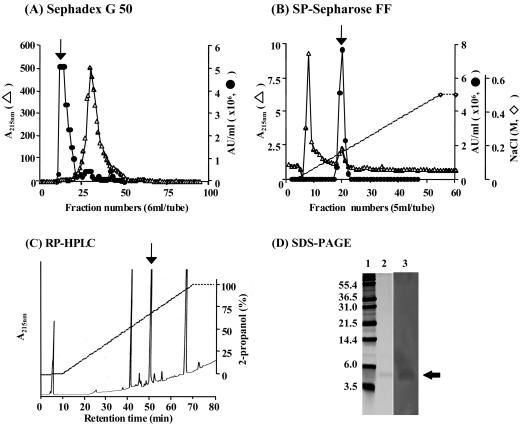
Bacteriocin was purified from the supernatant of a 2-liter C. piscicola CS526 culture propagated at 30°C for 12 h. Bacteriocin was precipitated with ammonium sulfate (50%[wt/vol]), collected by centrifugation (15,000 × g, 30 min, 4°C), and solubilized in 20 mM sodium phosphate buffer (pH 6.0) (buffer A). The sample was applied onto a 300-ml Sephadex G50 (Amersham Biosciences, Tokyo, Japan) gel-filtration gel equilibrated with buffer A, and the bacteriocin was eluted. The active fraction was applied onto a 30-ml SP-Sepharose Fast Flow (Amersham Biosciences) cation-exchange column equilibrated with 25 mM sodium N-morpholinoethanesulfonic acid (MES) containing 3.0 M urea, pH 6.0. (buffer B). After the column was washed with 120 ml of buffer B, the bacteriocin was eluted with linear NaCl gradient (0 to 0.5 M) at pH 6.0, and loaded on a Sep-Pack C18 cartridge (Waters, Milford, Mass.). The cartridge was washed with 20% 2-propanol in 0.1% trifluoroacetic acid (TFA), and the bacteriocin was eluted with 80% 2-propanol in 0.1% TFA. After drying, the bacteriocin was dissolved in 0.05% TFA and applied to C18 reverse-phase column (ODS-80Ts; 4.6 by 150 mm; Tosho, Tokyo, Japan) connected to a high-performance liquid chromatograph. Elution was performed by using a linear gradient from 100% 0.05 TFA to 100% 2-propanol in 60 min at a flow rate of 0.5 ml/min.
The purification was approximately a 1.6 × 104-fold overall, with a 7% yield. The inhibitory fraction after reverse-phase high-performance liquid chromatography (RP-HPLC) was analyzed further by Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) methods (5). SDS-PAGE revealed a single band of protein (Fig. 2D, lane 2) that inhibited L. monocytogenes IID581 (Fig. 2D, lane 3). It was estimated to be a 4.0- to 4.5-kDa peptide.
FIG. 2.
Purification of piscicocin CS526 by gel filtration (A), cation-exchange (B) and RP-HPLC (C), and antibacterial detection SDS-PAGE of piscicocin CS526 (D). Purified piscicocin CS526 was analyzed by SDS-PAGE. The gel was removed and cut into two parts. The first half, containing molecular weight markers (lane 1) and the purified piscicocin CS526 (lane 2), was stained with silver. The other half, containing the purified bacteriocin (lane 3), was overlaid with L. monocytogenes IID581 and incubated at 30°C for 24 h. The arrows indicate the bacteriocin activities.
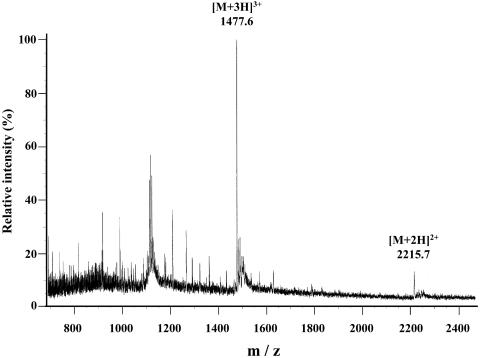
The purified piscicocin CS526 was subjected to mass spectral measurement by electrospray ionization spectrometry (model JM-700TZ mass spectrometer); JEOL, Peabody, Mass. Two unequivocal pseudomolecular ions [M + nH]n+ were observed at m/z corresponding to n = 2 and 3 protonated species, whose averaged molecular mass was 4,429.6 Da (Fig. 3).
FIG. 3.
Electrospray ionization mass spectrometry analysis of purified piscicocin CS526.
The partial NH2-terminal amino acid sequence, comprising 43 amino acid residues, was determined by Edman degradation with Procise 492-HT Protein Sequencer (Applied Biosystems, Foster City, Calif.) (Table 1). Piscicocin CS526 shared significant homology with munditicin and piscicolin 126, produced, respectively, by Enterococcus mundtii (3) and C. piscicola JG126 (11), belonging to class IIa bacteriocins. However, there were no identical sequences in the database. Class IIa bacteriocins, according to the system proposed by Klaenhammer (12), have antilisterial activity and possess the highly conserved N-terminal motif, YGNGV (15). Bhugaloo-Vial et al. (4) also indicated that it was possible to increase the consensus sequence as YGNGVXCX(K/N)XXCXV(N/D)(W/K/R)X(G/A/S)(A/N). Piscicocin CS526 exhibited strong antilisterial activity but possessed a novel N-terminal sequence that contained a change of Val to Leu at position 7. Only three other bacteriocins, bacteriocin 31 (19), sakacin 5X (20), and plantaricin C19 (2), possess this altered N-terminal sequence. The YGNGV consensus motif has been involved in a recognition step of the mechanism of action of class IIa bacteriocins (7, 8), but the YGNGV consensus motif did not appear to be involved in the initial binding step for pediocin PA-1 (6) Carnobacteriocin B2 activity was reduced by a substitution within the Y3GNGV motif (Tyr3 to Phe) (16). Pediocin AcH activity was dramatically reduced by a mutation within the YGN5GV motif (Asn5 to Lys) (14). In this study, we found that piscicocin CS526, which possessed YGNGL sequence, had anti-Listeria activity typical of class IIa bacteriocins. Accordingly, the presence of Val7 within the YGNGV7 motif could not be a prerequisite for the antimicrobial activity of class IIa bacteriocins.
TABLE 1.
Sequence alignment of piscicocin CS526 produced by C. piscicola CS526 and other class IIa bacteriocins
| Bacteriocin (reference) | Sequence and positiona |
|---|---|
| 110203040 | |
| Piscicocin CS526 | KYYGNGLSXN KKGXTVDWGT AIGIIGNNAA ANXATGGAAG XNK |
| Mundticin (3) | KYYGNGVSCN KKGCSVDWGK AIGIIGNNSA ANLATGGAAG WSK |
| Piscicolin126 (11) | KYYGNGVSCN KNGCTVDWSK AIGIIGNNAA ANLTTGGAAG WNKG |
| Bavaricin A (12a) | KYYGNGVHXG KHSXTVDWGT AIGNIGNNAA ANXATGXNAG G |
| Pediocin PA-1 (9) | KYYGNGVTCG KHSCSVDWGK ATTCIINNGA MAWATGGHQG NHKC |
| Sakacin 5X (20) | KYYGNGLSCN KSGCSVDWSK AISIIGNNAV ANLTTGGAAG WKS |
| Bacteriocin 31 (19) | ATYYGNGLYCN KQKCWVDWNK ASREIGKIIV NGWVQHGPWA PR |
| Divercin V41 (13) | TKYYGNGVYCN SKKCWVDWGQ ASGCIGQTVV GGWLGGAIPG KC |
| Carnobacteriocin BMI (15) | AISYGNGVYCN KEKCWVNKAE NKQAITGIVI GGWASSLAGM GH |
| Carnobacteriocin B2 (16) | VNYGNGVSCS KTKCSVNWGQ AFQERYTAGI NSFVSGVASG AGSIGRRP |
| Piscicocin V1a (4) | KYYGNGVSCN KNGCTVDWSK AIGIIGNNAA ANLTTGGAAG WNKG |
| Bavaricin MN (11a) | TKYYGNGVYCN SKKCWVDWGQ AAGGIGQTVV XGWLGGAIPG K |
| Consensus | YGNGCC |
X indicates unidentified residues. The altered L residues and consensus motif residues are underlined.
REFERENCES
- 1.Ahn, C., and M. E. Stiles. 1990. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl. Environ. Microbiol. 56:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrih, A., N. Rekhif, A. J. G. Moir, A. Lebrihi, and G. Lefebvre. 2001. Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int. J. Food Microbiol. 68:93-104. [DOI] [PubMed] [Google Scholar]
- 3.Bennik, M. H. J., B. Vanloo, R. Brasseur, L. G. M. Gorris, and E. J. Smid. 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii: full characterization and interaction with target organisms. Biochim. Biophys. Acta 1373:47-58. [DOI] [PubMed] [Google Scholar]
- 4.Bhugaloo-Vial, P., X. Dousset, A. Metivier, O. Sorokine, P. Anglade, P. Boyaval, and D. Marion. 1996. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 62:4410-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhunia, A. K., M. C. Johnson, and B. Ray. 1987. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Ind. Microbiol. 2:319-322. [Google Scholar]
- 6.Chen, Y., R. D. Ludescher, and T. J. Montville. 1997. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl. Environ. Microbiol. 63:4770-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fimland, G., O. R. Blingsmo, K. Sletten, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1996. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 62:3313-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 9.Henderson, J. T., A. L. Chopko, and P. D. van Wassenaar. 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 295:5-12. [DOI] [PubMed] [Google Scholar]
- 10.Holck, A. L., L. Axelsson, and U. Schillinger. 1994. Purification and cloning of piscicolin 61, a bacteriocin from Carnobacterium piscicola LV61. Curr. Microbiol. 29:63-68. [DOI] [PubMed] [Google Scholar]
- 11.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. H. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Kaiser, A. L., and T. J. Montville. 1996. Purification of the bacteriocin bavaricin MN and characterization of its mode of action against Listeria monocytogenes Scott A cells and lipid vesicles. Appl. Environ. Microbiol. 62:4529-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced bylactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 12a.Larsen, A. G., F. K. Vogensen, and J. Josephsen. 1993. Antimicrobial activity of lactic acid bacteria isolated from sour doughs: purification and characterization of bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. J. Appl. Bacteriol. 75:113-122. [DOI] [PubMed] [Google Scholar]
- 13.Metivier, A., M.-F. Pilet, X. Dousset, O. Sorokine, P. Anglade, M. Zagorec, J.-C. Piard, D. Marion, Y. Cenatiempo, and C. Fremaux. 1998. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergenes V41: primary structure and genomic organization. Microbiology 144:2837-2844. [DOI] [PubMed] [Google Scholar]
- 14.Miller, K. W., R. Schamber, O. Osmanagaoglu, and B. Ray. 1998. Isolation and characterization of pediocin AcH chimeric protein mutants with altered bactericidal activity. Appl. Environ. Microbiol. 64:1997-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quadri, L. E. N., M. Sailers, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 16.Quadri, L. E. N., L. Z. Yan, M. E. Stiles, and J. C. Vederas. 1997. Effect of amino acid substitutions on the activity of carnobacteriocin B2. J. Biol. Chem. 272:3384-3388. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels, G., I. F. Nes, and A. Guomundsdottir. 1992. Isolation and properties of a bacteriocin-producing Carnobacterium piscicola isolated from fish. J. Appl. Bacteriol. 73:309-316. [DOI] [PubMed] [Google Scholar]
- 18.Tagg, J. R., A. S. Dajani, and L. W. Wannamaker. 1976. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 40:722-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan, A., V. G. H. Eijsink, T. F. O'Sullivan, K. O'Hanlon, and D. van Sinderen. 2001. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 91:131-138. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki, K., M. Suzuki, Y. Kawai, N. Inoue, and T. J. Montville. 2003. Inhibition of Listeria monocytogenes in cold-smoked salmon by Carnobacterium piscicola CS526 isolated from frozen surimi. J. Food Prot. 66:1420-1425. [DOI] [PubMed] [Google Scholar]