Abstract
The study was carried out to determine the role of plasma fibrinogen in diagnosis of neonatal sepsis and to evaluate its role in predicting short term outcome. Sixty five neonates with clinical features suggestive of neonatal sepsis were included in this study. Seventy five neonates served as the control group. Plasma fibrinogen, prothrombin time, activated partial thromboplastin time and platelet counts were performed in all the neonates. Cut-off value of plasma fibrinogen for diagnosis of neonatal sepsis was determined with the help of receiver operating characteristic curve. Plasma fibrinogen level was found to be significantly higher among neonates with sepsis when compared to neonates in control group (p < 0.0001). It had sensitivity of 70.8 %, specificity of 82.7 %, positive predictive value (PPV) of 72.3 % and negative predictive value (NPV) of 81.6 % for diagnosis of neonatal sepsis at cut-off value of 301.90 mg/dL. When neonates with septic shock and/or disseminated intravascular coagulation (DIC) were excluded from study population, sensitivity and NPV rose to 91.9 % and 95.4 % at the same cut-off value while specificity and PPV remained the same. Lower level of plasma fibrinogen was detected in neonates with septic shock and/or DIC (p < 0.0001) and in neonates who died (p < 0.0001). Hence plasma fibrinogen can serve as an effective tool in diagnostic work up of neonatal sepsis as well as in assessing development of complications and outcome.
Keywords: Fibrinogen, Neonatal sepsis, Neonate, Septic shock
Introduction
Neonatal sepsis is a clinical syndrome characterized by presence of signs and symptoms of sepsis with or without accompanying bacteremia [1]. It is the leading cause of neonatal mortality in the developing countries, accounting for 30–50 % of neonatal deaths [2–4]. According to National Neonatal Perinatal Database (NNPD), incidence of culture proven neonatal sepsis in India was reported as 8.5 per 1000 live births in 2002–2003 [5].
Signs of sepsis are often subtle and non specific in the initial phase [1]. Early suspicion is of vital importance as most of the sepsis related deaths are preventable if appropriate treatment is instituted promptly. Definitive diagnosis is based on blood culture which is time consuming and has low yield. Presumptive diagnosis is based on certain indirect evidences of sepsis including some acute phase reactants. Plasma fibrinogen is one such acute phase reactant.
Fibrinogen, also known as factor I of coagulation pathway, is a high molecular weight glycoprotein synthesized in liver and plays an important role in haemostasis. Thrombin splits off fibrinopeptides A and B from fibrinogen to form fibrin monomers which polymerize subsequently. Fibrin is then stabilized to form a dense aggregate by covalent cross-linkage catalyzed by activated factor XIII. Decrease in fibrinogen level is observed in disseminated intravascular coagulation (DIC) where it is consumed in the coagulation process. On the other hand, fibrinogen level is raised in inflammation where it acts as a positive acute phase reactant [6–8]. Mean values of plasma fibrinogen level in healthy preterm and full-term neonates aged up to 30 days range from 2.43 to 2.54 g/L and 2.70 to 2.83 g/L respectively [9, 10].
In this study we have evaluated the role of plasma fibrinogen level in diagnosis as well as prediction of complications and outcome.
Subjects and Method
This study was conducted in the neonatal care unit of the authors’ institute after obtaining requisite permission from the Institutional Ethics Committee. Informed consent was obtained from next of kin of all the neonates who were included in the study. Newborn babies were assessed for signs and symptoms suggestive of sepsis like poor feeding, lethargy, respiratory distress, apnea, retraction, grunting, convulsion, bulging anterior fontanelle, hypothermia, hyperthermia, loose stool and abdominal distension. Initially 65 neonates with suspected sepsis were included in the study; those with negative blood culture were later excluded from the study population. Seventy five neonates admitted with diagnosis of neonatal jaundice were taken as the controls. Cases and controls were matched in all aspects except the condition under consideration. In all cases complete clinical assessment was performed. Moreover, perinatal, nosocomial and environmental risk factors were reviewed. Blood culture was performed in cases using BacT/ALERT® PF Plus (bioMerieux, Inc. Durham, North Carolina) which contains complex medium and absorbent polymeric beads. Blood samples were drawn with aseptic precautions from each of the neonates with suspected sepsis before instituting antimicrobial therapy. Blood samples for measurement of plasma fibrinogen level were collected with tri-sodium citrate as anticoagulant. It was measured in all the neonates during initial work-up at admission by turbidimetric immunoassay for the quantitative determination of fibrinogen in human plasma [Quantia®Fibrinogen, Tulip Diagnostics (P) Ltd, Goa, India]. It is based on the principle of agglutination reaction carried out in a semiautomated analyzer. Prothrombin time (PT), activated partial thromboplastin time (aPTT) and platelet count were estimated using standard haematological methods. Course of illness was closely followed in each of the neonates especially in terms of development of complications like septic shock and disseminated intravascular coagulation (DIC). Septic shock was defined as presence of sepsis and cardiovascular organ dysfunction defined as decrease in blood pressure (BP) (hypotension) <5th percentile for age or systolic BP <2 SD below normal for age despite administration of isotonic fluid bolus OR Need for vasoactive drug to maintain BP in normal range OR Two of the following: Unexplained metabolic acidosis: base deficit >5.0 mEq/L, Increased arterial lactate >2 X upper limit of normal, Oliguria: urine output <0.5 ml/kg/h, Prolonged capillary refill: >5 s, and Core to peripheral temperature gap >3 °C [11]. Sepsis triggered DIC was defined as consumption of platelets, procoagulant clotting factors and anticoagulant proteins leaving the haemostatic system unbalanced and prone to bleeding and clotting [12]. Neonates with sepsis were further subdivided in two groups—those with septic shock and/or DIC were termed as ‘complicated sepsis’ and those without this complication were termed as ‘uncomplicated sepsis’. Plasma fibrinogen, PT, aPTT and platelet count were compared between these two groups. We also, compared these parameters between neonates who survived and those who died. Statistical analyses were performed using SPSS version 17.0. Different subgroups as well as controls were compared using independent t test. Receiver operating characteristic (ROC) curve was used to determine sensitivity and specificity of plasma fibrinogen in diagnosis of neonatal sepsis. All statistical analyses were carried out at 5 % level of significance and p value <0.05 was considered significant.
Results
In the present study we found that among 65 neonates suspected of sepsis, blood culture was positive in 48 (73.84 %) cases. Only culture positive cases were included in the study, others were excluded. Most common organism isolated was Klebsiella pneumonae (n = 27), followed by Escherichia coli (n = 11), Pseudomonas aeruginosa (n = 4), Staphylococcus aureus (n = 3), Staphylococcus haemolyticus (n = 2) and Enterobacter aerogenes (n = 1). Eleven neonates developed features of complicated sepsis in form of septic shock/and or DIC during the course of hospital stay; 7 succumbed to death.
There was no significant difference in gestational age (p value 0.151) and birth weight (p value 0.182) of the neonates between cases and controls. However, plasma fibrinogen level (p value <0.0001), PT (p value <0.0001), and aPTT (p value <0.0001) were significantly higher and platelet count (p value 0.026) was significantly lower in cases than controls. Values of all clinical and laboratory parameters among cases and controls are given in Table 1.
Table 1.
Comparison of various clinical and laboratory parameters among cases and controls
| Parameters | Cases (n = 48) | Control (n = 75) | p value |
|---|---|---|---|
| Gestation (weeks) | 36.56 ± 3.43 | 37.29 ± 2.17 | 0.151 |
| Birth weight (g) | 2428.91 ± 649.57 | 2555.46 ± 396.13 | 0.182 |
| Fibrinogen (mg/dL) | 347.82 ± 131.20 | 279.32 ± 48.51 | <0.0001 |
| PT (s) | 16.85 ± 7.90 | 13.24 ± 1.66 | <0.0001 |
| aPTT (s) | 35.85 ± 9.48 | 29.69 ± 4.42 | <0.0001 |
| Platelet (per mm3) | 155,145 ± 87,389 | 24,890 ± 76,740 | 0.026 |
All values are given as mean ± standard deviation
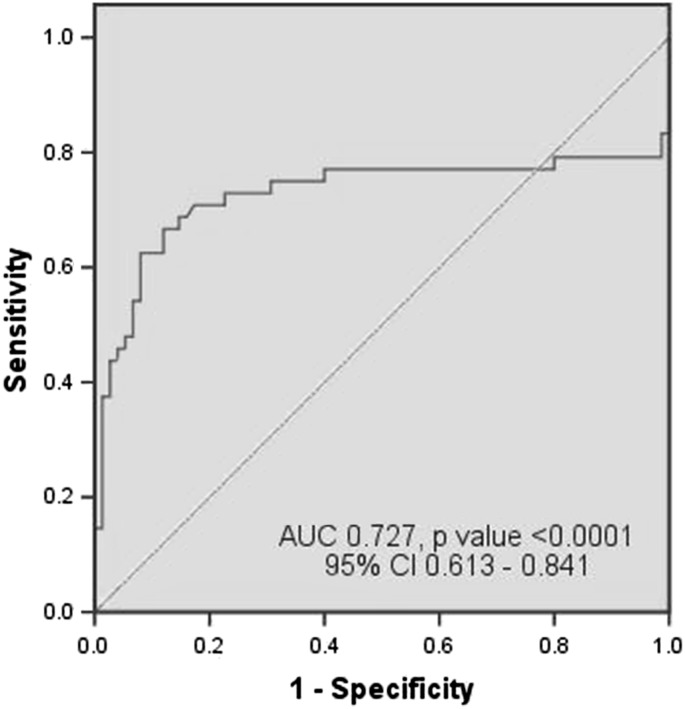
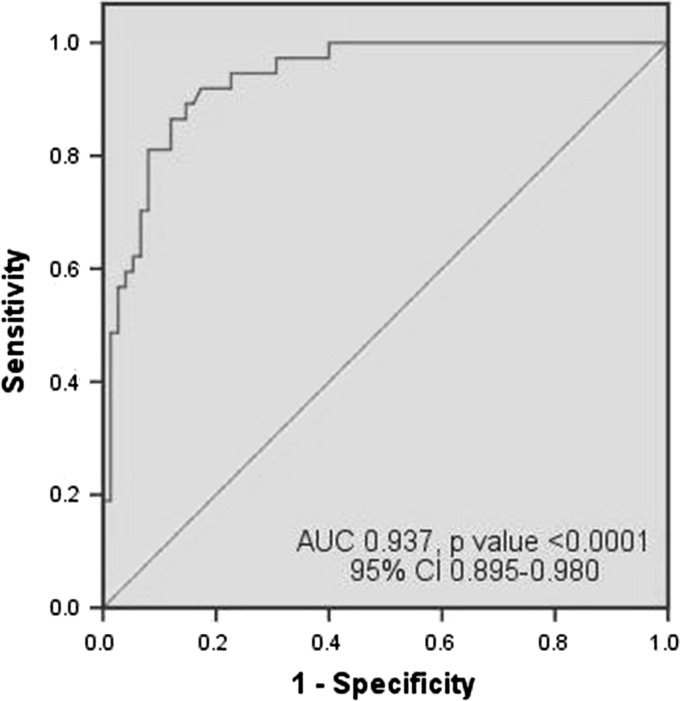
With the help of ROC curve, we have tried to determine the best cut-off value of plasma fibrinogen as a diagnostic marker of neonatal sepsis. We have calculated sensitivity and specificity at different plasma fibrinogen levels taking into account all the cases and also considered the uncomplicated sepsis cases separately. When all the septic neonates were considered together, it had sensitivity of 70.8 %, specificity of 82.7 %, PPV of 72.3 % and NPV of 81.6 % [area under the curve (AUC) 0.727, p value <0.0001, 95 % CI 0.613–0.841] for diagnosis of neonatal sepsis at cut-off value of 301.90 mg/dL. When neonates with septic shock and DIC were excluded (considering only the uncomplicated cases), sensitivity and negative predictive value rose to 91.9 % and 95.4 % [AUC 0.937, p value <0.0001, 95 % CI 0.895–0.980] at the same cut-off value. The ROC curves are given in Figs. 1 and 2.
Fig. 1.
ROC curve of plasma fibrinogen in diagnosis of neonatal sepsis when all cases of sepsis (n = 48) were compared with controls (n = 75)
Fig. 2.
ROC curve of plasma fibrinogen in diagnosis of neonatal sepsis when cases of uncomplicated sepsis (n = 37) were compared with controls (n = 75)
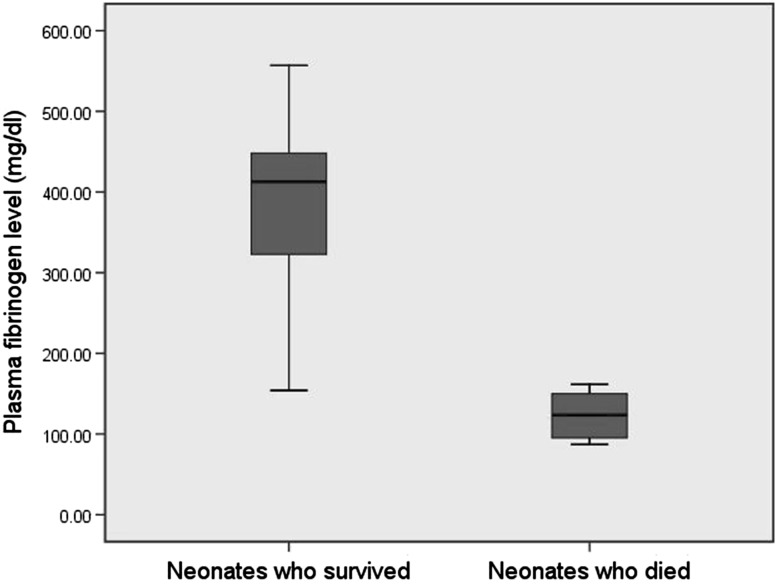
We compared various laboratory parameters of neonates considering complicated sepsis (n = 11) versus uncomplicated sepsis (n = 37) (Table 2). Plasma fibrinogen and platelet count were significantly lower (p value <0.0001) while PT and aPTT were significantly higher (p value <0.0001) among neonates with complicated sepsis than in the uncomplicated sepsis group. We also compared various laboratory parameters among patients who survived (n = 41) and who died (n = 7) (Table 2). Here also, neonates who died had significantly lowered values of plasma fibrinogen, platelet count, PT and aPTT (p value <0.0001) compared to the neonates who survived. Box plot comparing plasma fibrinogen levels in these two groups has been shown in Fig. 3.
Table 2.
Comparison of various laboratory parameters among subgroups
| Parameter | Complicated sepsis (n = 11) | Uncomplicated sepsis (n = 37) | p value | patients who survived (n = 41) | Patients who died | p value (n = 7) |
|---|---|---|---|---|---|---|
| Fibrinogen (mg/dL) | 145.73 ± 45.42 | 407.90 ± 75.56 | <0.0001 | 386.16 ± 98.72 | 123.24 ± 31.16 | <0.0001 |
| PT (s) | 30.55 ± 4.55 | 12.8 ± 1.20 | <0.0001 | 14.23 ± 4.73 | 32.14 ± 4.53 | <0.0001 |
| aPTT (s) | 50.82 ± 6.85 | 31.41 ± 3.94 | <0.0001 | 32.88 ± 6.12 | 53.29 ± 6.26 | <0.0001 |
| Platelet (per mm3) | 44,818 ± 17116 | 187,945 ± 71,161 | <0.0001 | 173,512 ± 80,846 | 47,571 ± 21,014 | <0.0001 |
All values are given as mean ± standard deviation
Fig. 3.
Box plot diagram comparing plasma fibrinogen level between babies who survived (n = 41) and who died (n = 7)
Discussion
In the present study, we evaluated the ability of plasma fibrinogen in diagnosis as well as prediction of short term outcome in neonatal sepsis. We found that plasma fibrinogen level was significantly higher in neonates with sepsis compared to those without sepsis (p < 0.0001). We observed that in patients with ‘complicated sepsis’, fibrinogen was low but PT and aPTT were prolonged where as in the ‘uncomplicated sepsis’ group, fibrinogen was raised but PT and aPTT were within normal range. The ‘cases’ group was a heterogeneous population—some had complicated sepsis while others had uncomplicated one and the latter outnumbered the former. So, plasma fibrinogen was high with prolonged PT and aPTT levels in the cases.
After comparing sepsis patients with our control population, we got sensitivity and specificity values of 70.8 % and 82.7 % respectively at the cut-off value of 301.90 mg/dL. Low levels of fibrinogen can indicate a systemic activation of the clotting system with consumption of clotting factors being faster than the synthesis. In any situation leading to DIC, including sepsis, its level falls. In the present study, we had 11 neonates with complicated sepsis. They behaved as false negative cases and subsequently decreased sensitivity and NPV when we compared all the cases with controls. When we excluded these cases with complicated sepsis, the results improved. When we excluded the cases with complicated sepsis we could show a sensitivity of 91.9 % at same cut-off value.
There is dearth of literature regarding role of plasma fibrinogen in diagnosis and prediction of outcome in neonatal sepsis. Guibourdenche et al. [13] have found that serum fibrinogen level is significantly higher in neonates with sepsis compared to normal with p value of 0.03. To determine optimum cut-off values, they have performed analysis by ROC curve and expressed test characteristics in terms of sensitivity, specificity and positive and negative predictive values. On the first day of life, ROC analysis gave optimum cut-off values of 3.0 g/L for plasma fibrinogen with sensitivity, specificity, PPV and NPV 66 %, 91 %, 80 %, and 84 % respectively for diagnosis of early onset neonatal sepsis. Our study showed similar results.
Erosy et al. showed that control group had higher mean fibrinogen value compared to the patients group which is contradicting our results. We assume the probable reason being they had almost equal number of cases in ‘only septicemia’ (n = 16) and ‘septic shock and/or DIC’ (n = 18) groups. On the other hand, in our study cases with uncomplicated sepsis (n = 37) were more than twice the complicated ones (n = 11). However they found that patients with ‘only septicemia’ had significantly higher (p value <0.01) initial plasma fibrinogen level when compared with those having ‘septic shock and/or DIC’ [14]. They also found that initial plasma fibrinogen level was significantly higher (p value <0.01) among patients who survived than those who did not survive. We confirmed the latter observations in the present study.
Speer et al. [15] studied 93 preterm and term infants with proven neonatal septicemia and/or meningitis. However, they found that fibrinogen was neither helpful in identifying neonatal infections nor a valuable follow-up parameter of successful treatment.
During early phase of sepsis (as seen in neonates with uncomplicated sepsis), fibrinogen behaves like an acute phase reactant and its plasma level rises. Later, it plays the role of a coagulation factor when patient’s condition deteriorates and septic shock and/or DIC ensue (as seen in neonates with complicated sepsis). Fibrinogen is consumed at this point leading to decrease in its plasma level. So, the increased level points towards development of sepsis while a low level predicts development of complications as well as poor outcome. It is not possible to predict complications at presentation on clinical grounds but we may forecast it to some extent by analyzing plasma fibrinogen level.
The present study has some potential limitations. Sample size was small. We could not measure the plasma fibrinogen level serially which would have been helpful in monitoring the course of sepsis more precisely.
We conclude that in case of suspected neonatal sepsis, initial plasma fibrinogen level can be used as a diagnostic tool. A value higher than 301.90 mg/dL should raise the suspicion of sepsis. Very low level should alert the neonatologist to become more vigilant to detect complications like septic shock or DIC at the earliest and thereby predict outcome.
Acknowledgments
Dr. Sharmistha Bhattacharjee and Dr. Nilanjana Ghosh, Department of Community Medicine, North Bengal Medical College and Hospital, Sushruta Nagar, Darjeeling, West Bengal, India for assisting in statistical analysis.
References
- 1.Bang AT, Bang RA, Bactule SB, Reddy HM, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet. 1999;354:1955–1961. doi: 10.1016/S0140-6736(99)03046-9. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ. The global impact of neonatal infection. Clin Perinatol. 1997;24:1–21. [PubMed] [Google Scholar]
- 3.Sankar MJ, Agarwal R, Deorari AK, Paul VK. Sepsis in the newborn. Indian J Pediatr. 2008;75:261–266. doi: 10.1007/s12098-008-0056-z. [DOI] [PubMed] [Google Scholar]
- 4.Lawn JE, Cowsens S, Zupan S, Lancet neonatal survival steering team 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 5.NNPD Network (2005) National Neonatal-Perinatal Database 2002–2003. NNPD Nodal Center at Department of Pediatrics, AIIMS, New Delhi. http://newbornwhocc.org/pdf/HRRC-Report_2002-03.pdf. Accessed on 19 Dec 2014
- 6.Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129–147. doi: 10.1016/0049-0172(90)90055-K. [DOI] [PubMed] [Google Scholar]
- 7.Eckersall PD. Acute phase proteins as markers of inflammatory lesions. Comp Haematol Int. 1995;5:93–97. doi: 10.1007/BF00638925. [DOI] [Google Scholar]
- 8.Kilicarslan A, Uysal A, Roach EC. Acute phase reactants. Acta Med. 2013;2:2–7. [Google Scholar]
- 9.Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–172. [PubMed] [Google Scholar]
- 10.Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V, Powers P. Development of the human coagulation system in the healthy premature infant. Blood. 1988;72:1651–1657. [PubMed] [Google Scholar]
- 11.Frankel LR, Kache S. Shock. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18. Philadelphia: Saunders; 2007. pp. 413–420. [Google Scholar]
- 12.Scott JP, Montgomery RR. Disseminated intravascular coagulation. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. 18. Philadelphia: Saunders; 2007. pp. 2080–2081. [Google Scholar]
- 13.Guibourdenche J, Bedu A, Petzold L, Marchand M, Mariani-Kurdjian P, Hurtaud-Roux MF, Aujard Y, Porquet D. Biochemical markers of neonatal sepsis: value of procalcitonin in the emergency setting. Ann Clin Biochem. 2002;39:130–135. doi: 10.1258/0004563021901874. [DOI] [PubMed] [Google Scholar]
- 14.Ersoy B, Nehir H, Altinoz S, Yilmaz O, Dundar PE. Prognostic value of initial antithrombin levels in neonatal sepsis. Indian Pediatr. 2007;44:581–584. [PubMed] [Google Scholar]
- 15.Speer CP, Gahr M, Schröter W. Early diagnosis of neonatal infection. Monatsschr Kinderheilkd. 1985;133:665–668. [PubMed] [Google Scholar]