Abstract
Escherichia coli O157:H7 is an important cause of diarrhea, hemorrhagic colitis, and potentially fatal human illness. Cattle are considered a primary reservoir of infection, and recent experimental evidence has indicated that the terminal rectum is the principal site of bacterial carriage. To test this finding in naturally colonized animals, intact rectum samples from 267 cattle in 24 separate lots were obtained immediately after slaughter, and fecal material and mucosal surfaces were cultured for E. coli O157 by direct and enrichment methods. Two locations, 1 and 15 cm proximal to the recto-anal junction, were tested. In total, 35 animals were positive for E. coli O157 at at least one of the sites and 232 animals were negative as determined by all tests. The frequency of isolation and the numbers of E. coli O157 cells were higher at the site closer to the recto-anal junction, confirming our previous experimental findings. We defined low- and high-level carriers as animals with E. coli O157 levels of <1 × 103 CFU g−1 or <1 × 103 CFU ml−1 and animals with E. coli O157 levels of ≥1 × 103 CFU g−1 or ≥1 × 103 CFU ml−1 in feces or tissues, respectively. High-level carriage was detected in 3.7% of the animals (95% confidence interval, 1.8 to 6.8%), and carriage on the mucosal surface of the terminal rectum was associated with high-level fecal excretion. In summary, our results support previous work demonstrating that the mucosal epithelium in the bovine terminal rectum is an important site for E. coli O157 carriage in cattle. The data also support the hypothesis that high-level fecal shedding (≥1 × 103 CFU g of feces−1) of enterohemorrhagic E. coli O157 results from colonization of this site.
Escherichia coli O157:H7 is an important cause of diarrhea, hemorrhagic colitis, and potentially fatal systemic sequelae in humans (14). Human infections may arise through a variety of routes, but as the organism has been frequently isolated from cattle (3, 4, 7), this species is considered a primary reservoir of infection (11, 22). E. coli O157 prevalence studies have estimated that fewer than 10% of cattle carry the pathogen in feces (11), and in the bovine host the organism generally colonizes the gastrointestinal tract without causing disease. Recent evidence obtained by our group showed that the terminal rectum is the principal site of colonization in experimentally infected calves (23). Following this finding there have been no large surveys of the extent of E. coli O157 carriage in the terminal rectum or of the relationship between tissue carriage and fecal shedding. The specific objectives of this study were to compare in animals at slaughter the E. coli O157 numbers at two mucosal sites that were 1 and 15 cm proximal to the recto-anal junction (RAJ) and to examine the relationship between mucosal carriage at these sites and fecal shedding of the organism.
MATERIALS AND METHODS
Abattoir methods.
This study was carried out at a single Scottish abattoir where approximately 200 to 400 cattle from throughout Scotland are slaughtered daily. Rectal samples were collected in the abattoir gut room from the first 10 to 12 animals slaughtered on Mondays from 21 October 2002 to 21 April 2003; 24 lots were sampled. The animals tested were all under 30 months old, and the terminal rectum was bunged on the slaughter line and then tied and cut from the remainder of the gastrointestinal tract. Each rectum was bagged individually and placed in a cool box for direct transportation to the testing laboratory. All samples were received at the laboratory within 3 h of slaughter and were kept in a chill room at 4°C until testing.
The abattoir generally receives animals from single farms in groups containing between 4 and 15 animals. In general, the animals in groups are transported and penned together before killing, and since samples were collected from consecutive animals on the slaughter line, most probably the animals had been grouped together prior to slaughter. However, in this study, as there was a perceived need to maintain confidentiality of the animals' source, the exact relationship of animals within each slaughtered lot to the submitted group or groups was unknown.
Sampling methods.
In the laboratory, sterile scissors were used to open the rectum from the proximal end, and the mucosa and contents were viewed. The RAJ was identified, and two sampling sites, which were 1 and 15 cm proximal to the RAJ, were selected. First, at least 1 g of feces from the 15-cm site was placed in a sterile container by using a sterile microbiological loop. Then at least 1 g of feces from the 1-cm site was similarly collected and placed into another sterile container.
Consistent with our previously described tissue sampling method (23), a block of the gut wall 15 cm proximal to the RAJ was cut out with a sterile scalpel. The mucosal sample was trimmed from the submucosa and muscle layers and then placed on sterile benching and trimmed to obtain 1-cm2 piece. Any feces were lifted off the mucosal surface, and the tissue was placed in a sterile container. The sampling procedure was repeated with a similar piece of mucosa cut from a site 1 cm proximal to the RAJ.
Laboratory methods.
The 1-cm2 mucosal tissue samples were weighed and suspended in maximum recovery diluent at a volume-to-weight ratio of 9:1. The samples were vortexed for 1 min to remove the adherent bacteria (23). The supernatant fluid from each tissue washing and a 10-fold dilution prepared in maximum recovery diluent were used for direct plating to obtain counts of adherent E. coli O157. Briefly, duplicate 0.1-ml aliquots of the 10−1 and 10−2 dilutions were pipetted onto sorbitol MacConkey agar plates (Oxoid CM813) supplemented with cefixime (0.05 mg liter−1) and potassium tellurite (2.5 mg liter−1) (CTSMAC agar) (Oxoid SR172E; Mast Diagnostic, Merseyside, United Kingdom).
Nine volumes of buffered peptone water was added to approximately 1 g of each fecal sample. The suspension was thoroughly mixed and allowed to stand for a short period before direct plating of 0.1 ml of this 10−1 dilution was spread onto duplicate CTSMAC agar plates. The remainder of the suspension was incubated for 6 h at 42°C before immunomagnetic separation (IMS). The samples that were not tested immediately were refrigerated overnight and warmed for 1 h in a 37°C incubator prior to IMS processing. For the IMS procedure we used 0.02 ml of Captivate O157 immunomagnetic beads (LabM, Bury, United Kingdom) with three washes in phosphate-buffered saline containing 0.05% Tween 20 (pH 7.4) and resuspension in 0.1 ml of buffer before duplicate plating onto CTSMAC agar plates.
All agar plates were incubated at 42°C for 20 ± 2 h, and the identity of an isolated non-sorbitol-fermenting colony typical of E. coli O157 was confirmed by using a latex agglutination kit (Oxoid DR620) (24, 27). For directly plated samples, positive colony counts were recorded for each sample dilution and replicate, and the estimated bacterial counts were expressed as numbers of cells per milliliter of the tissue wash fluid or per gram of feces. IMS test results were recorded as positive or negative.
The E. coli O157 isolates collected from lot 18 in the latter part of the study were stored at −20°C before phage typing (16) at the E. coli O157 Scottish Reference Laboratory. Thirty-three isolates were examined for verocytotoxin virulence determinants by PCR, and the amplification products were separated by restriction fragment length polymorphism (2, 21).
Statistical analyses.
The direct culture colony counts were summarized by using generalized linear mixed models (6) with a Poisson error distribution, a logarithmic link function, an overdispersion parameter at the count level, and the logarithm of the dilutions included as an offset variable to adjust for the different dilutions associated with different observations. The animal identifier was fitted as a random effect. From this analysis, based on the estimated counts from tissue washes and feces samples, animals were categorized as negative or low- or high-level carriers of E. coli O157. These data were analyzed by using a generalized linear model (20) by incorporating a binomial error distribution, a logit link function, and an overdispersion parameter at the lot stratum. All generalized linear mixed models and generalized linear models were fitted and statistical procedures, such as Fisher's exact test, were carried out by using Genstat, 6th ed. (26).
The IMS data were analyzed by assuming that the presence or absence observations at the 1- and 15-cm sites were independent Bernoulli variables, with the probability of a positive observation depending on three parameters: the proportion of positive animals in the population and the test sensitivity at each of the two sites. There was no statistically significant evidence for a difference in sensitivity at the two sites, so the latter two parameters were assumed to be equal. Confidence intervals for the two model parameters were calculated by using the chi-square approximation for the profile log-likelihood ratio (1). This approach allowed estimation of the prevalence of IMS-positive animals with an adjustment for the sensitivity of the IMS test.
RESULTS AND DISCUSSION
In previous experimental reports (5, 9, 12, 23) workers have proposed a variety of colonization sites for E. coli O157 in the bovine host. However, E. coli O157 has not previously been enumerated in studies of slaughtered animals in which the oral cavity, rumen, or colon was sampled (15, 17, 18). This study is the first study in which significant numbers of E. coli O157 cells were identified on intestinal mucosal surfaces of naturally colonized cattle. In this study the terminal recta of 267 animals in 24 separate lots were collected, and direct counts of E. coli O157 were obtained for washes from fixed amounts of mucosal tissue taken from sites 1 and 15 cm proximal to the RAJ. For most recta samples adjacent to both tissue sites were collected; the exceptions were two recta from which no fecal contents were obtained at the 1-cm sites. All the fecal samples were tested to enumerate E. coli O157 and, except for two unprocessed samples, were also examined by IMS for the presence of E. coli O157.
Consistent with previous reports (11, 18, 24, 25, 28, 29), the majority of animals were negative for E. coli O157. Thirty-five animals were positive for E. coli O157 at any site as determined by either the direct culture or IMS method. E. coli O157 was enumerated in feces or tissue washes from 16 animals. The direct culture count data for each dilution were analyzed by using generalized linear mixed models, and the estimated numbers in feces and tissue washes from the different sites were collated for each animal (Table 1). The region close to the RAJ was the principal site for E. coli O157 carriage in these naturally colonized cattle. For the five animals in which there were enumerable bacteria at both mucosal tissue sites the E. coli O157 counts were higher at the 1-cm site and ranged from 3.8 × 104 to 1.0 × 106 CFU ml−1. In five animals E. coli O157 was detected only in the 1-cm site tissue washes, and the estimated counts were between 8.9 × 101 and 7.1 × 104 CFU ml−1. For one animal the organism was enumerated only in tissue washes from the 15-cm site, and the estimated count was 6.1 × 103 CFU ml−1.
TABLE 1.
Estimated E. coli O157 counts for each sample site for the 16 animals from which the organism was enumerated by direct plating
| Animal | Estimated counts
|
|||
|---|---|---|---|---|
| Tissue at 1 cm | Tissue at 15 cm | Feces at 1 cm | Feces at 15 cm | |
| (CFU ml−1) | (CFU ml−1) | (CFU g−1) | (CFU g−1) | |
| 70 | 9.4 × 105 | 6.4 × 104 | 1 × 105 | 2.9 × 104 |
| 143 | 3.8 × 104 | 1.3 × 102 | 5.6 × 103 | 1.4 × 103 |
| 144 | 5.2 × 105 | 1.2 × 105 | 1 × 105 | 4.5 × 104 |
| 146 | 1.0 × 106 | 1.5 × 104 | NSa | 9.8 × 102 |
| 195 | 1.1 × 105 | 3.2 × 102 | 7.8 × 105 | 1.8 × 103 |
| 28 | 3.8 × 104 | NDb | ND | ND |
| 86 | 8.9 × 101 | ND | 3.1 × 103 | ND |
| 156 | 1.3 × 103 | ND | ND | ND |
| 197 | 9.3 × 101 | ND | ND | ND |
| 200 | 7.1 × 104 | ND | 2.1 × 104 | ND |
| 186 | ND | 6.1 × 103 | 6.0 × 103 | 2.8 × 102 |
| 39 | ND | ND | ND | 1 × 101 |
| 150 | ND | ND | ND | 4.6 × 101 |
| 157 | ND | ND | ND | 2.4 × 102 |
| 159 | ND | ND | 1.4 × 102 | ND |
| 190 | ND | ND | 3.9 × 101 | ND |
NS, no sample at site.
ND, E. coli O157 not detected by direct plating.
In 30 of the 35 positive animals feces samples were positive as determined by IMS. A contingency table was generated from the IMS data (Table 2), and the subset of nonmissing data was analyzed by using Fisher's exact test. More positive IMS results were obtained for fecal samples taken from the 1-cm site than for fecal samples taken from the 15-cm site. However, detecting E. coli O157 in feces at the 1-cm site by IMS was very significantly (P < 0.001) associated with a greater chance of detecting the organism in the 15-cm site samples. Thus, this work confirmed our previous experimental findings (23) concerning the importance of terminal rectal carriage and indicated that in slaughtered cattle E. coli O157 carriage may occur up to 15 cm proximal to the RAJ. It was also apparent that there was considerable variation between individual animals in the E. coli O157 counts in the terminal rectum.
TABLE 2.
Relationship between the IMS results for the presence of E. coli O157 in rectal feces at sites 1 and 15 cm proximal to the recto-anal junction
| Feces at 1-cm sitea | No. of samples of feces at 15-cm siteb
|
|||
|---|---|---|---|---|
| Negative | Positive | Missing | Total | |
| Negative | 236 | 2 | 1 | 239 |
| Positive | 7 | 18 | 25 | |
| Missing | 3 | 3 | ||
| Total | 243 | 23 | 1 | 267 |
The sampling site was 1 cm proximal to the recto-anal junction.
The sampling site was 15 cm proximal to the recto-anal junction.
Since longitudinal studies of E. coli O157 carriage on the mucosal surface of the terminal rectum have not been conducted, the cause of the variation between animals is unclear. It may be that carriage at the 1-cm site is a stage in more extensive carriage at proximal rectal sites that may occur in any animal. Alternatively, animals may differ in the extent to which E. coli O157 carriage occurs in the terminal rectum depending on the regional distribution of lymphoid follicles (19), where E. coli O157 is thought to attach (23 ; unpublished data).
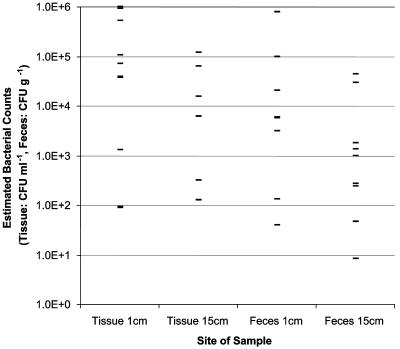
The estimated counts for samples in which E. coli O157 was detected by direct plating are shown by site in Fig. 1. The estimated counts for the whole data set suggested that there were two subpopulations of animals that could be categorized by whether the E. coli O157 levels were above or below a cutoff value of 103 CFU ml−1 or 103 CFU g−1. Ten cattle (animals 28, 70, 86, 143, 144, 146, 156, 186, 195, and 200) had high levels of E. coli O157 carriage. In 9 of these 10 animals >103 CFU ml−1 was detected in the tissue washes from the terminal rectum, and the highest numbers of E. coli O157 were found most frequently close to the RAJ. This high-level mucosal carriage was associated with high-level fecal excretion, as seven animals had >9.8 × 102 CFU g−1 in their feces and all fecal samples were IMS positive. For only one of the animals (animal 86) was the tissue count below 103 CFU ml−1; however, the fact that the fecal count for this individual was 3.1 × 103 CFU g−1 suggests that the area of high-level tissue carriage may occasionally be restricted and difficult to sample. Despite this result, the study showed that mucosal carriage of E. coli O157 in the terminal rectum is generally associated with high levels of the organism in feces.
FIG. 1.
Estimated counts for each site at which E. coli O157 was detected by direct plating.
For two animals (animals 28 and 156) the estimated counts in tissue washes were 3.8 × 104 and 1.3 × 103 CFU ml−1, but there was no detectable shedding of E. coli O157 as determined by direct plating of the feces. This suggests that there are fluctuations in the manner in which the surface of the fecal bolus becomes coated with the organism, and therefore, fecal counts should only be used as an indirect reflection of mucosal carriage at the terminal rectum. We therefore support the proposal resulting from studies of experimentally and naturally colonized animals (27) that rectal mucosal swabbing is likely to be more reliable than fecal testing for detection of E. coli O157 carriage.
The animal subpopulation with a low level of E. coli O157 carriage included 19 animals that were positive only as determined by IMS testing of feces. Additionally, positive direct counts and variable IMS results were obtained for six other animals, but the levels of E. coli O157 isolated were consistently <103 CFU ml−1 or <103 CFU g−1. In only 1 of these 25 animals was E. coli O157 detected in washes of rectal mucosal tissues, and it seems possible that samples from these animals were positive through passive carriage of the organism acquired by ingestion. An alternative explanation is that there was low-level carriage on mucosal surfaces of the terminal rectum but the levels were below the detection limits of the test. For either situation we believe that this low-level carriage category is likely to be consistent with the animals described by Rice et al. (27) as transient carriers that would be negative by rectal mucosal swabbing.
It was noted after sampling and testing of lot 15 that high-level carrier animals appeared to be associated with low levels of bacterial carriage in other animals in the lot sampled. Therefore, the bacterial carriage data for individual animals from tissue washes and feces samples were placed into three categories, as follows: negative for all tests; positive only when IMS was used or low level of E. coli O157 (<103 CFU ml−1 or <103 CFU g−1) detected at any site; and high level of E. coli O157 (≥103 CFU ml−1 or ≥103 CFU g−1) at any site. The categorized results from each day's sampling are shown in Table 3. In total, 35 animals were E. coli O157 culture positive, and these animals were found on 10 of the 24 sampling days. A generalized linear model was fitted to the numbers of negative and low-level carriage animals, and the presence of high-level carriage was shown to be very significantly (P = 0.001) associated with a higher risk of animals in the same lot having IMS-positive results or low direct counts (log odds ratio = 3.1, standard error = 0.84). It was not possible to confirm that high-level E. coli O157 carriage was the cause of the low-level E. coli O157 carriage. However, after making this observation, we explored the hypothesis by characterizing the isolates from animals in lot 18, which included two animals with high-level carriage. All isolates were confirmed to be verocytotoxigenic E. coli O157, and isolates indistinguishable by phage typing were recovered from the high-level carriers and from seven low-level carriers.
TABLE 3.
Numbers of animals by ordered category of E. coli O157 carriage in each lot
| Lot | No. of animals in bacterial carriage categoriesa
|
|||
|---|---|---|---|---|
| Negative | Low level | High level | Total | |
| 1 | 10 | 10 | ||
| 2 | 9 | 1 | 10 | |
| 3 | 7 | 2 | 1 | 10 |
| 4 | 9 | 1 | 10 | |
| 5 | 10 | 10 | ||
| 6 | 8 | 2 | 10 | |
| 7 | 9 | 1 | 10 | |
| 8 | 10 | 10 | ||
| 9 | 9 | 1 | 10 | |
| 10 | 10 | 10 | ||
| 11 | 12 | 12 | ||
| 12 | 12 | 12 | ||
| 13 | 12 | 12 | ||
| 14 | 1 | 7 | 3 | 11 |
| 15 | 8 | 3 | 1 | 12 |
| 16 | 12 | 12 | ||
| 17 | 12 | 12 | ||
| 18 | 3 | 7 | 2 | 12 |
| 19 | 9 | 2 | 1 | 12 |
| 20 | 12 | 12 | ||
| 21 | 12 | 12 | ||
| 22 | 12 | 12 | ||
| 23 | 12 | 12 | ||
| 24 | 12 | 12 | ||
| Total | 232 | 25 | 10 | 267 |
Negative, negative as determined by all tests; low level, positive as determined only by IMS or by bacterial levels of <103 CFU ml−1 or <103 CFU g−1 for all samples; high level, bacterial levels of ≥103 CFU ml−1 or ≥103 CFU g−1 at either tissue site or in either fecal sample.
We therefore assumed that sensitive techniques, such as IMS, may identify low-level carriage of E. coli O157 that can arise from contact with high-level carrier animals in the lairage, during transport, or on the farm of origin. These low-level carriers may not be colonized on the rectal mucosa but presumably could become colonized if bacterial attachment does occur. The hypothesis that high-level E. coli O157 carriage can be a source for low-level E. coli O157 carriage has some appeal as it may explain why single pulsed-field types have been shown to be dominant in slaughter pens (15).
Prevalence estimation was not a primary objective of this study, and the animal sampling was not formally structured. However, prevalence estimates were made to confirm that our sample population was representative of the larger slaughtered cattle population by comparison with previous observational studies (8, 24, 25, 28). The prevalence of E. coli O157 fecal shedding was calculated based only on IMS-positive results when testing of two samples allowed estimation of both the shedding prevalence and the sensitivity of the IMS test. The latter was estimated to be 81.0%. By using these data, the prevalence of E. coli O157 fecal shedding was estimated to be 11.7%, with a 95% confidence interval of 8.1 to 16.1%. This value is higher than the proportion of IMS-positive animals in the study, as the statistical model adjusted for the 3.6% of the shedding population that the duplicate sampling was presumed to have failed to identify. The result is probably more accurate than the results given in previous studies (8, 24, 25, 28) since the testing of two separate fecal samples from each animal increased the sampling sensitivity from 81.0% to 96.4%.
However, the feces from the 1-cm site alone provided the data that are most comparable to previous cattle prevalence data. The data for the feces from the 1-cm site indicated that 25 of 264 animals were positive as determined by IMS, and this corresponded to a point prevalence of 9.5% with a 95% confidence interval of 6.2 to 13.7%. This is consistent with the previously reported Scottish cattle prevalence values of 8.6% (28) and 7.5% (24) and is similar to other slaughtered cattle estimates from the United Kingdom (8, 25). The prevalence estimate for animals with high-level E. coli O157 carriage (defined as ≥103 CFU ml−1 or ≥103 CFU g−1) was 3.7%, with a 95% confidence interval of 1.8 to 6.8%. Hence, it may be assumed that approximately one in three animals that can be detected by IMS have significant mucosal carriage at the terminal rectum. Interestingly, in a slaughterhouse study (24) of cattle in the United Kingdom in which bacterial enumeration was used, the data showed that 1.86% of the cattle exhibited fecal shedding of 103 CFU g−1 and that one-quarter of all positive animals had fecal E. coli O157 counts of >103 CFU g−1. A study (17) in which most-probable-number estimates for 38 samples were used also resulted in data that showed that a similar proportion of animals had high-level fecal carriage.
In conclusion, the findings are entirely consistent with our experimental results (23) and support the proposal that the terminal rectum is the principal site of E. coli O157 carriage in the bovine host. Mucosal carriage at the terminal rectum is associated with high-level fecal excretion, and this may have epidemiological significance and lead to transient carriage in other animals. Previous studies (10) have shown correlations between fecal prevalence and initial carcass contamination, and there is a need to produce prevalence estimates based on bacterial enumeration and not merely on presence-absence techniques, such as IMS. The concept of high-level fecal excretion has major implications not only for epidemiological studies but also for the control of E. coli O157 in the abattoir and on the farm. It has been suggested that the best ways to protect the food chain are to reduce E. coli O157 shedding in feces (13) and to target colonized animals where there is potential for persistent shedding (27). Studies should therefore be conducted to understand the dynamics of colonization at the terminal rectum, and interventions aimed primarily at colonized animals may prove to be particularly successful in reducing E. coli O157 carriage levels in the major animal reservoir.
Acknowledgments
J.C.L., D.F., and I.J.M. acknowledge the financial support of SEERAD. We acknowledge funding of S.W.N. from a DEFRA veterinary fellowship to D.L.G.
We thank Pam Taylor and Jimmy Wilson for technical assistance.
REFERENCES
- 1.Barndorff-Nielsen, O. E., and D. R. Cox. 1989. Asymptotic techniques for use in statistics. Chapman and Hall, London, England.
- 2.Bastian, S. N., I. Carle, and F. Grimont. 1998. Comparison of 14 PCR systems for the detection and subtyping of stx genes in Shiga-toxin-producing Escherichia coli. Res. Microbiol. 149:457-472. [DOI] [PubMed] [Google Scholar]
- 3.Besser, R. E., P. M. Griffin, and L. Slutsker. 1999. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50:355-367. [DOI] [PubMed] [Google Scholar]
- 4.Borczyk, A. A., M. A. Karmali, H. Lior, and L. M. C. Duncan. 1987. Bovine reservoir for verotoxin-producing Escherichia coli O157:H7. Lancet i:98. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, H., and R. Prescott. 1999. Applied mixed models in medicine. Wiley and Sons, Chichester, England.
- 7.Chapman, P. A., C. A. Siddons, D. J. Wright, P. Norman, J. Fox, and E. Crick. 1993. Cattle as a possible source of verocytotoxin-producing Escherichia coli O157 infections in man. Epidemiol. Infect. 111:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman, P. A., C. A. Siddons, A. T. Cerdan Malo, and M. A. Harkin. 1997. A 1-year study of Escherichia coli O157 infections in cattle, sheep, pigs and poultry. Epidemiol. Infect. 119:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder, R. O., J. Keen, G. R. Siragusa, G. A. Barkocy-Gallacher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gansheroff, L. J., and A. D. O'Brien. 2000. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA 97:2959-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan, D., S. A. McEwen, A. M. Lammerding, W. B. McNab, and J. B. Wilson. 1999. Pre-slaughter control of Escherichia coli O157 in beef cattle: a simulation study. Prev. Vet. Med. 41:55-74. [DOI] [PubMed] [Google Scholar]
- 14.Karmali, M., M. Petric, C. Lim, P. Fleming, and B. Steele. 1983. Escherichia coli cytotoxin, hemolytic uremic syndrome and hemorrhagic colitis. Lancet ii:1299-1300. [DOI] [PubMed] [Google Scholar]
- 15.Keen, J. E., and R. O. Elder. 2002. Isolation of Shiga-toxigenic Escherichia coli O157 from hide surfaces and the oral cavity of finished beef feedlot cattle. J. Am. Vet. Med. Assoc. 220:756-763. [DOI] [PubMed] [Google Scholar]
- 16.Khakria, R., D. Duck, and H. Lior. 1990. Extended phage-typing scheme for Escherichia coli O157:H7. Epidemiol. Infect. 105:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahti, E., O. Ruoho, L. Rantala, M.-L. Hanninen, and T. Honkanen-Buzalski. 2003. Longitudinal study of Escherichia coli O157 in a cattle finishing unit. Appl. Environ. Microbiol. 69:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laven, R. A., A. Ashmore, and C. S. Stewart. 2003. Escherichia coli in the rumen and colon of slaughter cattle, with particular reference to E. coli O157. Vet. J. 165:78-83. [DOI] [PubMed] [Google Scholar]
- 19.Liebler, E. M., J. F. Pohlenz, and G. N. Woode. 1988. Gut-associated lymphoid tissue in the large intestine of calves. I. Distribution and histology. Vet. Pathol. 25:503-508. [DOI] [PubMed] [Google Scholar]
- 20.McCullagh, P., and J. A. Nelder. 1989. Generalised linear models, 2nd ed. Chapman and Hall, London, England.
- 21.McNally, A., A. J. Roe, S. Simpson, F. M. Thomson-Carter, D. E. Hoey, C. Currie, T. Chakraborty, D. G. E. Smith, and D. L. Gally. 2001. Differences in levels of secreted locus of enterocyte effacement proteins between human disease associated and bovine Escherichia coli O157. Infect. Immun. 69:5107-5114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle dense mucosa at the terminal rectum is the principal site of colonization of Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omisakin, F., M. MacRae, I. D. Ogden, and N. J. C. Strachan. 2003. Concentration and prevalence of Escherichia coli O157 in cattle feces at slaughter. Appl. Environ. Microbiol. 69:2444-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paiba, G. A., J. C. Gibbens, S. J. S. Pascoe, J. W. Wilesmith, S. A. Kidd, C. Byrne, J. B. M. Ryan, R. P. Smith, I. M. McLaren, R. J. Futter, et. al. 2002. Faecal shedding of verocytotoxin-producing Escherichia coli O157 in cattle and sheep at slaughter in Great Britain. Vet. Rec. 150:593-598. [DOI] [PubMed] [Google Scholar]
- 26.Payne, R. W. 2000. The guide to Genstat, part 2: statistics. Lawes Agricultural Trust, Rothamstead, England.
- 27.Rice, D. H., H. Q. Sheng, S. A. Wynia, and C. Hodve. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Synge, B., and G. Paiba. 2000. Verocytotoxin producing E. coli O157. Vet. Rec. 147:27. [PubMed] [Google Scholar]
- 29.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]