Abstract
We have analyzed the method used in our laboratory to detect the most elusive, clinically significant alloantibody: the Kidd alloantibodies and find the most convenient procedure. A retrospective analysis of the method used in our laboratory for determining Kidd alloantibodies from January 2013 to May 2015 was conducted. The details of the event that sensitized the patient for red cell antibody formation and procedure used to detect the alloantibody were retrieved from the departmental records. Of 405 red cell antibody identification cases, 24 (5.9 %) had Kidd antibody (anti-Jka in 12: 50 % cases; anti-Jkb in 4: 16.7 % cases; multiple antibodies in 8: 32 % cases). Thirteen of 24 patients (54.2 %) had autocontrol positive of which 6 cases needed adsorption procedures whereas antibody/ies could be identified without adsorption procedure in the remaining 7 cases. All the 7 cases had autocontrol of 1+ strength. Of the 11 patients (45.8 %) with autocontrol negative, the antibody was identified using solid phase in 7 cases whereas tube panels were also used in the remaining 4 cases. Kidd alloantibodies though deceptive can be identified by sensitive techniques like the solid phase and simple but laborious techniques using the tube cell panels. Depending upon the reaction strength of the autocontrol, the routine autoadsorption process may be skipped and tube cell enzyme treated cells or solid phase techniques be used to get the results.
Keywords: Kidd, Alloantibody, Adsorption, SPRCA
Introduction
Kidd antigen system, ISBT number 009, is known for some uniqueness in the alloantibodies that it produces. Alloantibodies to the Kidd antigens are relatively uncommon. The triggering events are transfusion of blood and blood components, pregnancy and transplantation. Kidd antigens are less antigenic as compared to Rh and Kell. Kidd alloantibodies (anti-Jka and anti-Jkb) share a special importance in the field of transfusion medicine as they show evanescence i.e. their titers tend to reduce with time, sometimes to undetectable levels [1]. This makes their detection difficult. These Kidd antibodies also demonstrate dosage phenomena which make the detection more challenging. Missing them may result in a delayed hemolytic transfusion reaction or an anamnestic transfusion reaction. This is due to the fact that the memory lymphocytes produced on the first antigenic exposure mount a flared up antibody response on subsequent encounter with the same antigen.
The antibodies anti-Jka and Jkb are most often found along with other alloantibodies. These antibodies are mainly IgG in nature. Nearly 50 % of these antibodies bind complement and cause intravascular hemolysis [2]. Due to the dosage effect, the panel of the antibody identification needs to have cells with homozygous expression of the Kidd antigens [1, 3]. Reaction of the Kidd antibody with the antigen positive cells at immediate spin is most often weak. Tests performed at antiglobulin phase (AHG) or enzyme treated cells help in their detection [1].
We attempt to compile all the cases of Kidd alloantibodies detected in our laboratory and find the prevalence as a single or one of the multiple alloantibodies. We have also analyzed the method and the techniques used in our laboratory to detect the antibody/ies.
Materials and Methods
A retrospective analysis of all the cases where Kidd alloantibodies were detected in the Department of Transfusion Medicine and Immunohaematology, Indraprastha Apollo Hospitals, New Delhi from January 2013 to May 2015 was conducted. All the data was retrieved from the departmental records. The data collected included: age, sex, diagnosis, history of transfusion, transplantation, pregnancy; duration since the last transfusion, blood component which was transfused, number of units transfused during the last transfusion episode.
The details of the workup done were noted. This included: autocontrol, direct antiglobulin test (DAT), screen for red cell antibody, red cell antibody identification using 10 cell and 16 cell panel, autoadsorbtion/alloadsorbtion and application of ‘select cells’. Screening for red cell antibodies was done by Capture-R Ready-Screen (Immucor, Inc. Norcross, GA). Antibody identification panels used were liquid panel and ficin treated panel- Panocell-20 and Panocell-10, Ficin-Treated (Immucor, Inc. Norcross, GA) and Capture-R Ready-ID (Immucor, Inc. Norcross, GA). Autoadsorption was done using Warm Autoantibody Removal Medium (W.A.R.M) (Immucor, Inc. Norcross, GA) whereas alloadsorbtion was done with in-house prepared panel of R1R1, R2R2, rr cells. Autoadsorption procedure was done as per manufacturer’s kit insert [4]. Alloadsorbtion procedures were performed as described in American Association of Blood Banks (AABB) Technical Manual [5].
Data regarding the type (auto/allo) and number of antibodies detected, number of units of PRC crossmatched and issued were noted. All the data were entered in the excel sheet and necessary analysis were done. The Fig. 1 shows the protocol we use for antibody identification where serum tests positive with our 4 cell screen panel.
Fig. 1.
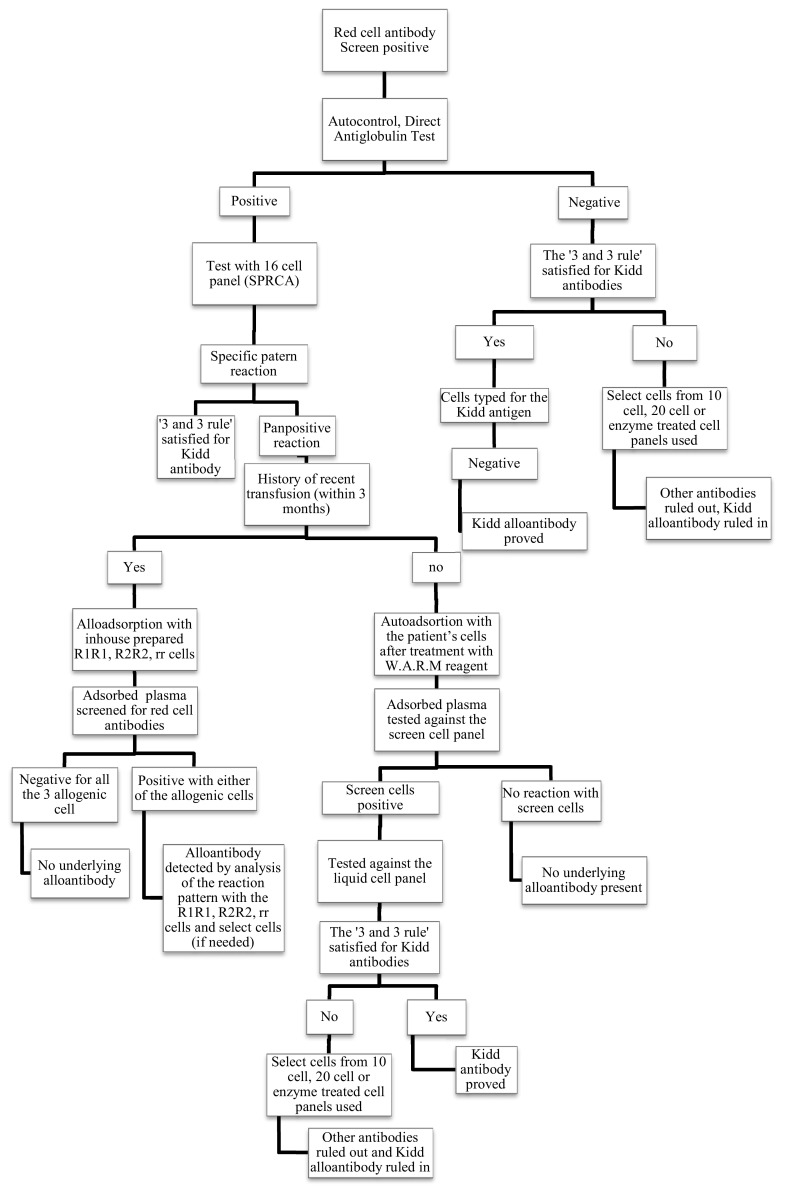
Flowchart showing the procedure we use to identify red cell antibodies in screen for red cell antibody positive cases. An elution study of the positive direct antiglobulin test cases, not included in our protocol, should also be ideally performed
Results
During our study period, antibody identification was done for 405 patients who were positive for screen for red cell antibodies. Kidd antibody was identified in 24 of the 405 patients’ sera (5.9 % cases). The 24 cases of Kidd antibody detected during the studied period which included 15 female and 9 male, the average age of the patients was 47.7 (18–78) years. Eight (32 %) of the 24 cases had other antibodies associated with the Kidd alloantibodies. While anti-Jka was the only alloantibody detected in 12 (50 %) cases, anti-Jkb was found alone in 4 (16.7 %) cases.
Twenty-two of the 24 patients (91.7 %) had history of transfusion of blood and/or blood components. Two (8.3 %) of the patients had no history of transfusion but were multiparous females. Out of the 22 patients who received transfusion, 21 patients (95.5 %) were transfused red cells whereas 1 (4.5 %) of the patients had multiple transfusions of platelets only.
The ‘3 and 3 rule’, according to which at least three antigen positive red cells that react and three antigen negative red cells that do not react [6] should be observed, was followed to identify the alloantibody/ies. Of the 24 patients, anti-Jka was identified in 16 cases (66.7 %), out of which 3 cases had autoantibodies and 1 case had autoantibody as well as alloantibody anti-E. Anti-Jkb was found in 8 cases (33.3 %), of which it was found alone in 4 patients and along with warm autoantibody and various other alloantibodies in 4 patients (Table 1).
Table 1.
Antibodies detected with anti-Jka and anti-JKb
| Anti-Jka detected alone = 12 cases | Anti-JKb detected alone = 4 cases | ||
|---|---|---|---|
| Other antibodies with anti-Jka | Other antibodies with anti-JKb | ||
| Warm autoantibodies | 3 cases | Warm autoantibodies, anti-E | 1 case |
| Warm autoantibodies, anti-E | 1 case | Warm autoantibodies, anti-C | 1 case |
| Anti-c, anti-E, anti-K | 1 case | ||
| Anti-s, anti-Lea | 1 case | ||
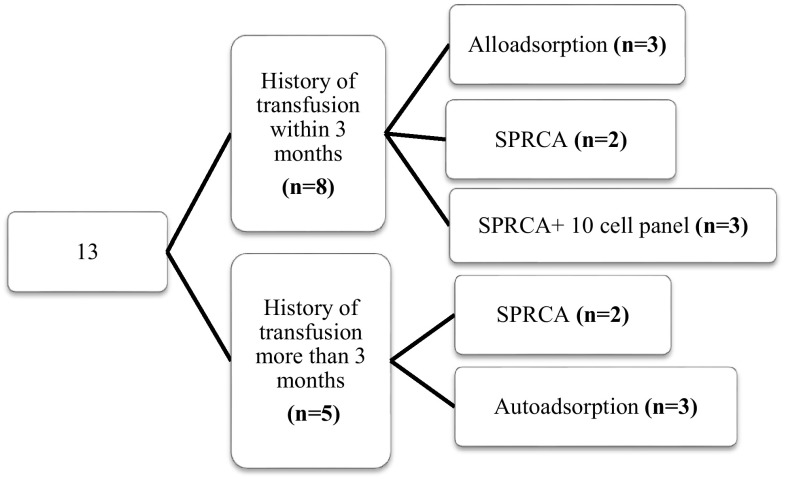
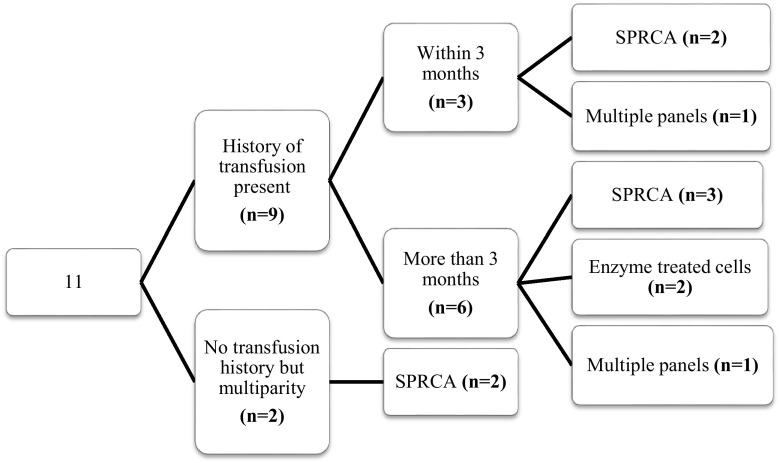
Out of the 24 cases, 13 patients (54.2 %) had autocontrol positive. Adsorption procedure was required in 6 cases (3 autoadsorption; 3 alloadsorption) whereas in the remaining 7 cases, it could be avoided. All these 7 cases had autocontrol of low strength (1+). The Fig. 2 describes the method used to detect the antibody/ies with respect to the gap between the last transfusion and the day of immunohaematological workup for the 13 cases which had autocontrol positive. Similarly, Fig. 3 shows the methods used for the 11 patients (45.8 %) who had autocontrol negative, in relation with the transfusion history.
Fig. 2.
Flowchart showing the transfusion history and technique used to detect the alloantibody/ies in the patients who had autocontrol positive. The numbers in the brackets depicts the number of cases/patients
Fig. 3.
Flowchart showing the transfusion history and technique used to detect the alloantibody/ies in the patients who had autocontrol negative. The numbers in the brackets depicts the number of cases/patients
Out of the total crossmatch compatible units, 88 units typed negative for the respective antigens of which 51 units were issued for transfusion. Thus 57.9 % of crossmatched blood was utilized with the maximum number of units transfused to a single patient was 16. Crossmatching was done with patient plasma or with the adsorbed plasma wherever adsorption was done. No adverse transfusion reaction was recorded.
Discussion
The Kidd alloantibodies have the capacity to cause severe acute hemolytic transfusion reaction (AHTR) and delayed hemolytic transfusion reaction (DHTR) [7]. In case the Kidd alloantibodies are missed, it may either result in a mild DHTR or may be as severe as intravascular hemolysis and associated complications like acute renal failure, shock, DIC etc. More severe reactions are a result of the anamnestic reactions and its ability to fix complements. It has been estimated that over one-third of DHTRs are caused by anti-Jka [8, 9]. Case studies have also pointed to anti-Jkb as being responsible for severe DHTR [10, 11]. These typical characteristics of the Kidd alloantibody make it an important but elusive antibody in the blood bank serology and prompted us for an analysis.
During the study period of nearly 2 years, we detected 24 cases of Kidd alloantibodies. Kidd antibodies are predominantly found in combination with other antibodies [1]. Interestingly, in the present study, 2/3rd of the Kidd antibodies were detected alone and only 1/3rd were detected in combination with other antibodies. However, in concordance to the available literature [3], we found anti-Jka antibody as the solitary antibody more commonly than anti-Jkb in a transfused individual.
Detecting Kidd alloantibodies is a challenge due to several reasons. First and foremost challenge is due to the dosage effect [12]. The panels used need to have cells with homozygous expression of Jka and Jkb to be able to detect a distinct reaction pattern. All the panels we used, commercial as well as in-house, abided to this requirement. Second problem is that the titer of Kidd antibody most often falls faster than other antibodies [12], becoming undetectable beyond 3 months from the triggering event. Hence it becomes a challenge for the serologist to diagnose it. This is in contrary to our experience as many of our patients had the sensitizing event beyond 3 months from the day of evaluation. Majority of our study population had a history of blood/blood component transfusion. Two patients did not receive transfusion ever. Both of the patients were multiparous females. It is known that the Kidd antigens present on the fetal red cell surface, inherited from the father, are capable of causing alloimmunization of the mother [13]. The third problem associated, as described by Nguyen et al. [7]., the newly formed allo anti-Jkb, are loosely bound to the red cell surface which may disperse if the manual agitation during shaking the tubes is stronger thus may be easily missed. Use of enhancing media like enzyme treated cells and the solid phase in our laboratory has helped in avoiding this problem.
The solid phase being an automated platform not only saves time but also is less laborious. The solid phase has helped us in detecting the Kidd antibodies amidst other alloantibodies. Incidentally, the cases with low strength of the autocontrols gave specific reaction pattern in the solid phase and no auto/allo adsorption was needed. Thus the adsorption procedure could be avoided and precious time saved. Also, the fact that the Kidd antibody reactivity is enhanced by the use of enzymes like ficin and papain [14] and the titer of the Kidd alloantibody is better within the 3 months of sensitizing trigger [12], helped in determining the antibody itself.
Antigen typed and crossmatch compatible units were provided to all these patients who required it. The 24 patients included cases admitted for transplants of liver or kidney. Providing antigen negative, crossmatch compatible units in these surgeries which are known to demand a larger number of red cell units pose a challenge to the blood bank. However the challenge was successfully met each time. None of the transfusions reported any adverse transfusion reaction.
Our study had certain limitations which included the smaller sample size. Being a retrospective study, the possibility of delayed hemolytic transfusion reaction of the cases with positive autocontrol was neither reported nor analyzed. Performing elution study could have established the DHTR cases, which was missed. A comparison of all the techniques we included in our study to analyze each sample for auto/alloantibodies could have been a better way to conclude the efficacy of the techniques in detecting the antibody.
Conclusions
Kidd alloantibodies though deceptive can be identified by sensitive techniques like the solid phase and simple but laborious techniques using the tube cell panels. Depending upon the strength of the autocontrol reaction, the routine autoadsorption process may be skipped and tube cell enzyme treated cells or solid phase techniques be used to get the results.
References
- 1.Storry JR. Other blood group systems and antigens. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical manual. 18. Bethesda: American Association of Blood Banks; 2014. pp. 337–363. [Google Scholar]
- 2.Klein HG, Anstee DJ. Other red cell antigens. In: Klein HG, Anstee DJ, editors. Mollison’s blood transfusion in clinical medicine. 12. Oxford: Wiley Blackwell; 2014. pp. 214–258. [Google Scholar]
- 3.Leger RM. Blood group terminology and other blood groups. In: Harmening DM, editor. Modern blood banking & transfusion practices. 6. Philadelphia: F. A. Davis Company; 2012. pp. 172–215. [Google Scholar]
- 4.Immucor Inc. W.A.R.M. manufacturer’s package insert. Package insert code 0057319: Revised in October 2011
- 5.Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical manual. 18. Bethesda: American Association of Blood Banks; 2014. [Google Scholar]
- 6.Trudell KS. Detection and identification of antibodies. In: Harmening DM, editor. Modern blood banking & transfusion practices. 6. Philadelphia: F. A. Davis Company; 2012. pp. 216–237. [Google Scholar]
- 7.Nguyen DM, Lee HJ, Mirabella D, Wu DW. Delayed hemolytic transfusion reaction due to anti-Jkb: case report highlighting the importance of early blood bank consultation and literature review. N Am J Med Sci. 2010;3(4):187–193. doi: 10.7156/v3i4p187. [DOI] [Google Scholar]
- 8.Pineda AA, Taswell HF, Brzica SM. Transfusion reaction. An immunologic hazard of blood transfusion. Transfusion. 1978;18:1–7. doi: 10.1046/j.1537-2995.1978.18178118550.x. [DOI] [PubMed] [Google Scholar]
- 9.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688–693. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 10.Morgan P, Wheeler CB, Bossom EL. Delayed transfusion reaction attributed to anti-Jkb. Transfusion. 1967;7:307–308. doi: 10.1111/j.1537-2995.1967.tb05523.x. [DOI] [PubMed] [Google Scholar]
- 11.Holland PV, Wallerstein RO. Delayed hemolytic transfusion reaction with acute renal failure. JAMA. 1968;204:1007–1008. doi: 10.1001/jama.1968.03140240063023. [DOI] [PubMed] [Google Scholar]
- 12.Hussain SS, Ebbs AM, Curtin NJ, Keidan AJ. Delayed haemolytic transfusion reaction due to anti-Jkb in a patient with non-Hodgkin’s lymphoma-transient nature of anti-Jkb and the importance of early serological diagnosis. Transfus Med. 2007;17(3):197–199. doi: 10.1111/j.1365-3148.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Geifman-Holtzman O, Wojtowycz M, Kosmas E, Artal R. Female alloimmunization with antibodies known to cause hemolytic disease. Obstet Gynecol. 1997;89:272–275. doi: 10.1016/S0029-7844(96)00434-6. [DOI] [PubMed] [Google Scholar]
- 14.Walker PS, Hamilton JR. Other Blood group systems and antigens. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical manual. 18. Bethesda: American Association of Blood Banks; 2014. pp. 391–424. [Google Scholar]