Abstract
Despite a wealth of experimental evidence concerning the efficacy of the biocidal action associated with the TiO2 photocatalytic reaction, our understanding of the photochemical mechanism of this particular biocidal action remains largely unclear. It is generally accepted that the hydroxyl radical (·OH), which is generated on the surface of UV-illuminated TiO2, plays the main role. However, our understanding of the exact mode of action of the hydroxyl radical in killing microorganisms is far from complete, and some studies report that other reactive oxygen species (ROS) (H2O2 and O2·−, etc.) also play significant roles. In particular, whether hydroxyl radicals remain bound to the surface or diffuse into the solution bulk is under active debate. In order to examine the exact mode of action of ROS in inactivating the microorganism, we tested and compared the levels of photocatalytic inactivation of MS-2 phage and Escherichia coli as representative species of viruses and bacteria, respectively. To compare photocatalytic microbial inactivation with the photocatalytic chemical degradation reaction, para-chlorobenzoic acid, which rapidly reacts with a hydroxyl radical with a diffusion-limited rate, was used as a probe compound. Two different hydroxyl radical scavengers, tert-butanol and methanol, and an activator of the bulk phase hydroxyl radical generation, Fe2+, were used to investigate their effects on the photocatalytic mode of action of the hydroxyl radical in inactivating the microorganism. The results show that the biocidal modes of action of ROS are very different depending on the specific microorganism involved, although the reason for this is not clear. It seems that MS-2 phage is inactivated mainly by the free hydroxyl radical in the solution bulk but that E. coli is inactivated by both the free and the surface-bound hydroxyl radicals. E. coli might also be inactivated by other ROS, such as O2·− and H2O2, according to the present results.
Since photoelectrochemical disinfection with platinum-doped TiO2 was first reported by Matsunaga et al. (16) almost 20 years ago, many photocatalytic-inactivation studies with TiO2 have been conducted. Many of these disinfection studies were carried out either to identify the effective disinfection factors, such as the TiO2 concentration, light intensity, and pH (1, 12, 14, 26, 27), or to investigate the disinfection kinetics (9, 10, 15, 26) for practical purposes.
However, although many mechanistic investigations into the photocatalytic degradation of chemicals have been published (2, 6, 13, 18), there are few available reports on photocatalytic inactivation of microorganisms that consider the role of complex photooxidants, such as the hydroxyl radical (·OH), the superoxide radical (O2·−), and hydrogen peroxide (H2O2), etc. It is frequently assumed that the hydroxyl radical is the major factor responsible for the antimicrobial activity observed in the TiO2 photocatalytic reaction (11, 22, 26). On the other hand, Maness et al. (15) reported that other reactive oxygen species (ROS) (H2O2 and O2·−, etc.), as well as the hydroxyl radical, play significant roles in microorganism inactivation. Therefore, depending on the kind of microorganism, there is an increasing requirement for quantitative evidence supporting a role for ROS in the photocatalytic disinfection system. It is important to make a comparative study with microorganisms, since the biocidal actions of photooxidants generated in TiO2 photocatalytic reactions may differ depending on the kind of microorganism.
Recently, two kinds of hydroxyl radicals, one in the bulk solution phase and the other on the surfaces of TiO2 particles, were reported to act in the heterogeneous TiO2 photocatalytic reaction (6, 13). For example, Kim and Choi (13) reported that the TiO2 photocatalytic oxidation of (CH3)4N+ under acidic conditions proceeded through the intermediary of free hydroxyl radicals present in the solution, rather than on the surface of TiO2, by using the hydroxyl radical scavenger tert-butanol (t-BuOH) as a diagnostic probe to elucidate the mechanism. On the other hand, El-Morsi et al. (6) concluded that the photocatalytic degradation of 1,10-dichlorodecane should be ascribed to surface-bound hydroxyl radicals (in aqueous suspensions of TiO2) on the basis of the results of kinetic experiments that used various scavengers of valence band holes (h+vb), hydroxyl radicals, and conduction band electrons (e−eb). The methodologies employed for probing the mode of action of the ROS in the photocatalytic degradation of organic chemicals could also be applied to investigating the mechanism of action of the ROS, including the surface-bound hydroxyl radical and free hydroxyl radical, in the photocatalytic inactivation of microorganisms.
Therefore, this study was carried out to determine the biocidal modes of action of hydroxyl radicals and other photooxidants of two microorganisms, MS-2 phage and Escherichia coli. In the drinking water supply research field, these two microorganisms are well-known and popular viral and bacterial test microorganisms causing waterborne diseases (11, 22).
MATERIALS AND METHODS
Culture and analysis of MS-2 phage and E. coli.
MS-2 phage and E. coli were selected as representative microorganisms of viruses and bacteria, respectively. MS-2 phage has a size (ca. 25 nm in diameter), shape (round and icosahedral), and type of nucleic acids (RNA) similar to those of waterborne viral pathogens, such as enteric viruses, including enteroviruses, caliciviruses, and rotaviruses, etc. E. coli also has characteristics similar to those of major waterborne bacterial pathogen genera, such as Salmonella, Shigella, Vibrio, Campylobacter, and Yersinia, etc. Many previous studies have been conducted with these indicator microorganisms.
The MS-2 phages (ATCC 15597) were prepared and quantified by the soft-agar overlay (double-agar layer) method of plaque assay using a mutant strain of E. coli HS(pFamp)R as the host (5). The selected host strain is resistant to somatic coliphages T2 to T7 and φ174 and has a resistance marker to an antibiotic, such as ampicillin, on the F plasmid and the genes for resistance to streptomycin and nalidixic acid on the chromosome. E. coli HS(pFamp)R was grown and assayed in medium containing 1% tryptone, 0.05% glucose, 0.8% NaCl, 0.03% CaCl2 · 2H2O, and 15 mg each of ampicillin and streptomycin/liter. The growth broth also contained 0.1% yeast extract. The media for the top and bottom agars used in the plaque assays contained 0.7 and 1.5% agar (final concentrations), respectively, as well as 1.5 mg of MgSO4/liter. The host was used for the plaque assay from the exponential phase to the early stationary phase, and the host lawn medium was incubated overnight. The incubation temperature was always 37°C. MS-2 phage stocks were prepared from overlay agar plates after lyses to confluence; after the soft-agar overlay was suspended by the addition of 10 ml of phosphate-buffered saline (PBS) (150 mM sodium phosphate plus 150 mM sodium chloride [pH 7.2] [P4244]; Sigma Co., St. Louis, Mo.) to the plate and collected, the supernatant obtained after centrifugation for 15 min at 3,000 × g was saved.
E. coli (ATCC 8739) was inoculated in tryptic soy broth (Difco Co., Detroit, Mich.) and grown for 18 h at 37°C. The bacteria were harvested by centrifugation with a 50-ml conical tube at 1,000 × g for 10 min and washed two more times with 50 ml of PBS. Stock solution of E. coli was prepared by resuspending the final pellets in 50 ml of PBS. The cell concentration was determined by the spread plate method on nutrient agar after growing the E. coli for 24 h at 37°C. This nonselective medium of nutrient agar was chosen for E. coli counting since it gives a conservative estimation of the bacterial population.
The initial populations of E. coli and MS-2 phage for each disinfection experiment ranged from approximately 4.4 × 105 to 8.4 × 105 CFU or PFU/ml. During the experiment, 1 ml of suspension was withdrawn at each sampling time and was diluted to 1/10 and 1/100. Three replicates of the diluted and undiluted 0.1-ml suspensions were used to count the CFU, and results showed good reproducibility within a standard deviation of 10%. For all experiments, this procedure was repeated three times.
Experimental procedures.
All solutions and reagents were prepared with deionized and distilled water, and analytical-reagent-grade chemicals were used throughout (Aldrich Co.). All glassware used was washed with distilled water and then autoclaved at 121°C for 15 min. The reaction temperature was controlled at 20 ± 1°C with a thermostatic chamber (Jeio Tech Co.). The pH was maintained at 7.1 by use of phosphate buffer stock solution (500 mM, potassium phosphate plus sodium hydroxide). The resulting buffer solution was maintained at a concentration of 20 mM.
TiO2 particles (P25, 1 g/liter; Degussa Co.) were sonicated for 30 min before the experiment in order to uniformly disperse the particles. The reaction was carried out with 50 ml of solution, with the MS-2 phage or E. coli being held in a 60-ml Pyrex flask in the presence of the presonicated TiO2 particles (UV cutoff, <300 nm) and mixed by magnetic stirring. The t-BuOH (30 mM) and methanol (MeOH; 30 mM), which were used as hydroxyl radical scavengers, or Fe2+ (2 μM) (20, 21, 22), which was used as an enhancer of the bulk phase hydroxyl radical reaction, was injected, depending upon the specific experimental conditions. Illumination was provided by 18-W black-light blue lamps (BLB; Philips Co.), which emit wavelengths in the range of 300 to 420 nm and were positioned on the four sides of the reactor. The light intensity, which was measured by ferrioxalate actinometry (8), was 7.9 × 10−6 einsteins/liter/s. In general, four to seven samples were collected for 30 to 210 min to measure the populations of the microorganisms. To separate the MS-2 phage from TiO2 particles, sampled solution was centrifuged at 10,770 × g for 10 min, and then the supernatant was harvested, as described previously by Sjogren and Sierka (22). The E. coli concentration was measured without separating the microorganism from the TiO2 particles, as previously described by Wei et al. (27). To determine the concentrations of MS-2 phage and E. coli, 1 ml of sample solution was withdrawn at each sampling time and diluted to 1/10 and 1/100. One-tenth of a milliliter of the undiluted and diluted solutions was used to count the number of MS-2 phage or E. coli cells. Three replicate plates were used for each dilution. All of the disinfection experiments were repeated three times, and their averaged values with statistical deviations were used for the data analysis. Statistical analysis, which includes the error bar, standard deviation, averaged values, and log scale expression, was conducted in this study by using the Microsoft Excel program.
The same experiments were carried out with or without microorganisms to measure the concentration of para-chlorobenzoic acid (pCBA), a hydroxyl radical probe compound, for each disinfection experimental condition. In all cases, the pCBA degradation measurement for each experimental condition was conducted during three different reaction periods, such as the initial, middle, and final stages of the disinfection experiments. In the control test, the degradation of pCBA was not influenced by the absence or presence of either MS-2 phage or E. coli. Thereafter, the pCBA experiments were conducted without the microorganisms being present.
Analysis of pCBA.
As described in our previous study (3), pCBA (rate constant with hydroxyl radical, 5 × 109 M−1 s−1) (7) is well known as a hydroxyl radical probe compound in the water system (3, 7). The concentration of pCBA (initial concentration, 1.92 μM) was analyzed by means of high-pressure liquid chromatography (Waters Co.). A C18 reverse-phase column (XTerra Rp18 5-μm; 2.1 mm by 150 mm) was used with a UV detector (UV/VIS-151; Gilson Co., Lewis Center, Ohio) at a wavelength of 230 nm for measuring the concentration of pCBA. A solvent mixture of 35% acetonitrile-65% water containing 40 mM phosphate buffer was employed as the mobile phase.
TiO2 chemistry.
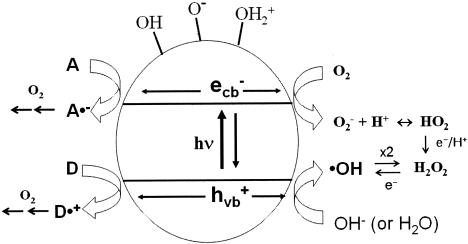
In general, the photochemical reaction involving TiO2 can be explained by the following series of elementary reaction steps (see equations 1 through 8 below) (2), and the simple scheme of the photocatalytic TiO2 reaction is described in Fig. 1. The first step is the light-induced (λ < 390 nm) generation of a valence band hole (h+vb) and a conduction band electron (e−eb) pair (equation 1). This e−eb is available for electron donation to reducible species that are adsorbed to the TiO2 surface. e−eb reduces oxygen to produce the superoxide radical (O2·−, equation 2) and subsequently the O2·− to produce H2O2 (equation 3). O2·− can react with H2O2 to produce the hydroxyl radical (Haber-Weiss reaction). The reduction of H2O2 by e−eb can generate the hydroxyl radical (equation 5) as well. h+vb abstracts electrons from absorbed oxidizable species and reacts with the surface OH− or H2O to form the hydroxyl radical (equations 6 and 7). The recombination of hydroxyl radicals also produces H2O2 (equation 8). Therefore, the production of H2O2 can be ascribed to either a reductive pathway (equation 3) or an oxidative pathway (equation 8).
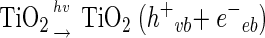 |
(1) |
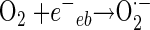 |
(2) |
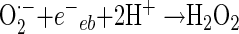 |
(3) |
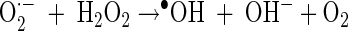 |
(4) |
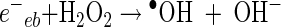 |
(5) |
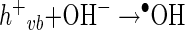 |
(6) |
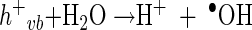 |
(7) |
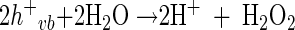 |
(8) |
FIG. 1.
Simple scheme of photocatalytic TiO2 chemistry. hν, UV light; A, electron acceptor; D, electron donor; ecb−, conduction band electron; e−, electron.
When Fe2+ is added, free hydroxyl radicals are produced in the solution as a result of the Fenton reaction (equation 9). Fe3+ can be reduced by e−eb or O2·− (equations 10 and 11). This enhancement of free hydroxyl radical formation in the presence of Fe2+ was used to evaluate its role in the inactivation of each microorganism in the bulk phase of the solution (19, 20).
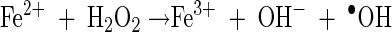 |
(9) |
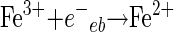 |
(10) |
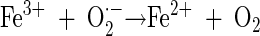 |
(11) |
The relative effects of the two different modes of action of the hydroxyl radical (either in the bulk phase or in the surface phase) on microbial inactivation were investigated. Two well-known scavengers, t-BuOH and MeOH, were used to suppress the activity of the hydroxyl radical. Excess t-BuOH is known to scavenge all hydroxyl radicals in the bulk solution phase and some of the adsorbed hydroxyl radicals in the surface phase (13). On the other hand, an excess of MeOH, especially of the MeOH radical, is known to act not only as an efficient scavenger of both the bulk phase and surface-bound hydroxyl radicals but also as an h+vb scavenger (6, 23). The MeOH radical, which is generated by the reaction of MeOH and h+vb, also reduces the surface hydroxyl radical (4).
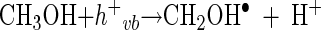 |
(12) |
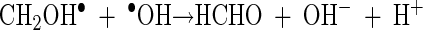 |
(13) |
RESULTS AND DISCUSSION
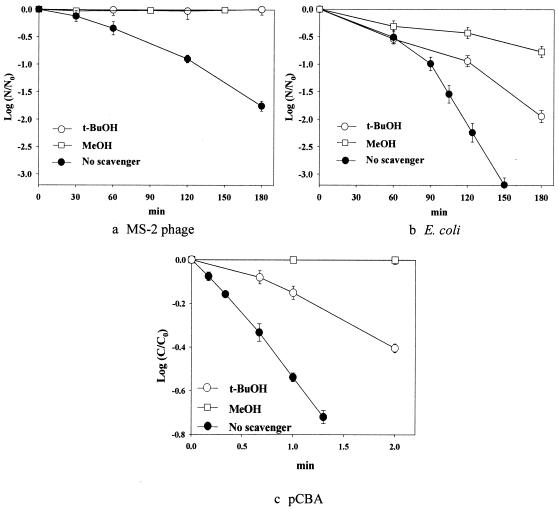
The inactivation behaviors of the two microorganisms, which were expressed with log scale, were observed with pCBA degradation in the presence of the scavenger, t-BuOH or MeOH, under the same experimental conditions (Fig. 2). In the control test, when either TiO2 or light was not applied, no inactivation of the microorganisms was observed during the time scale of this disinfection study, and all of the triplicate disinfection experiments in this study showed good reproducibility, with a 10% standard deviation. Three important observations can be made from the results shown in Fig. 2. First, MS-2 phage is more resistant than E. coli to TiO2 photocatalytic inactivation. Irradiation for 120 min was required for the 0.95- and 2.25-log inactivations of MS-2 phage and E. coli, respectively. The result that MS-2 phage was more resistant than E. coli, as shown in Fig. 1, was also observed with other popular chemical disinfectants, such as chlorine, ozone, and chlorine dioxide (25). The rapid pCBA degradation shown in Fig. 2c indicates that the hydroxyl radical is related to the inactivation of MS-2 phage and E. coli to some extent.
FIG. 2.
Inactivation of two types of microorganisms (MS-2 phage [a] and E. coli [b]) in illuminated TiO2 suspensions, compared with the photocatalytic degradation of pCBA (c). N, concentration of microorganisms (in cfu or pfu per milliliter); N0, initial N; C, concentration of pCBA; C0, initial C.
Second, the impacts of the hydroxyl radical scavenger on microorganism inactivation differ greatly between the two microorganisms. No inactivation of MS-2 phage was observed in the presence of either t-BuOH or MeOH as a hydroxyl radical scavenger, whereas the photocatalytic inactivation of E. coli (Fig. 2b) took place even in the presence of the scavengers, and the extent to which the inactivation was hindered depended on which scavenger was used. The inactivation of E. coli in the presence of t-BuOH or MeOH was reduced by 58 or 78%, respectively. It is also noteworthy that pCBA degradation in the presence of MeOH was inhibited completely but that pCBA degradation was only partially hindered in the presence of t-BuOH. The different responses in the photocatalytic kinetics of pCBA degradation, which were induced upon the addition of MeOH or t-BuOH, could be understood on the basis of the facts that the presence of excessive t-BuOH does not scavenge all of the surface hydroxyl radicals on TiO2 particles (13) and that MeOH scavenges not only the free hydroxyl radicals but also surface-bound hydroxyl radicals along with surface-trapped h+vb (6).
The results displayed in Fig. 2 imply that the surface-associated hydroxyl radicals that might survive even in the presence of t-BuOH are capable of inactivating E. coli to some extent but that they are not able to inactivate MS-2 phage. The photocatalytic inactivation of the observed inactivation behavior of MS-2 phage is hypothesized to be mediated by the bulk phase free hydroxyl radical, not by the surface-bound one. This can be rationalized based on the electrostatic interaction between the TiO2 surface and the cell surface. Since the surface of TiO2 (isoelectric point, 6.3) at pH 7.1 is predominantly negatively charged, and the MS-2 phage surface has both hydrophobic and negatively charged hydrophilic regions (22), the electrostatic repulsion between the TiO2 particles and MS-2 phage should be present. Therefore, the adsorption of MS-2 phage onto the surfaces of the TiO2 particles is not favored, and the direct contact between the cells and the illuminated TiO2 surface should be minimal. Under this condition, the inactivation of MS-2 phage is likely to proceed mainly through the bulk phase hydroxyl radical. However, the difficulty of extending this hypothesis to E. coli, which faces a similar situation of electrostatic repulsion between the TiO2 surface and the cell surface, requires further elaborate investigation.
In addition, it should be noted that more than one-fourth of the inactivation of E. coli was still observed, although the hydroxyl radical should have been completely scavenged in the presence of MeOH, judging from the complete deactivation of pCBA degradation under the same condition. This means that ROS such as O2·− and H2O2, which are known to be generated in the TiO2 photocatalytic system, may be partly responsible for E. coli inactivation as well as the hydroxyl radical. Kikuchi et al. (12) reported that E. coli inactivation can occur via the reaction between O2·− and H2O2 (equation 4) when these two species successfully pass through the cell membrane (11, 24).
The different inactivation behaviors of MS-2 phage and E. coli with ROS produced by TiO2 photocatalytic activity might be explained by the differences in the sizes and surface structures of these two microorganisms. MS-2 phage, which is one of the small, round RNA bacteriophages, is 25 to 28 nm in diameter. It has a capsid of cubic symmetry and is assumed to be the capsid of an icosahedron with 180 molecules of a coat protein. The high resistance of viruses over OH radical attack has been explained to be associated with the lack of enzymes and other sensitive systems (17). On the other hand, E. coli is a straight rod with a size of 1.1 by 2.0 to 1.5 by 6.0 μm and consists of complex structures of lipopolysaccharide, a peptidoglycan cell wall, and lipid bilayers of inner and outer membranes, etc. Even the slightest damage to the E. coli cell surface can destroy metabolic systems of the bacterium, such as respiration or other active systems (17). This finding seems to allow the less active oxygen species, such as O2·− and H2O2, to play a role in the inactivation of E. coli, to a certain extent. On the other hand, the inactivation of MS-2 phage is induced by denaturing the protein of the capsid, whose structure is simple and rigid and thereby requires more oxidizing power. As ROS (free and surface hydroxyl radicals O2·− and H2O2) produced by TiO2 photocatalytic activity compete for the reaction of coat protein of MS-2 phage, it is plausible that hydroxyl radicals, which have the highest oxidizing power, are the species responsible for MS-2 phage inactivation and that the species other than hydroxyl radicals (O2·− and H2O2) play a negligible role on MS-2 phage, as shown in Fig. 2.
Fe2+-aided photocatalytic inactivation.
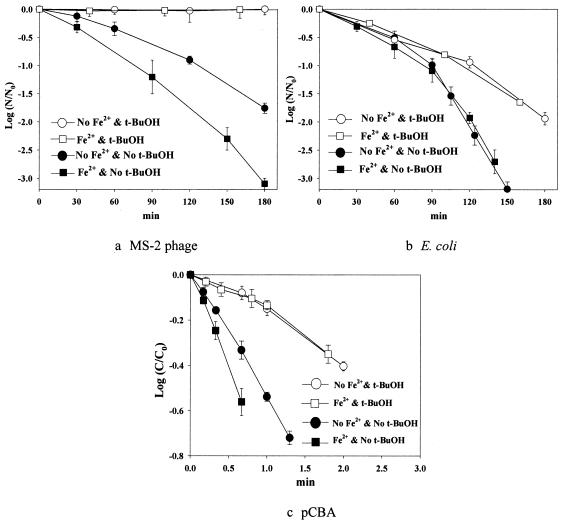
The time profiles of inactivation of MS-2 phage and E. coli were observed in conjunction with that of pCBA degradation, when Fe2+ and/or t-BuOH was added to the TiO2 suspension (Fig. 3). The results of the inactivations of MS-2 phage and E. coli and the degradation of pCBA presented in Fig. 2, which were obtained without the addition of Fe2+, were also included in Fig. 3 for comparison. The presence of Fe2+ enhances the generation of the bulk phase free hydroxyl radicals (through equation 9) and hence the inactivation path through free hydroxyl radicals. This effect of Fe2+ addition is also analyzed in pCBA degradation, as shown in Fig. 3c. Without t-BuOH, the presence of Fe2+ resulted in the increased degradation of pCBA, which indicated that free hydroxyl radicals were formed to a greater extent. With t-BuOH, the addition of Fe2+ had no effect on the pCBA degradation rate. This observation is consistent with the hypothesis that excess t-BuOH effectively scavenges all free hydroxyl radicals. As shown in equations 9 and 10, the presence of Fe2+ can help to prevent recombination of the charge pairs and eventually increase the formation of free or surface hydroxyl radicals.
FIG. 3.
Inactivation of MS-2 phage (a) and E. coli (b) in the presence or absence of Fe2+ (2 μM) and/or t-BuOH (30 mM) in illuminated TiO2 suspensions, compared with the degradation of pCBA (c). N, concentration of microorganisms (in cfu or pfu per millimeter); N0, initial N; C, concentration of pCBA; C0, initial C.
As shown in Fig. 3a and b, the addition of Fe2+ in the absence of t-BuOH induced the different inactivation behaviors of MS-2 phage and E. coli. The enhancement of inactivation was observed only with MS-2 phage, not with E. coli. However, in the presence of t-BuOH, with which all hydroxyl radicals present in the bulk phase, if any, should be scavenged, the Fe2+-induced enhancement of inactivation was not observed for either MS-2 or E. coli. These observations further support the hypotheses that MS-2 phage inactivation occurs mainly via the free hydroxyl radical in the solution bulk and that the same free hydroxyl radicals hardly play a role in the inactivation of E. coli, which are consistent with the results shown in Fig. 2. The increased inactivation of MS-2 phage in the photocatalytic disinfection of iron-loaded TiO2 observed in this study is similar to the results obtained by Sjogren and Sierka (22).
On the other hand, the possibility of the use of the non-Fenton route, which suggests that Fe2+ addition does not enhance the bulk phase hydroxyl radical reaction, can be effectively excluded, since the concentration of Fe2+ added (and, accordingly, that of its reduced form [Fe3+]) is small compared to that of oxygen. If the increased concentration of the hydroxyl radical occurred mainly via equations 2 and 3, then it is not clear which type of hydroxyl radical (bulk phase hydroxyl radical or surface phase hydroxyl radical) is responsible for this increase. However, no significant impact on the competitive reactions of oxygen or O2·− (equations 2 and 3) is to be expected.
This study clearly shows that the inactivation behaviors, which are mediated by the hydroxyl radicals generated on the illuminated TiO2 surface, are quite different, depending on the kind of microorganism (MS-2 phage or E. coli). It was found that the inactivation of MS-2 phage was carried out predominantly through the action of the bulk phase free hydroxyl radical but that E. coli was inactivated by both free and surface-bound hydroxyl radicals as a major path and by other ROS such O2·− and H2O2 as a minor path.
Acknowledgments
This research was partially supported by the Brain Korea 21 Program (of the Ministry of Education). This support was greatly appreciated.
REFERENCES
- 1.Bahnemann, D., D. Bockelmann, and R. Goslich. 1991. Mechanistic studies of water detoxification in illuminated TiO2 suspensions. Sol. Energy Mater. 24:564-583. [Google Scholar]
- 2.Bolton, J. R., and S. R. Cater. 1994. Homogeneous photodegradation of pollutants in contaminated water: an introduction, p. 467-490. In G. Heltz, R. G. Zepp, and D. Crosby (ed.), Surface and aquatic photochemistry. Lewis Publishers, Boca Raton, Fla.
- 3.Cho, M., H. Chung, and J. Yoon. 2003. Disinfection of water containing natural organic matter by using ozone-initiated radical reactions. Appl. Environ. Microbiol. 69:2284-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, W., and M. R. Hoffmann. 1995. Photoreductive mechanism of CCl4 degradation on TiO2 particles and effects of electron donors. Environ. Sci. Technol. 29:1646-1654. [DOI] [PubMed] [Google Scholar]
- 5.Debartolomeis, J., and V. J. Cabelli. 1991. Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages. Appl. Environ. Microbiol. 57:1301-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Morsi, T. M., W. R. Budakowski, A. S. Abd-El-Aziz, and K. J. Friesen. 2000. Photocatalytic degradation of 1,10-dichlorodecane in aqueous suspensions of TiO2: a reaction of adsorbed chlorinated alkane with surface hydroxyl radicals. Environ. Sci. Technol. 34:1018-1022. [Google Scholar]
- 7.Elovitz, M. S., U. von Gunten, and H. P. Kaiser. 2000. Hydroxyl radical/ozone ratios during ozonation processes. II. The effect of temperature, pH, alkalinity, and DOM properties. Ozone Sci. Eng. 22:123-150. [Google Scholar]
- 8.Hatchard, C. G., and C. A. Parker. 1956. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. A 235:518-536. [Google Scholar]
- 9.Huang, N., Z. Xiao, D. Huang, and C. Yuan. 1998. Photochemical disinfection of Escherichia coli with a TiO2 colloid solution and a self-assembled TiO2 thin film. Supermol. Sci. 5:559-564. [Google Scholar]
- 10.Huang, Z., P. C. Maness, D. M. Blake, E. J. Wolfrum, S. L. Smolinksi, and W. A. Jacoby. 2000. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 130:163-170. [Google Scholar]
- 11.Ireland, J. C., P. Klostermann, E. W. Rice, and R. M. Clark. 1993. Inactivation of Escherichia coli by titanium dioxide photocatalytic oxidation. Appl. Environ. Microbiol. 59:1668-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi, Y., K. Sunada, T. Iyada, K. Hashimoto, and A. Fujishima. 1997. Photocatalytic bactericidal effect of TiO2 thin films: dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A 160:51-56. [Google Scholar]
- 13.Kim, S., and W. Choi. 2002. Kinetics and mechanisms of photocatalytic degradation of (CH3)nNH4−n+ (0 ≤ n ≤ 4) in TiO2 suspension: the role of OH radicals. Environ. Sci. Technol. 36:2019-2025. [DOI] [PubMed] [Google Scholar]
- 14.Lee, S., N. M. Otaki, and S. Ohgaki. 1997. Photocatalytic inactivation of phage QB by immobilized titanium dioxide mediated photocatalyst. Water Sci. Technol. 35:101-106. [Google Scholar]
- 15.Maness, P.-C., S. Smolinski, D. M. Blake, Z. Huang, E. J. Wolfrum, and W. A. Jacoby. 1999. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 65:4094-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsunaga, T., R. Tomodam, T. Nakajima, and H. Wake. 1985. Photochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 29:211-214. [Google Scholar]
- 17.Montgomery, J. M. 1985. Water treatment principles and design, p. 262-283. John Wiley & Sons, New York, N.Y.
- 18.Prairie, M. R., L. R. Evans, B. M. Stange, and S. L. Martinez. 1993. An investigation of TiO2 photocatalysis for the treatment of water contaminated with metals and organic chemicals. Environ. Sci. Technol. 27:1776-1782. [Google Scholar]
- 19.Rupper, G., R. Bauer, and G. J. Hesler. 1993. The photo-fenton reaction—an effective photochemical wastewater treatment process. J. Photochem. Photobiol. A 73:75-78. [Google Scholar]
- 20.Safarzadeh-Amiri, A., J. R. Bolton, and S. R. Cater. 1996. The use of iron in advanced oxidation processes. J. Adv. Oxid. Technol. 1:29-36. [Google Scholar]
- 21.Scalfani, A., L. Palmisano, and E. Davi. 1991. Photocatalytic degradation of phenol in aqueous polycrystalline TiO2 dispersions: the influence of Fe3+, Fe2+ and Ag+ on the reaction rate. J. Photochem. Photobiol. A 56:113-123. [Google Scholar]
- 22.Sjogren, J. C., and R. A. Sierka. 1994. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 60:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun, Y., and J. J. Pignatello. 1995. Evidence for a surface dual hole-radical mechanism in the TiO2 photocatalytic oxidation of 2,4-dichlorophenoxyacetic acid. Environ. Sci. Technol. 29:2065-2072. [DOI] [PubMed] [Google Scholar]
- 24.Sunanda, K., Y. Kikuchi, K. Hashimoto, and A. Fujishima. 1998. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci. Technol. 32:726-728. [Google Scholar]
- 25.U.S. Environmental Protection Agency. 1999. Alternative disinfectants and oxidations: guidance manual. U.S. Environmental Protection Agency, Washington, D.C.
- 26.Watts, R. J., S. Kong, M. P. Orr, G. C. Miller, and B. E. Henry. 1995. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 29:95-100. [Google Scholar]
- 27.Wei, C., W. Lin, Z. Zainal, N. E. Zhu, K. Kruzic, R. L. Smith, and K. Rajeshwar. 1994. Bactericidal activity of TiO2 photocatalyst in aqueous media: toward a solar-assisted water disinfection system. Environ. Sci. Technol. 28:934-938. [DOI] [PubMed] [Google Scholar]