Abstract
Highly respirable particles (diameter, <1 μm) constitute the majority of particulate matter found in indoor air. It is hypothesized that these particles serve as carriers for toxic compounds, specifically the compounds produced by molds in water-damaged buildings. The presence of airborne Stachybotrys chartarum trichothecene mycotoxins on particles smaller than conidia (e.g., fungal fragments) was therefore investigated. Cellulose ceiling tiles with confluent Stachybotrys growth were placed in gas-drying containers through which filtered air was passed. Exiting particulates were collected by using a series of polycarbonate membrane filters with decreasing pore sizes. Scanning electron microscopy was employed to determine the presence of conidia on the filters. A competitive enzyme-linked immunosorbent assay (ELISA) specific for macrocyclic trichothecenes was used to analyze filter extracts. Cross-reactivity to various mycotoxins was examined to confirm the specificity. Statistically significant (P < 0.05) ELISA binding was observed primarily for macrocyclic trichothecenes at concentrations of 50 and 5 ng/ml and 500 pg/ml (58.4 to 83.5% inhibition). Of the remaining toxins tested, only verrucarol and diacetylverrucarol (nonmacrocyclic trichothecenes) demonstrated significant binding (18.2 and 51.7% inhibition, respectively) and then only at high concentrations. The results showed that extracts from conidium-free filters demonstrated statistically significant (P < 0.05) antibody binding that increased with sampling time (38.4 to 71.9% inhibition, representing a range of 0.5 to 4.0 ng/ml). High-performance liquid chromatography analysis suggested the presence of satratoxin H in conidium-free filter extracts. These data show that S. chartarum trichothecene mycotoxins can become airborne in association with intact conidia or smaller particles. These findings may have important implications for indoor air quality assessment.
The World Health Organization made the first attempt to define sick building syndrome (SBS) in 1982. SBS has proven to be difficult to define, and no single cause has ever been identified (2, 34). The complaints associated with poor indoor air quality (IAQ) range in severity and include difficulty in breathing, headaches, watering of the eyes, and flu-like symptoms, but they are not limited to these complaints (35). Numerous research groups have tried to determine the underlying cause(s) of SBS and poor IAQ. Fungi and their secondary metabolites, such as mycotoxins, are hypothesized contributors that have been closely examined (3, 5, 6, 9, 18, 21). The fungi isolated from buildings with poor IAQ include a wide variety of genera and species. Recent research has shown that, along with airborne conidia, highly respirable fungal fragments can lead to human exposure because the fragments can be aerosolized simultaneously with conidia. The amounts of these fragments could be as large as 320 times the amounts of conidia (16). Kildeso et al. (29) claimed that for a typical spore size (diameter, 3 μm; density, 1 g/cm3) the average exposure to spores may be approximately 0.14 μg/m3. Expanding on previous studies which concluded that the average concentration of respirable particles in a typical Danish office building was approximately 50 μg/m3 of air (28), conidia could be only a small fraction of the potential particulates (possibly less than 1%) in contaminated buildings. This suggests that fungal fragments and other small particulates, such as dust and nonorganic debris, could be potential carriers of the majority of aerosolized mycotoxins and therefore be a cause for concern and further study. A number of different fungi, including Stachybotrys chartarum, have been hypothesized to be important contributors to problems such as the adverse human health effects associated with indoor fungal growth (6, 15, 37). S. chartarum has been proposed to be associated with human adverse health effects on a limited scale (8, 12, 13, 22, 23). Numerous compounds have been characterized from S. chartarum. This fungus can produce anticomplement compounds and phenylspirodrimanes (30). Recently, a novel hemolysin named stachylysin has been described (42). A group of compounds known as atranones has also been described recently (20). S. chartarum is also known to produce cyclosporins, trichoverrols, trichoverrins, spirolactams, spirolactones (24), and spirocyclic drimanes (31). None of these compounds, however, have been a main focus of study for ailments arising from exposure to S. chartarum in indoor air. Instead, there has been much interest in the trichothecene mycotoxins that S. chartarum produces (1, 27, 32, 33, 39, 40). These toxins include, but are not limited to, the macrocyclic trichothecenes verrucarins B and J, roridin E, satratoxins F, G, and H, and isosatratoxins F, G, and H (19, 25). Recently, satratoxin G was found to be localized primarily in the conidia, followed by other fungal constituents, such as the phialides and hyphae (17). Several of these mycotoxins are known to react primarily with mucous membranes of the upper respiratory tract and eyes, leading to irritating erythema, inflammation, and pain (10). Inhalational studies in animals have shown that the respiratory route of exposure to trichothecene mycotoxins is highly effective (7).
While the consequences of exposure to mycotoxins in buildings with poor IAQ are essentially unexplored (36), there is a substantial body of case studies and some laboratory evidence which suggest that these toxins may contribute to reported complaints such as headaches, eye and throat irritation, nausea, dizziness, nose bleeds, and both physical and mental fatigue in subjects occupying such interiors (11, 36, 41). The members of the macrocyclic trichothecene family of mycotoxins are known inhibitors of protein synthesis in eukaryotes (14, 34, 43). In some studies workers have hypothesized that these compounds may also play a role in neurotoxicity and could therefore be particularly detrimental to humans (26). In light of the potential consequences of airborne mycotoxins for human health, it is important to know the possible ways in which mycotoxins can become airborne. The aim of this study was to determine if airborne macrocyclic trichothecenes can exist separate from S. chartarum conidia.
MATERIALS AND METHODS
Fungal growth.
A mycotoxin-producing strain of S. chartarum (ATCC 201212) was used in our experiments. The strain used has previously been shown to produce macrocyclic trichothecenes by high-performance liquid chromatography (HPLC) analysis (24, 25). Stock cultures were maintained on sterile cellulose ceiling tiles (7 by 7 cm) in a 25°C incubator. To grow the organism, ceiling tile squares were first allowed to absorb 50 ml of autoclaved pyrogen-free water in sterile Pyrex glass jars (100 by 80 mm). One milliliter of an S. chartarum conidium suspension at a concentration of 1 × 106 conidia per ml of phosphate-buffered saline (PBS) was evenly dispersed over the entire surface of each piece of water-saturated ceiling tile. The conidia were collected from confluent cultures in 20 ml of PBS at pH 7.4. Collection was performed with sterile 25-ml disposable serological pipettes (Fisher Scientific, Hampton, N.H.). Briefly, the cultures were washed with the PBS by repeated gentle aspiration (approximately 20 times or until the majority of the visible growth was removed). The conidia were then counted with a hemacytometer and diluted to the concentration mentioned above. This working suspension was used for immediate inoculation of ceiling tiles used in the experiments. Similar to stock cultures, ceiling tiles were considered ready for air sampling experiments when the growth was confluent (after 14 days).
Air sampling setup and particulate collection.
Air sampling and particulate collection experiments were performed a total of 12 times (including controls) with various sampling times (see below). The air sampling apparatus used for collecting macrocyclic trichothecenes on particulates is shown in Fig. 1. Pieces of ceiling tile that contained confluent 14-day-old S. chartarum cultures were cut into 1-in. squares with a sterile scalpel blade under a class II biological safety cabinet. The pieces were wet at the time of cutting (which minimized loss of conidia and mycelial fragments) but were dried quickly (due to the relatively high flow rate) as collection began. The supplied desiccant was removed from gas-drying tubes (Drierite, Xenia, Ohio), and the tubes were thoroughly cleaned with a soapy water-2% bleach solution and dried before use. Fourteen squares of ceiling tile were placed in each tube. Six tubes connected in series with polyvinyl chloride tubing (Nalgene, Rochester, N.Y.) were used for air sampling. Incoming air was filtered with an EPM-2000 glass microfiber filter (Whatman, Clifton, N.J.). EPM filter material was selected by the United States Environmental Protection Agency as the standard for use in high-volume air sampling. According to the manufacturer, such filters are 99.99% efficient for 0.3-μm-diameter dioctyl phthalate particles (the standard particles used for testing filter efficiency) at a flow rate of 5 cm/s. For this reason, EPM filters were chosen to ensure that clean, particulate-free air was entering the experimental apparatus. Prior to each sampling period, the sealed tubes were vigorously shaken by hand (25 times up and down). This was done similarly each time to initially generate intact conidia, mycelia, and fragments from the fungal constituents. Air moving at a flow rate of 30 liters/min, as measured with a flow meter (Gilmont Instruments, Barrington, Ill.) at the exit, was passed through the connected tubes, and there was a negligible drop in pressure throughout the apparatus. Particulates were collected on a series of membrane filters housed in 47-mm Fisherbrand gas line low-pressure filter holders (Fisher Scientific) that were placed at the exit of the air sampling setup in order of decreasing pore size. For these experiments, polycarbonate filters (Millipore, Billerica, Mass.) with pore sizes of 5.0, 1.2, and 0.4 μm were used. The sampling times were 1, 3, 6, 12, 24, 48, and 72 h. Additionally, for collection of material used for HPLC analysis, the same procedure was used for an extended sampling time (120 h). Tubes containing squares of sterile ceiling tile alone were used as controls. They were similarly sampled at 1, 6, 12, and 24 h for comparison. Each collection period was performed once.
FIG. 1.
Experimental air sampling apparatus. Filtered house air at a flow rate of 30 liters/min (LPM) was passed over cellulose ceiling tile with confluent S. chartarum growth for various periods of time. A total of six gas-drying tubes representing approximately 1,176 cm2 of S. chartarum growth were connected by using polyvinyl chloride (PVC) tubing. Particles were separated and collected on 47-mm-diameter polycarbonate membrane filters with pore sizes of 5.0 μm (filter A), 1.2 μm (filter B), and 0.4 μm (filter C) and later were analyzed for the presence of macrocyclic trichothecenes.
SEM.
One-quarter of the area (approximately 435 mm2) was cut from each of the test filters by using a sterile scalpel blade immediately following 72 h of air sampling. This was done in duplicate in order to retrieve the best-quality images for scanning electron microscopy (SEM). The remaining portion of each filter was used for an enzyme-linked immunosorbent assay (ELISA) and HPLC confirmation. The pieces used for SEM were individually placed in clean, sterile, 20-ml scintillation vials. Samples were mounted on studs and gold coated. After coating, samples were kept at 0°C in the gold coater until scanning in order to prevent any outside contamination. They were scanned with an Hitachi S-500 scanning electron microscope (Hitachi America, Ltd.).
Sample extraction and preparation.
Following air sampling, filters were removed from the filter holders and examined macroscopically for any tears or large holes. If no tears or holes were seen, the filters were then individually placed in 20-ml scintillation vials. Each filter was submerged in 15 ml of HPLC-grade methanol. The vials were vortexed for 60 s, and the filters were immediately removed with sterile forceps. The filter extracts were evaporated to dryness with a TurboVap II concentration workstation (Zymark Corporation, Hopkinton, Mass.). The dried extracts were resuspended in 1 ml of 5% HPLC-grade methanol in PBS and filtered through 13-mm-diameter 0.22-μm-pore-size nylon syringe filters (Millipore). Filtering the samples in this manner had a negligible effect on the trichothecene concentrations. These samples were the final working samples used for the ELISA. Sterile, unexposed filters were also treated in the same manner.
Macrocyclic trichothecene mycotoxin detection.
Macrocyclic trichothecenes were detected with a QuantiTox kit for trichothecenes (EnviroLogix, Portland, Maine). This competitive ELISA kit includes trichothecene-specific antibodies developed and previously described by Chung et al. (4) immobilized on polystyrene microtiter wells. All reagents and antibody-coated wells were allowed to equilibrate to room temperature before use. Briefly, 170-μl portions of filter extract were mixed with 170 μl of horseradish peroxidase-conjugated satratoxin G in separate 1.5-ml centrifuge tubes. The tubes were vortexed to ensure proper mixing. For testing, 100 μl of a sample or a control mixture was added to wells in triplicate. The wells were covered and incubated at room temperature on a plate rocker for 45 min. Following incubation, the wells were washed five times with PBS (pH 7.4) by using an MRW plate washer (Dynatech, Chantilly, Va.). The wells were blotted dry on clean paper towels. Immediately, 100 μl of a tetramethylbenzidine substrate solution was added to each well. The preparation was incubated at room temperature under reduced lighting for 15 min. To stop the reaction, 100 μl of 1 N hydrochloric acid (stop solution) was added to each well. The wells were read at 450 nm by using an EL-312 microtiter plate reader (Bio-Tek Instruments, Winooski, Vt.). The inhibition values were based on comparisons of samples and appropriate controls and represent the levels of inhibition that the test samples had on the ability of the satratoxin G-horseradish peroxidase conjugate to bind to the immobilized antibody. The cross-reactivities to other trichothecenes and two nontrichothecene mycotoxins were investigated to confirm the efficacy of the ELISA. All toxins were diluted to the same concentrations (50 and 5 ng/ml and 500 and 50 pg/ml) in PBS containing 5% (vol/vol) HPLC-grade methanol and tested in triplicate wells. Satratoxins G and H were purified as described by Hinkley and Jarvis (19) in our laboratory. Roridin A, verrucarin A, deoxynivalenol, verrucarol, diacetylverrucarol, neosolaniol, and T-2 toxin were purchased from Sigma (St. Louis, Mo.). Altenuene (Sigma) and sterigmatocystin (Acros Organics), which are nontrichothecene mycotoxins, were also tested. Roridin A at a concentration of 1 μg/ml was used as a positive control for each ELISA performed.
ELISA interpretation and statistical analysis.
Percent inhibition was calculated as described by Schick et al. (38) by using the following equation: percent inhibition = 100 × 1 − [(optical density at 450 nm of sample − background optical density at 450 nm)/(optical density at 450 nm of control − background optical density at 450 nm)].
Statistical analyses were performed by using the SigmaStat 2.0 software (Systat Software, Inc., Point Richmond, Calif.). Toxin concentrations were compared to solvent alone (5% methanol in PBS) and were individually compared by using a Student's t test (P < 0.05). Filter extracts were compared to controls (similar unused filters) by using a one-way analysis of variance followed by Tukey's post hoc analysis (P < 0.05).
For the filter extracts, data were also expressed as relative concentrations of macrocyclic trichothecene mycotoxins. This was done by using an ELISA-based macrocyclic trichothecene standard curve. The standard curve was developed by testing a mixture of equal amounts of four macrocyclic trichothecenes (satratoxins G and H, verrucarin A, and roridin A) by the ELISA as described above. Dilutions were made in PBS from a concentrated stock solution of the toxins in methanol (250 μg of each toxin per ml), which resulted in the following 12 test concentrations: 500, 250, 100, 50, 25, 10, 5, 2.5, 1, 0.5, 0.25, and 0.1 ng/ml. Average values for ELISA absorbance at 450 nm (based on three replicates) were then plotted against toxin concentrations (based on dilutions) to generate a standard curve. The statistical analyses used were the same as those described above for the inhibition comparisons.
HPLC analyses.
HPLC analyses were performed by using an 1100 Series HPLC system (Agilent Technologies, Palo Alto, Calif.) equipped with a UV-visible diode array detector. An Eclipse C8 analytical column (400 mm [250 plus 150 mm] by 4.6 mm; particle size, 5 μm) and a 12.5-mm guard column set at 40°C were used for the analyses. The flow rate was 1.0 ml/min, and the injection volumes were 10 μl for purified satratoxin H and 100 μl for the filter extracts. Filter extract samples (as described above for the ELISA analysis) were chromatographed in a mobile phase in which the gradient changed from 35% of 5% acetonitrile in water to 70% acetonitrile in 14 min. Samples were read at 260 nm and were analyzed by using the Chemstation software (Agilent). The method was quantitatively calibrated for satratoxin H.
RESULTS
SEM.
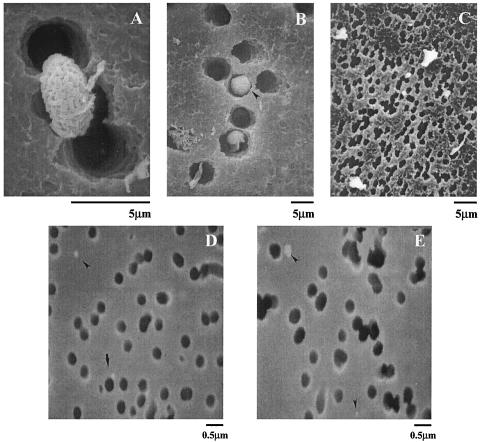
SEM demonstrated that particle separation and collection with our air sampling apparatus were successful. S. chartarum conidia were clearly identified on the 5.0-μm-pore-size filters (Fig. 2A) that we hypothesized would capture most conidia. However, we did not rule out the possibility that conidia could still pass through pores of this size. Figure 2B shows an S. chartarum conidium oriented perpendicular to the filter and lodged in a pore. This implies that some conidia could have passed through to the next filter in the series. However, based on our observations, this was not the case. We did not find intact S. chartarum conidia on the 1.2-μm-pore-size filters, but we found various particulates and other debris (Fig. 2C). Figures 2D and E show that we also captured extremely small particles (<0.5 μm based on the scale) on the 0.4-μm-pore-size filters.
FIG. 2.
Scanning electron micrographs of polycarbonate membrane filters following 72 h of sampling from the air sampling apparatus. The filter pores are clearly distinguishable from captured particulate matter (dark round and irregularly shaped bodies, respectively). (A) Filter (pore size, 5.0 μm) with a captured S. chartarum spore. Magnification, ×5,000. (B) The same type of filter with an intact S. chartarum spore lodged in a pore (arrowhead). Magnification, ×2,000. (C) Filter (pore size, 1.2 μm) with no S. chartarum spores but with a significant amount of debris. Magnification, ×2,500. (D and E) Filters (pore size, 0.4 μm) with extremely small captured particulates (arrowheads). Magnification, ×10,000.
ELISA cross-reactivity characterization.
As Table 1 shows, the QuantiTox ELISA kit proved to be suitable for detection of macrocyclic trichothecenes at several dilutions. No toxin showed statistically significant competitive inhibition at a concentration of 50 pg/ml. Significance was defined as a P value of ≤0.05 as determined by Student's t test. Significant values were obtained for satratoxin G at 50 and 5 ng/ml but not at 500 pg/ml. Significant inhibition was observed for satratoxin H, roridin A, and verrucarin A at 50 and 5 ng/ml and 500 pg/ml. Verrucarol, a nonmacrocyclic trichothecene mycotoxins, exhibited competitive inhibition at concentrations of 50 and 5 ng/ml but did not exhibit the same high levels of inhibition as the macrocyclic trichothecenes. Diacetylverrucarol, a derivative of verrucarol, exhibited relatively high binding only at 50 and 5 ng/ml. At a concentration of 500 pg/ml, the average inhibition for neosolaniol was significant, but a large standard deviation (±12.6%) was observed at this concentration. All other toxins tested were negative or relatively unreactive.
TABLE 1.
Competitive ELISA inhibition of select trichothecenes and two nontrichothecene mycotoxins
| Toxina | Concnb | % Inhibitionc |
|---|---|---|
| Satratoxin G | 50 ng/ml | 58.4 ± 2.85d |
| 5 ng/ml | 24.4 ± 1.75d | |
| 500 pg/ml | 9.23 ± 6.14 | |
| 50 pg/ml | 0.00e | |
| Satratoxin H | 50 ng/ml | 79.3 ± 1.56d |
| 5 ng/ml | 62.5 ± 0.62d | |
| 500 pg/ml | 28.2 ± 2.24d | |
| 50 pg/ml | 0.00e | |
| Roridin A | 50 ng/ml | 83.5 ± 0.51d |
| 5 ng/ml | 59.8 ± 5.16d | |
| 500 pg/ml | 43.3 ± 10.1d | |
| 50 pg/ml | 0.00e | |
| Verrucarin A | 50 ng/ml | 78.6 ± 1.82d |
| 5 ng/ml | 51.5 ± 1.60d | |
| 500 pg/ml | 14.2 ± 2.22d | |
| 50 pg/ml | 0.00e | |
| Deoxynivalenol | 50 ng/ml | 0.195 ± 3.33 |
| 5 ng/ml | 0.00e | |
| 500 pg/ml | 0.038 ± 2.59 | |
| 50 pg/ml | 0.00e | |
| T-2 toxin | 50 ng/ml | 1.19 ± 2.06 |
| 5 ng/ml | 0.00e | |
| 500 pg/ml | 0.00e | |
| 50 pg/ml | 0.00e | |
| Verrucarol | 50 ng/ml | 18.2 ± 5.65d |
| 5 ng/ml | 11.1 ± 2.95d | |
| 500 pg/ml | 3.40 ± 2.22 | |
| 50 pg/ml | 0.00e | |
| Diacetylverrucarol | 50 ng/ml | 51.7 ± 4.11d |
| 5 ng/ml | 25.3 ± 9.20d | |
| 500 pg/ml | 12.1 ± 4.82d | |
| 50 pg/ml | 0.00e | |
| Neosolaniol | 50 ng/ml | 5.43 ± 4.08 |
| 5 ng/ml | 6.96 ± 3.47 | |
| 500 pg/ml | 20.5 ± 12.6d | |
| 50 pg/ml | 6.64 ± 2.72 | |
| Altenuene | 50 ng/ml | 0.00e |
| 5 ng/ml | 0.00e | |
| 500 pg/ml | 0.00e | |
| 50 pg/ml | 0.00e | |
| Sterigmatocystin | 50 ng/ml | 0.00e |
| 5 ng/ml | 0.00e | |
| 500 pg/ml | 8.49 ± 1.01 | |
| 50 pg/ml | 0.00e |
Satratoxins G and H, roridin A, and verrucarin A are macrocyclic trichothecene mycotoxins. Deoxynivalenol, T-2 toxin, verrucarol, diacetylverrucarol, and neosolaniol are nonmacrocyclic trichothecene mycotoxins. Altenuene and sterigmatocystin are nontrichothecene mycotoxins.
Dilutions were made by using 5% methanol in PBS.
The results are based on solvent-only (5% methanol in PBS) controls. The values represent the results for triplicate wells. The values are averages ± standard deviations.
Significant as determined by Student's t test (P < 0.05).
Negative inhibition value converted to 0.00%.
Airborne macrocyclic trichothecene mycotoxin detection.
The existence of airborne macrocyclic trichothecenes was confirmed by using our filter apparatus with S. chartarum conidia, equivalent-size particulates, and smaller particles. Values considered to be statistically significant (compared with solvent alone) had P values of ≤0.05 as determined by one-way analysis of variance. Table 2 shows competitive inhibition percentages and semiquantitative macrocyclic trichothecene estimates for the polycarbonate filter extracts. The trichothecene equivalents were determined by using the standard curve shown in Fig. 3. The data show that all inhibition rates were high with the first filter extracts (pore size, 5 μm); the average was 94.5%. The average trichothecene equivalents were also high (average concentration, >500 ng/ml). The second filter extracts (pore size, 1.2 μm) also exhibited high levels of inhibition and toxin concentrations (averages, 89.3% and 260.2 ng/ml, respectively). Overall, the 48-h samples contained smaller amounts of toxin. The third filters (pore size, 0.4 μm) collected extremely small (diameter, <0.5 μm) conidium-free particulates, as demonstrated by SEM. The extracts showed significant reactivity in the ELISA, although the values were not as high as those obtained with the 5- and 1.2-μm-pore-size filters. The average inhibition percentage was 45.1%, and the average trichothecene equivalent value was 1.1 ng/ml; there was a general trend of increasing toxicity as the sampling time increased. Again, the 48-h sample had a significantly lower macrocyclic trichothecene content. Extracts from filters used for sampling ceiling tile alone showed moderate binding that increased with time (specifically on the 5-μm-pore-size filters). However, the percent inhibition values were much lower than the values for extracts from filters when S. chartarum was used.
TABLE 2.
Competitive ELISA inhibition for the polycarbonate filter extract-ceiling tile setup
| Sampling time (h)a | Filter pore size (μm) | % Inhibitionb | Avg Trichothecene equivalent (ng/ml)c |
|---|---|---|---|
| 1 | 5.0 | 96.17 ± 0.08d | >500d |
| 1.2 | 94.82 ± 0.08d | 335.96 ± 13.09d | |
| 0.4 | 52.50 ± 1.00d | 1.00 ± 0.06d | |
| 3 | 5.0 | 96.08 ± 0.20d | >500d |
| 1.2 | 94.57 ± 0.24d | 298.72 ± 33.95d | |
| 0.4 | 37.24 ± 3.03 | 0.48 ± 0.06 | |
| 6 | 5.0 | 95.99 ± 0.16d | >500d |
| 1.2 | 94.88 ± 0.14d | 348.34 ± 24.65d | |
| 0.4 | 38.36 ± 0.32d | 0.50 ± 0.01d | |
| 12 | 5.0 | 96.42 ± 0.14d | >500d |
| 1.2 | 95.45 ± 0.14d | 473.71 ± 39.42d | |
| 0.4 | 46.61 ± 4.19d | 0.75 ± 0.17d | |
| 24 | 5.0 | 96.30 ± 0.26d | >500d |
| 1.2 | 94.50 ± 0.28d | 289.75 ± 37.42d | |
| 0.4 | 71.93 ± 1.18d | 3.99 ± 0.42d | |
| 48 | 5.0 | 85.28 ± 0.66d | 21.76 ± 2.62d |
| 1.2 | 60.16 ± 3.23d | 1.61 ± 0.33d | |
| 0.4 | 17.35 ± 4.90d | 0.23 ± 0.04d | |
| 72 | 5.0 | 95.13 ± 0.36d | 402.48 ± 72.68d |
| 1.2 | 90.71 ± 0.45d | 73.05 ± 8.84d | |
| 0.4 | 51.57 ± 0.75d | 0.95 ± 0.04d | |
| 1 | 5.0e | 15.71 ± 1.82d | 0.22 ± 0.01d |
| 1.2e | 11.83 ± 3.01d | 0.20 ± 0.02d | |
| 0.4e | 3.44 ± 1.43 | 0.15 ± 0.01 | |
| 6 | 5.0e | 5.97 ± 1.61 | 0.17 ± 0.01 |
| 1.2e | 12.08 ± 0.49d | 0.20 ± 0.00d | |
| 0.4e | 0.00f | 0.14 ± 0.01 | |
| 12 | 5.0e | 32.07 ± 4.31d | 0.39 ± 0.07d |
| 1.2e | 9.35 ± 1.88d | 0.18 ± 0.01d | |
| 0.4e | 18.50 ± 3.52d | 0.24 ± 0.03d | |
| 24 | 5.0e | 37.46 ± 2.56d | 0.49 ± 0.05d |
| 1.2e | 15.75 ± 6.92d | 0.23 ± 0.05d | |
| 0.4e | 14.62 ± 1.85d | 0.21 ± 0.01d | |
| NAg | 5.0 | 0.00f | 0.13 ± 0.02 |
| 1.2 | 0.00f | 0.14 ± 0.01 | |
| 0.4 | 0.00f | 0.12 ± 0.01 |
Filters are grouped based on the order of the series and sampling time. Each sampling period experiment was performed one time.
The results are based on solvent-only (5% methanol in PBS) controls. The data are the results for triplicate wells. The values are averages ± standard deviations. Test groups (Stachybotrys on ceiling tiles) at 1, 6, 12, and 24 h were compared to correlating sampling times by using ceiling tiles alone and were significantly different (P < 0.05).
The values are semiquantitative and are based on the macrocyclic trichothecene standard curve shown in Fig. 3. Statistical analyses were performed as described for the inhibition comparisons.
Significant as determined by a one-way analysis of variance (P < 0.05).
Tests performed with sterile ceiling tile alone.
Negative inhibition value converted to 0.00%.
NA, not applicable (tests performed with sterile filters alone).
FIG. 3.
ELISA-based macrocyclic trichothecene standard curve. Equal concentrations of satratoxins G and H, roridin A, and verrucarin A were mixed in methanol, and the preparation was diluted in PBS (500 to 0.1 ng/ml) and tested by using a macrocyclic trichothecene-specific ELISA. Optical densities at 450 nm (OD450) were plotted against toxin concentrations in a power curve. This standard curve was used to estimate macrocyclic trichothecene equivalents for experimental filter extracts. For reference, the trichothecene concentrations, optical densities at 450 nm, and percent inhibition values are indicated. The standard deviations (based on three replicates) for all values are also indicated.
HPLC analysis.
Figure 4 shows chromatograms of extracts from a 120-h sampling. A longer sampling time was chosen because the extracts for the other times (as shown in Table 2) did not contain sufficient amounts of sample for HPLC analysis (data not shown). Figure 4A shows the results for a satratoxin H standard at a concentration of 1 ng/μl. UV spectrum analysis confirmed the presence of satratoxin H. Figure 4B shows the chromatogram for the filter extract from the 5.0-μm-pore-size filter, which clearly demonstrated the presence of satratoxin H (inset). The retention time (9.842 min) is slightly different from the standard shown in Fig. 4A due to the larger injection volume. Figure 4C shows the chromatogram for the 1.2-μm-pore-size filter extract. Although there is no clear indication that satratoxin H was present, there is a major peak at 9.845 min where satratoxin H was found on the 5.0-μm-pore-size filter. The UV spectrum of this peak (inset) has qualities similar to those of the satratoxin H spectrum (major absorbance at approximately 235 and 265 nm). Figure 4D shows the chromatogram for the extract from the 0.4-μm-pore-size filter. As observed with the 1.2-μm-pore-size filter, a satratoxin-like peak eluted at 9.848 min.
FIG. 4.
HPLC chromatograms of filter extracts from 120-h sampling. The retention times (in minutes) are plotted on the x axis. The peak sizes (in milliabsorbance units) are plotted on the y axis. For UV spectrum analyses (insets), wavelengths (in nanometers) are plotted on the x axis. (A) Satratoxin H standard (indicated by an asterisk) at a concentration of 1 ng/μl, with a retention time of 9.777 min. The inset shows the UV spectrum of the toxin with a maximum absorbance near 235 nm. (B, C, and D) Chromatograms of extracts from the 5-, 1.2-, and 0.4-μm-pore-size filters, respectively, that were used in a 120-h sampling experiment. The 5-μm-pore-size filter extract clearly shows the presence of satratoxin H with a retention time of 9.482 min (indicated by an asterisk). The results of UV spectral analyses are also presented (inset). The 1.2- and 0.4-μm-pore-size filter extract chromatograms have major peaks at 9.845 and 9.848 min, respectively, that could be satratoxin H. UV analyses demonstrated that these two peaks were qualitatively similar to the peak for purified satratoxin H.
DISCUSSION
In this study, we demonstrated the presence of airborne S. chartarum macrocyclic (and possibly nonmacrocyclic) trichothecenes on filters in the absence of conidia. This finding has implications for indoor air quality investigations involving S. chartarum growth on building material and the possible occupant complaints since individuals could potentially be exposed to these potent toxins for extended periods of time via the respiratory route. Using a controlled airflow system that included S. chartarum growth on cellulose ceiling tile, we demonstrated that airborne macrocyclic trichothecenes were present on particles smaller than conidia. The initial mechanical generation of particles by shaking was a means to shorten the overall sampling times for the experiments. Such intense disturbance and consequent release of particulates that include S. chartarum conidia would not be expected to occur in native environments. However, subtler disturbance mechanisms (human and mechanical vibrations, fans, air conditioning units, etc.) are hypothesized to cause the persistent release of such particulate matter over a longer period of time. The SEM images generated showed that most of the intact conidia were captured on the 5.0-μm-pore-size filters and that there were very few, if any, conidia on the 1.2-μm-pore-size filters. SEM clearly showed that there were particles that were 0.5 μm in diameter and smaller on the 0.4-μm-pore-size filters after 72 h of sampling (Fig. 2D and E). It was unclear whether these particles were fungal fragments, pieces of substrate, or other debris. In addition, particles of this size were difficult to locate and observe on the final filter. However, this does not mean that the quantity of such particles was low. The fact that we observed very low numbers may simply have been due to the mechanical capture of the bulk of the smaller particles on the preceding filters. To further emphasize this phenomenon, workers in our laboratory conducted similar tests (data not shown) by using membrane filters with larger pore sizes (up to 20 μm). The results showed that the majority of Stachybotrys conidia were captured on these filters even though the pores were easily large enough to allow passage of conidia. We believe that this phenomenon occurred with the 5- and 1.2-μm-pore-size polycarbonate filters and that very few extremely minute particulates (carrying toxin) passed through to the final filter.
We showed that a novel ELISA that included an antibody specific for macrocyclic trichothecene mycotoxins can be used for determining airborne concentrations of these toxins. Our results correlate with data recently published by Chung et al. (4), thereby supporting the efficacy of the ELISA. It was not surprising to find cross-reactivities with nonmacrocyclic trichothecenes. All trichothecene mycotoxins share a central trichothecene moiety. The antibody used for detection in our analysis was raised against a low-molecular-weight compound (satratoxin G). When this compound was compared to an antibody against a very specific tertiary structure (such as a protein), it was expected that a small degree of cross-reactivity or nonspecific binding would occur. Because of this, other compounds with similar structures, such as other mycotoxins, may result in background noise. Nonetheless, we are confident that the ELISA used here is highly specific for macrocyclic trichothecenes.
Filter extracts exhibited significant inhibition proportionate to the length of the sampling time. Extracts obtained from sampling at 48 h skewed our results, and the reason for this is uncertain. One possible explanation is that the shaking process could have resulted in uneven disturbance for this particular trial. Nonetheless, we were still able to demonstrate the presence of mycotoxins. We also demonstrated that filter extracts resulting from sampling of sterile ceiling tile alone showed significant ELISA reactivity. This could be attributed to the sensitivity of the ELISA and may have falsely increased our values. The high concentration of particles initially generated by hand could have overwhelmed the test (essentially resulting in high background noise). We do not believe that trichothecenes were present on the sterile ceiling tile. If they were originally present (due to cross-contamination, for instance), they would have been destroyed by the autoclaving used to sterilize the ceiling tile (44). To further strengthen our conclusions, we performed similar air collection experiments using Stachybotrys-contaminated rice (data not shown). Sterile rice alone did not result in false positives. However, the particulate-trichothecene separation was still successful. The combined data obtained from the filter experiments demonstrated that macrocyclic trichothecenes were found without captured intact conidia. Based on correlations with standards of similar toxins, our filter extracts contained 500 parts per trillion to more than 50 ppb of macrocyclic trichothecenes. We also demonstrated (by HPLC analysis) the presence of satratoxin H associated with conidia, but satratoxin H was only speculatively present with smaller particulates, such as fungal fragments. We identified peaks that eluted at the same retention time as satratoxin H for the 5.0-μm-pore-size filter that were similar in nature but were not definitively satratoxin H. This lack of certainty was most likely due to the significantly smaller amount of satratoxin H present on the 1.2-μm-pore-size filter, as demonstrated by a 10-fold decrease in milliabsorbance units, in combination with the large injection volume and limit of detection. For the HPLC methods and equipment used in these experiments, the limit of detection was approximately 10 pg/μl when a large volume (100 μl) of purified toxin was injected. This indicates that the ELISA technology used in our experiments was more sensitive and/or specific than the HPLC methods used (limit of detection, 100 pg/ml versus 10 pg/μl). This is important because the levels of trichothecenes from S. chartarum in contaminated indoor environments are expected to be very low at any given time and a sensitive means of detection is necessary. To date, however, there are no data describing what airborne concentrations of these toxins are necessary to adversely affect human health.
Most indoor air quality investigations focus on surface growth and airborne conidium concentrations. As Górny et al. concluded, spore counts do not adequately represent the amount of fungal fragments that are present in the air at any given time (16). In fact, fragments and particles that are the same size greatly outnumber intact fungal spores. Based on our results, we feel that air sampling in S. chartarum-contaminated indoor environments should include a means of collecting particulates smaller than conidia, followed by a specific and sensitive test for mycotoxins. For example, collection and separation could be done by using a filtration setup similar to the setup that we describe here. Also, current cyclone technology that allows exclusion of particles that are a certain size (such as conidia) could be used in conjunction with the numerous types of air sampling devices that are available. Digital particle counters and analyzers would also be ideal for characterizing and enumerating the collected particles. Finally, it should be noted that background normal levels of such toxins, particularly as determined by the ELISA described here, have yet to be determined.
Acknowledgments
We thank Assured IAQ, Dallas, Tex., for their support and EnviroLogix, Portland, Maine, for donating the QuantiTox kits for this research. This work was supported by grant 010674-0006-2001from the Texas Higher Education Coordinating Board and by a Center of Excellence Award from Texas Tech University Health Sciences Center. Additional funding was provided by the UT-Houston School of Public Health Pilot Research Projects in Occupational Safety and Health.
We thank the staff of the Center for Indoor Air Quality Research at Texas Tech University Health Sciences Center for their contributions.
REFERENCES
- 1.American Academy of Pediatrics Committee on Environmental Health. 1998. Toxic effects of indoor molds. Pediatrics 101:712-714. [PubMed] [Google Scholar]
- 2.Brightman, H. S., and N. Moss. 2000. Sick building syndrome studies and the compilation of normative and comparative values, p. 3.1-3.32. In J. D. Spengler, J. M. Samet, and J. F. McCarthy (ed.), Indoor air quality handbook. McGraw-Hill, Washington, D.C.
- 3.Chao, H. J., D. K. Milton, J. Schwartz, and H. A. Burge. 2001. Dustborne fungi in large office buildings. Mycopathologica 154:93-106. [DOI] [PubMed] [Google Scholar]
- 4.Chung, Y.-J., B. Jarvis, T. Heekyung, and J. J. Pestka. 2003. Immunochemical assay for satratoxin G and other macrocyclic trichothecenes associated with indoor air contamination by Stachybotrys chartarum. Toxicol. Mech. Methods 13:247-252. [DOI] [PubMed] [Google Scholar]
- 5.Cooley, J. D., W. C. Wong, C. A. Jumper, J. C. Hutson, H. J. Williams, C. J. Schwab, and D. C. Straus. 2000. An animal model for allergic penicilliosis induced by the intranasal instillation of viable Penicillium chrysogenum conidia. Thorax 55:489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooley, J. D., W. C. Wong, C. A. Jumper, and D. C. Straus. 1998. Correlation between the prevalence of certain fungi and sick building syndrome. Occup. Environ. Med. 55:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creasia, D. A., J. D. Thurman, R. W. Wannemacher, Jr., and D. L. Bunner. 1990. Acute inhalation toxicity of T-2 mycotoxin in the rat and guinea pig. Fundam. Appl. Toxicol. 14:54-59. [DOI] [PubMed] [Google Scholar]
- 8.Croft, W. A., B. J. Jarvis, and C. S. Yatawara. 1986. Airborne outbreak of trichothecene toxicosis. Atmos. Environ. 20:549-552. [Google Scholar]
- 9.Dales, R. E., R. Burnett, and H. Zwanenburg. 1991. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 143:505-509. [DOI] [PubMed] [Google Scholar]
- 10.Dearborn, D. G., I. Yike, W. G. Sorenson, M. J. Miller, and R. A. Etzel. 1999. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ. Health Perspect. 107(Suppl. 3):495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmage, S., M. Bailey, J. Raven, K. Abeyawickrama, D. Cao, D. Guest, J. Rolland, A. Forbes, F. Thien, M. J. Abramson, and E. H. Walters. 2002. Mouldy houses influence symptoms of asthma among atopic individuals. Clin. Exp. Allergy 32:714-720. [DOI] [PubMed] [Google Scholar]
- 12.Elidemir, O., G. N. Colasurdo, S. N. Rossman, and L. L. Fan. 1999. Isolation of Stachybotrys from the lung of a child with pulmonary hemosiderosis. Pediatrics 104:964-966. [DOI] [PubMed] [Google Scholar]
- 13.Etzel, R. A., E. Montana, W. G. Sorenson, G. J. Kullman, T. M. Allan, D. G. Dearborn, D. R. Olson, B. B. Jarvis, and J. D. Miller. 1998. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch. Pediatr. Adolesc. Med. 152:757-762. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg, B., and C. S. McLaughlin. 1998. Biochemical mechanism of action of trichothecene mycotoxins, p. 27. In V. R. Beasley (ed.), Trichothecene mycotoxicosis: pathophysiologic effects, vol. 1. CRC Press, Boca Raton, Fla. [Google Scholar]
- 15.Fung, F., R. Clark, and S. Williams. 1998. Stachybotrys, a mycotoxin-producing fungus of increasing toxicologic importance. J. Toxicol. Clin. Toxicol. 36:79-86. [DOI] [PubMed] [Google Scholar]
- 16.Górny, R. L., T. Reponen, K. Willeke, D. Schmechel, E. Robine, M. Boissier, and S. A. Grinshpun. 2002. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 68:3522-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory, L., J. J. Pestka, D. G. Dearborn, and T. G. Rand. 2004. Localization of satratoxin-G in Stachybotrys chartarum spores and spore-impacted mouse lung using immunocytochemistry. Toxicol. Pathol. 32:26-34. [DOI] [PubMed] [Google Scholar]
- 18.Harrison, J., C. A. Pickering, E. B. Faragher, P. K. Austwick, S. A. Little, and L. Lawton. 1992. An investigation of the relationship between microbial and particulate indoor air pollution and the sick building syndrome. Respir. Med. 86:225-235. [DOI] [PubMed] [Google Scholar]
- 19.Hinkley, S., and B. Jarvis. 2000. Chromatographic method for Stachybotrys toxins. Methods Mol. Biol. 157:173-194. [PubMed] [Google Scholar]
- 20.Hinkley, S., E. P. Mazzola, J. C. Fettinger, Y. Lam, and B. Jarvis. 2000. Atranones A-G, from the toxigenic mold Stachybotrys chartarum. Phytochemistry 55:663-673. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson, M. 1992. Field studies on the sick building syndrome. Ann. N. Y. Acad. Sci. 641:21-36. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson, M. J., P. Morey, W.-Y. Leung, L. Morrow, D. Miller, B. B. Jarvis, H. Robbins, J. F. Halsey, and E. Storey. 1998. Building-associated pulmonary disease form exposure to Stachybotrys chartarum and Aspergillus versicolor. J. Environ. Med. 40:241-249. [DOI] [PubMed] [Google Scholar]
- 23.Hossain, M. A., M. S. Ahmed, and M. A. Ghannoum. 2004. Attributes of Stachybotrys chartarum and its association with human disease. J. Allergy Clin. Immunol. 113:200-208. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis, B. B., J. Salemme, and A. Morais. 1995. Stachybotrys toxins. 1. Nat. Toxins 3:10-16. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis, B. B., W. G. Sorenson, E. L. Hintikka, M. Nikulin, Y. Zhou, J. Jiang, S. Wang, S. Hinkley, R. A. Etzel, and D. Dearborn. 1998. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 64:3620-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johanning, E., R. Biagini, D. Hall, P. Morey, B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in a water damaged office environment. Int. Arch. Occup. Environ. Health 68:207-218. [DOI] [PubMed] [Google Scholar]
- 27.Kielsen, K. F., S. Gravesen, P. A. Nielsen, B. Andersen, U. Thrane, and J. C. Frisvad. 1999. Production of mycotoxins on artificially and naturally infested building materials. Mycopathologica 145:43-56. [DOI] [PubMed] [Google Scholar]
- 28.Kildeso, J., L. Tornvig, P. Skov, and T. Schneider. 1998. An intervention study of the effect of improved cleaning methods on dust concentration and on dust composition. Indoor Air 8:12-22. [Google Scholar]
- 29.Kildeso, J., H. Wurtz, K. F. Nielsen, P. Kruse, K. Wilkins, U. Thrane, S. Gravesen, P. A. Nielsen, and T. Schneider. 2003. Determination of fungal spore release from wet building materials. Indoor Air 13:148-155. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki, W., H. Tamaoka, M. Shinohara, H. Kaise, T. Izawa, Y. Nakano, T. Kinoshita, K. Hong, and K. Inoue. 1980. A complement inhibitor produced by Stachybotrys complementi, nov. sp. K-76, a new species of fungi imperfecti. Microbiol. Immunol. 24:1090-1108. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, K. F. 2003. Mycotoxin production by indoor molds. Fungal Genet. Biol. 39:103-117 [DOI] [PubMed] [Google Scholar]
- 32.Nielsen, K. F., K. Huttunen, A. Hyvärinen, B. Andersen, B. Jarvis, and M. Hirvonen. 2002. Metabolite profiles of Stachybotrys isolates from water-damaged buildings and their induction of inflammatory mediators and cytotoxicity in macrophages. Mycopathologica 154:201-205. [DOI] [PubMed] [Google Scholar]
- 33.Pasanen, A.-L., M. Nikulin, M. Tuomainen, S. Berg, P. Parikka, and E.-L. Hintikka. 1993. Laboratory experiments on membrane filter sampling of airborne mycotoxins produced by Stachybotrys atra corda. Atmos. Environ. 27A:9-13. [Google Scholar]
- 34.Rao, C. Y. 2000. Toxigenic fungi in the indoor environment, p. 46.1-46.19. In J. D. Spengler, J. M. Samet, and J. F. McCarthy (ed.), Indoor air quality handbook. McGraw-Hill, Washington, D.C.
- 35.Redlich, C. A., J. Sparer, and M. R. Cullen. 1997. Sick-building syndrome. Lancet 349:1013-1016. [DOI] [PubMed] [Google Scholar]
- 36.Revankar, S. G. 2003. Clinical implications of mycotoxins and Stachybotrys. Am. J. Med. Sci. 325:262-274. [DOI] [PubMed] [Google Scholar]
- 37.Scheel, C. M., W. C. Rosing, and A. L. Farone. 2001. Possible sources of sick building syndrome in a Tennessee middle school. Arch. Environ. Health 56:413-417. [DOI] [PubMed] [Google Scholar]
- 38.Schick, M. R., G. R. Dreesman, and R. C. Kennedy. 1987. Induction of an anti-hepatitis B surface antigen response in mice by noninternal image (Ab2α) anti-idiotypic antibodies. J. Immunol. 138:3419-3425. [PubMed] [Google Scholar]
- 39.Smoragiewicz, W., B. Cossette, A. Boutard, and K. Krzystyniak. 1993. Trichothecene mycotoxins in the dust of ventilation systems in office buildings. Int. Arch. Occup. Environ. Health 65:113-117. [DOI] [PubMed] [Google Scholar]
- 40.Sorenson, W. G., D. G. Frazer, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Straus, D. C. 2001. Consequences of mold exposure in buildings. Tex. J. Rural Health 19:8-13. [Google Scholar]
- 42.Vesper, S. J., M. L. Magnuson, D. G. Dearborn, I. Yike, and R. A. Haugland. 2001. Initial characterization of the hemolysin stachylysin from Stachybotrys chartarum. Infect. Immun. 69:912-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wannemacher, R. W., and S. L. Wiener. 1997. Trichothecene mycotoxins, p. 655-676. In R. Zajtchuk and R. F. Bellamy (ed.), Textbook of military medicine, part I. Warfare, weaponry, and the casualty, medical aspects of chemical and biological warfare. Office of the Surgeon General, Washington, D.C.
- 44.Yumbe-Guevara, B. E., T. Imoto, and T. Yoshizawa. 2003. Effects of heating procedures on deoxynivalenol, nivalenol and zearalenone levels in naturally contaminated barley and wheat. Food Addit. Contam. 20:1132-1140. [DOI] [PubMed] [Google Scholar]