Abstract
Myeloid cells, key players in atherosclerosis, take up and present antigens, leading to systemic and local T cell activation. The recruitment and activation of immune cells to the aorta in atherosclerosis is regulated by adhesion molecules, chemokines and cytokines. IL-27R is an immunoregulatory signaling nod in autoimmune and infectious pathologies. IL-27R was shown to suppress T cells activation in atherosclerosis, however it’s possible role in myeloid cell accumulation and activation is not understood. Here we demonstrate that Apoe −/− Il27ra −/− mice fed with “Western Diet” for 7 or 18 weeks developed significantly more atherosclerosis compared to Apoe −/− Il27ra +/− controls. Accelerated disease was driven by enhanced expression of adhesion molecules and chemokines causing the accumulation of immune cells. Myeloid cells produced more inflammatory cytokines and upregulated MHCII. Multiphoton microscopy revealed more efficient interactions between aortic myeloid cells and CD4+ T cells. Overall, we show that IL-27R signaling controls endothelial cells activation and myeloid cell recruitment at early and advanced stages of atherosclerosis. In the absence of IL-27R myeloid cells become hyperactivated, produce pro-inflammatory cytokines and act as more potent antigen presenting cells. Enhanced interactions between Il27ra −/− APC and CD4+ T cells in the aortic wall contribute to T cells re-activation and pro-atherogenic cytokine production.
Introduction
Atherosclerosis is a lipid-driven chronic inflammatory disease characterized by progressive atherosclerotic plaque growth accompanied by the accumulation, local proliferation and activation of various immune cells in the vessel wall1, 2. Cells accumulated in atherosclerotic plaques and surrounding tissues produce various “mediators of inflammation” such as cytokines and chemokines, fueling local inflammation and promoting atherosclerosis3–6. Healthy aortas contain small number of macrophages, T cells and other immune cells2, 4. At early stages of atherosclerosis, endothelial cell activation facilitates the initial phase of monocytes, neutrophils and T cells recruitment, and subsequent increase in production of chemokines and cytokines in the plaque results in further accumulation of inflammatory cells.
A key step in the immune response is the activation of T cells by antigens presented by antigen-presenting cells (APC). In a variety of inflammatory contexts, including atherosclerosis, such interactions take place in the lymph nodes7, 8. Activated T cells migrate to the site of inflammation, where they carry out their effector functions. However, local interaction of antigen-experienced CD4+ T cells with APC in the tissue was also reported to contribute to CD4+ T cells re-activation (recall response), which further fueled inflammatory response in various diseases, including atherosclerosis9, 10. In case of atherosclerosis, local abundance of lipoprotein-derived atherosclerotic antigens may constantly cause the activation of APC, antigen presentation and, thus, promote persistent local CD4+ T cells re-activation and as a consequence, upregulated cytokine production. However, mechanisms, which could negatively control this process, remain poorly understood.
Many cytokines have been implicated into pathogenesis of atherosclerosis11, 12, with only few of them, namely IL-10 and TGFβ, being athero-protective13–16. IL-27, a member of IL-6/IL-12 superfamily, is a critical regulator of immune responses. IL-27 cytokine is produced in response to inflammatory stimuli by myeloid cells and controls the activation and function of multiple hematopoietic cell subsets as well as some non-hematopoietic cells expressing IL-27 receptor (IL-27R)17–19. IL-27R is heterodimer of a unique IL-27ra and common gp130 chains. IL-27 consists of 2 subunits, p28 and Ebi320. The heterodimeric structure of IL-27 protein complicates the investigation of this cytokine function using inactivation of genes encoding IL-27 subunits, because each of its subunits on its own also participates in the formation of other cytokines. Ebi3 heterodimerizes with IL-12p35 to form IL-35, another anti-inflammatory cytokine21, while the p28 subunit potentially forms a homodimer22 or binds with cytokine-like factor 1(CLF) to produce p28/CLF, a complex that engages IL-6R23. Because of such complexity, IL-27R deficient mice (lacking Il27ra gene) represent the best tool to address the role of IL-27R signaling in various physiological settings.
IL-27R signaling was shown to negatively control Th17 activation and IL-17A production in models of infection and autoimmunity24, 25. It was also shown to modulate Th1 cells activation and IFNγ production26. T regulatory (Treg) cells survival and functions are also dependent on IL-27R signaling, however both suppressive and activating role of IL-27R signaling on Tregs were reported by different groups27, 28, perhaps reflecting differences in mouse models used in the studies. IL-27R expression was also reported on myeloid cells29. In vitro studies demonstrated that IL-27 promotes inflammatory gene expression in myeloid cells, however, in vivo data suggest suppressive role of IL-27R signaling as determined by the enhanced MHCII expression on dendritic cells (DC) isolated from Il27ra −/− mice30.
IL-27R signaling was shown to limit atherosclerosis in Ldlr −/− atherosclerosis-prone mice with global31 or hematopoietic IL-27R deficiency19. Hematopoietic ablation of Il27ra accelerated atherosclerosis due to enhanced activation of CD4+ T cells, in particular, Th17 cells, accompanied by increased IL-17A, TNF-α and IL-6 production, CCL2 chemokine expression and accumulation of myeloid cells19. In addition to its ability to regulate cells of adaptive immunity (i.e. lymphocytes), IL-27R signaling can also potentially control atherosclerosis via regulation of innate immune cells, particularly macrophages and APC. Indeed, IL-27R signaling was shown to suppress macrophage activation and foam cell formation as determined by the analysis of peritoneal macrophage function in Il27ra −/− Ldlr −/− or bone marrow transplanted mice31.
The present study was designed to examine whether IL-27R signaling regulates antigen presentation in atherosclerosis. Here we elucidated the role of IL-27R in the Apoe −/− model of atherosclerosis, which shares similarities with, but also has important differences from the Ldlr −/− model. We assessed the role of IL-27R in early and advanced stages of the disease. Finally, we determined the role of IL-27R in antigen presentation in the aortic wall. Our data show that IL-27R signaling is critical in limiting both early and late stages of atherosclerosis by controlling myeloid cell accumulation (via regulation of adhesion molecule and chemokine expression), and regulating myeloid cells activation and antigen presentation (by limiting MHCII expression and cytokine production) in the aortas, subsequently affecting CD4+ T cells activation and atherosclerosis progression.
Results
IL-27R signaling suppresses atherosclerosis development in Apoe−/− mice
To date a limited number of publications had tried to address the role of IL-27 in atherosclerosis, using Ldlr −/− model and bone marrow transplantation approach19, 31. Here we decided to test the role of IL-27R signaling in atherosclerosis progression in another atherosclerotic model- Apoe −/− mice and further characterize molecular and cellular mechanisms underlying IL-27R action. We crossed Il27ra −/− mice to Apoe −/− background to obtain Apoe −/− Il27ra −/− and Apoe −/− Il27ra +/− mice. Mice were fertile and healthy with no evidence for runting. It is known that even small differences in genetic background32 or microbiota33, 34 influences atherosclerosis and other inflammation-driven diseases. Therefore, we compared atherosclerosis progression in Apoe −/− Il27ra −/− and Apoe −/− Il27ra +/− cage mate and littermate controls, derived from the same parents and housed in the same cages through the entire duration of the experiment, to minimize these potentially confounding factors. Starting at 8 weeks after birth, these mice were fed with high fat “Western Diet” (WD) for 7 (“early” atherosclerosis) or 18 weeks (“advanced” atherosclerosis) to assess inflammatory changes in the aortic wall and atherosclerosis development at early and advanced stages of the model, respectively.
Macroscopic and histological analyses revealed accelerated atherosclerosis progression in Apoe −/− Il27ra −/− mice already after 7 weeks of WD feeding compared to Apoe −/− Il27ra +/− cage mate and littermate controls (Fig. 1A,B). Quantitative analysis of atherosclerotic lesion area also revealed enhanced lesion size in roots of Apoe −/− Il27ra −/− mice fed with WD for 7 weeks compared to controls (Fig. 1C). Lipid profile and weight of mice from both cohorts remained unchanged (Supplementary Fig. S1A–C), indicating that effects of IL-27R deficiency on atherosclerosis are not directly mediated by global alterations in lipid homeostasis driven by the absence of IL-27R signaling. The analysis of blood leukocyte count revealed a significant reduction of circulating monocytes in Apoe −/− Il27ra −/− mice fed with WD for 7 weeks compared to controls, suggesting possible increased recruitment of these cells into the aorta (Supplementary Fig. S1D). No significant difference in number of hematopoietic bone marrow precursors was found between cohorts (data not shown).
Figure 1.

Accelerated atherosclerotic lesions development in Apoe −/− Il27ra −/−mice. Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice were fed with Western diet (WD) for 7 (A –C) or 18 weeks (D–F). Atherosclerotic lesions in aortic arch (A) and aortic root sections (B) of Apoe −/− Il27ra +/− and Apoe −/− Il27ra −/− mice fed with WD for 7 weeks. (C) Quantitative comparison of aortic lesion size in Apoe −/− Il27ra +/− (n = 8) and Apoe −/− Il27ra −/− (n = 8) mice feeding with WD for 7 weeks. Atherosclerotic lesions in aortic arch (D) and aortic root sections of (E) Apoe −/− Il27ra +/− and Apoe −/− Il27ra −/− mice fed with WD for 18 weeks. (F) Quantitative comparison of aortic lesion size in Apoe −/− Il27ra +/− (n = 10) and Apoe −/− Il27ra −/− (n = 9) mice feeding with WD for 18 weeks. Data are mean ± SEM from 4 independent experiments.
Moreover, Apoe −/− Il27ra −/− mice also had significantly accelerated atherosclerosis progression at advanced stages of the disease, when fed with WD for 18 weeks (Fig. 1D,E). Atherosclerotic plaques in aortic roots of Apoe −/− Il27ra −/− mice were significantly enlarged compared to controls (Fig. 1F).
Taken together, our data demonstrate strong acceleration of atherosclerosis in an Apoe −/− mouse model at early and advanced stages of the disease in the absence of IL-27R signaling and for the first time specifically address the possible role of IL-27R during early stages of plaque development.
Increased expression of chemokines and adhesion molecules in Apoe−/−Il27ra−/− mice
Early atherosclerosis is characterized by endothelial cell activation, increased expression of adhesion molecules and chemokines, mediating the recruitment of inflammatory cells to the aortic wall3. We sought to examine if IL-27R signaling regulates adhesion molecules expression at early stages of the disease. RT-qPCR analysis revealed strong induction of the adhesion molecule ICAM-1 and VCAM-1 expression in aortas of Apoe −/− Il27ra −/− mice fed with WD for 7 weeks compared to controls (Fig. 2A).
Figure 2.

Upregulated expression of chemokines and adhesion molecules in aortas of Apoe −/− Il27ra −/−mice. (A,B) Relative gene expression of adhesion molecules ICAM-1 and VCAM-1 in aortas of Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (A) or 18 weeks (B) were normalized to L-32 and fold induction was calculated based on the gene expression in aortas of control Apoe −/− Il27ra +/− (n = 5) mice. (C,D) Relative gene expression of CCL2 and CCL5 in aortas of Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (C) or 18 weeks (D) were normalized to L-32 gene expression and fold induction was calculated based on the gene expression in aortas of control Apoe −/− Il27ra +/− (n = 5) mice. (E,F) CCL2 and CCL5 were measured by multiplex cytokines array in supernatants obtained from aortic cell suspensions of Apoe −/− Il27ra +/− (n = 5) or Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (E) or 18 weeks (F) stimulated with anti-CD3/anti-CD28 for 48 hours. (G) Relative gene expression of vascular adhesion molecule VCAM-1, P-selectin, E-selectin and PECAM-1 in endothelial cells (mLEC cell line) treated with acLDL (100 μg/ml) and rIL-27 (25ng/ml) were normalized to L-32 and fold induction was calculated based on the gene expression in untreated endothelial cells. Data are mean ± SEM from at least 2 independent experiments.
Various chemokines including CCL2 and CCL5 were shown to control the recruitment of myeloid cells to the aortas during atherosclerosis progression35. We examined chemokine expression in mice fed with WD for 7 weeks and found enhanced production of CCL2 and CCL5 chemokines in aortas of Apoe −/− Il27ra −/− mice compared to Apoe −/− Il27ra +/− controls (Fig. 2C,E). CCL2 and CCL5 chemokine productions were also enhanced in the spleen and paraaortic lymph node (paLN) of Apoe −/− Il27ra −/− mice (Supplementary Fig. S2A). Similar to the early stages of atherosclerosis, advanced atherosclerotic lesions were also characterized by elevated expression of adhesion molecules (Fig. 2B) and chemokines (Fig. 2D,F) in aortas, spleen and paLN (Supplementary Fig. S2B) of Apoe −/− Il27ra −/− mice fed with WD for 18 weeks.
Because vascular endothelial cells can be activated in the inflammatory environment in vivo by multiple stimuli including modified low-density lipoproteins, we decided to examine if IL-27 has direct effect on cultured endothelial cells in the presence of acetylated LDL (AcLDL). We pretreated stable cell line of lung endothelial cells (mLEC) with acLDL (100 μg/ml) for 6 hours followed by stimulation with rIL-27 (25ng/ml) and assessed changes in gene expression 24 h later. AcLDL activated the expression of several adhesion molecules including VCAM-1, P-selectin, E-selectin and PECAM-1, while this effect was strongly diminished in the presence of recombinant IL-27 (Fig. 2G). Taken together, these data suggest that IL-27 has a direct effect on endothelial cells preventing the excessive expression of potentially pro-inflammatory adhesion molecules.
Thus, our data suggest that during atherosclerosis development and progression IL-27R signaling may regulate endothelial cells function and drives the suppression of adhesion molecule and chemokine expression, thereby preventing excessive accumulation of immune cells.
IL-27R deficiency accelerates immune cell accumulation in the aorta
One key signature underlying atherosclerosis progression is the accumulation of various immune and inflammatory cells in the vessel wall both in the plaque area and surrounding adventitia36. We performed flow cytometry analysis and assayed the composition of immune cells in aortas of Apoe −/− Il27ra −/− and Apoe −/− Il27ra +/− control mice. We found increased accumulation of CD45+ leukocytes (Fig. 3A): including CD11b+CD11c−, CD11b+CD11c+ and CD11b−CD11c+ myeloid cell subsets (Fig. 3B) in aortas of Apoe −/− Il27ra −/− mice fed with WD for 7 weeks compared to Apoe −/− Il27ra +/− controls. Moreover, we also observed increased accumulation of T cells in aortas of IL-27R deficient mice (Fig. 3C). Further accumulation of immune cells subsets was also found in mice fed with WD for 18 weeks (Supplementary Fig. S3A–C).
Figure 3.
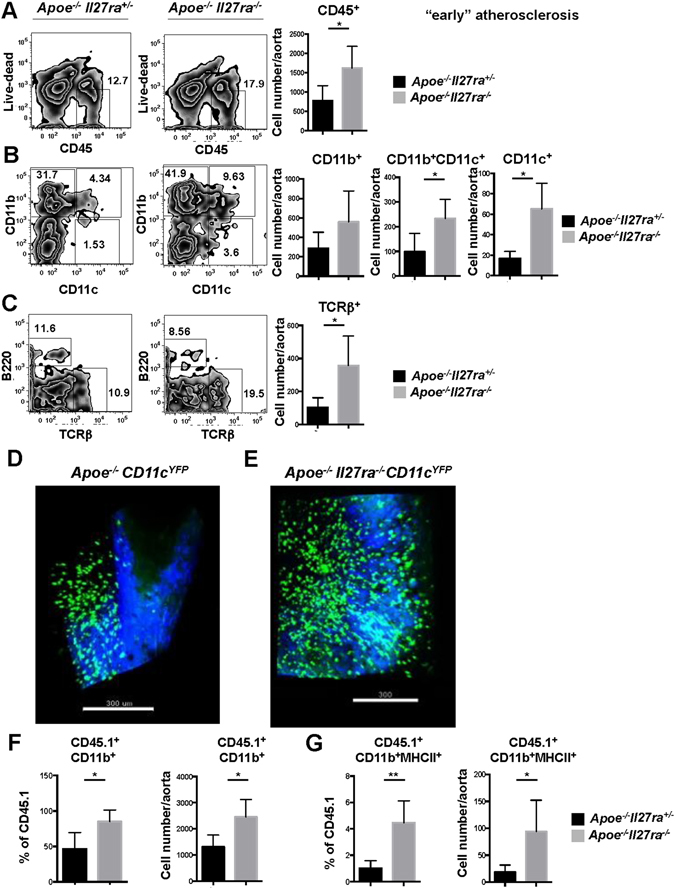
Enhanced accumulation of immune cells in aortas of Apoe −/− Il27ra −/− mice. Live CD45+ cells from aortas of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice fed with WD for 7 weeks were stained for CD45+, CD11b+, CD11c+ and TCRβ+. Percentage (left) and cell number (right) (A–C) of live CD45+, CD11b+CD11c−, CD11b+CD11c+ and CD11b- CD11c+ cells, TCRβ+ T cells in aortas of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice fed with WD for 7 weeks was quantified by flow cytometry. Data are mean ± SEM from at least 3 independent experiments. Accumulation of CD11cYFP+ APC in aortas of Apoe −/− (D) and Apoe −/− Il27ra −/− (E) mice was analyzed by 2 photon microscopy. Green – CD11cYFP+ APC cells, blue – collagen detected by second harmonics generation. CD45.1+ monocytes from B6 mice were adoptively transferred to Apoe −/− Il27ra +/− (n = 5) or Apoe −/− Il27ra −/− (n = 6) mice fed with WD for 7 weeks. Monocyte recruitment to the aortas was assessed by flow cytometry 48 hours after cell transfer. Percentage and absolute number of recruited monocytes (F) and MHCII expression by recruited CD45.1 CD11b+ monocytes (G) in the aortic wall of Apoe −/− Il27ra −/− and Apoe −/− Il27ra +/− mice. Data are mean ± SEM from 2 independent experiments.
Whole mount imaging of aortas to visualize CD11cYFP+ cells (Fig. 3D,E and Supplementary movies S1 and 2) and immunofluorescent staining demonstrated the localization and increased accumulation of myeloid cells in aortic roots of Apoe −/− Il27ra −/− mice compared to Apoe −/− Il27ra +/− controls (Supplementary Fig. S3D).
To determine if increased myeloid cells accumulation is due to enhanced recruitment of monocytes, we adoptively transferred CD45.1+ monocytes isolated from B6/CD45.1 congenic mice into Apoe −/− Il27ra −/− or Apoe −/− Il27ra +/− controls fed with WD for 7 weeks. Flow cytometry analysis revealed increased percentage and absolute number of recruited monocytes in the aortic wall of Apoe −/− Il27ra −/− mice 48 h after monocyte transfer (Fig. 3F). Recruited monocytes were characterized by elevated MHCII expression when transferred into Apoe −/− Il27ra −/− hosts (Fig. 3G). These results suggest that myeloid cells accumulation in aortas of Apoe −/− Il27ra −/− can be at least partially due to increased monocyte recruitment.
Enhanced activation of myeloid and T cells in Apoe−/−Il27ra−/− mice
To gain insights into functional role and activation perturbations of immune cells accumulated in atherosclerotic aortas, we first analyzed cytokines in supernatants obtained from the aortas of Apoe −/− Il27ra −/− mice and controls fed with WD for 7 weeks or 18 weeks. We found increased production of myeloid cell-derived cytokines, including IL-6, IL-1α and IL-1β (Fig. 4A–D) in aortas obtained from Apoe −/− Il27ra −/− mice. These data suggest that IL-27R signaling not only controls the recruitment of inflammatory cells to the aortic wall, but also regulates their activation and myeloid cell-derived pro-inflammatory cytokine production.
Figure 4.

Enhanced activation of myeloid cells and T cells in Apoe −/− Il27ra −/− mice. (A,B) Relative gene expression of IL-6, IL-1α and IL-1β in aortas of Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (A) or 18 weeks (B) were normalized to L-32 gene expression and then normalized to gene expression in aortas of control Apoe −/− Il27ra +/− (n = 5) mice. (C,D) IL-6, IL-1α and IL-1β were measured by bead array in supernatants of aortic cell suspension obtained from Apoe −/− Il27ra +/− (n = 5) or Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (C) or 18 weeks (D), stimulated with anti-CD3/anti-CD28 for 48 hours. (E,F) Relative gene expression of F4/80 and MHCII in aortas of Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (E) or 18 weeks (F) were normalized to L-32 gene expression and fold induction was calculated based on the gene expression in aortas of control Apoe −/− Il27ra +/− (n = 5) mice. (G) Expression of MHCII by CD11b+CD11c+ and CD11c+ cells in aorta of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice fed with WD for 7 weeks. (H) Expression of CD69, a marker of T cell activation, by CD4+ T cells in aortas of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice fed with WD for 18 weeks. Data are mean ± SEM from 3 independent experiments. (I) Localization of CD3+ T cells and MHCII+ cells in aortic roots of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice fed with WD for 7 weeks (early lesions) or 18 weeks (advanced lesions) as demonstrated by confocal imaging. Arrows show co-localization of CD3+ T cells and MHCII+ cells.
To assess the effect of IL-27R deficiency on antigen presentation, we measured MHCII gene expression by RT-qPCR and its surface protein expression by flow cytometry. This analysis revealed enhanced MHCII expression on CD11b+CD11c+ and CD11b−CD11c+ APC in the aorta (Fig. 4E–G) and spleen as well as in paLN (Supplementary Fig. S4A,B) of Apoe −/− Il27ra −/− mice.
Upregulation of MHCII surface expression indicates more profound maturation and activation of APC and enhanced antigen presentation, which in turn should promote T cells activation. Indeed, we found elevated expression of CD69 on T cells in aortas (Fig. 4H) and spleens (Supplementary Fig. S4C) of Apoe −/− Il27ra −/− mice, suggesting enhanced CD4+ T cells activation in these mice. Immunofluorescent staining revealed also increased co-localization of T cells with MHCII expressing cells in aortic roots of Apoe −/− Il27ra −/− mice compared to controls (Fig. 4I). Taken together, our data show that in atherosclerosis, IL-27R signaling controls myeloid cell activation in early and advanced atherosclerosis and suggest that in the absence of competent IL-27R signaling, excessive myeloid cell activation may contribute to heightened T cell responses.
IL-27R deficiency enhances local antigen presentation
Atherosclerosis progression in part is modulated by local interactions between APC and CD4+ T cells in the aortic wall9. Such interactions serve to promote local re-activation of CD4+ T cells, subsequent production of inflammatory pro-atherogenic cytokines9.
To determine if IL-27R signaling regulates local antigen presentation in the aortas, we employed 2 photon microscopy to visualize and characterize interactions between APC and CD4+ T cells. To assay and image antigen presentation in aortas of atherosclerotic mice, we bred composite Apoe −/− Il27ra −/−CD11cYFP double knockout-transgenic mice and compared them to Apoe −/−CD11cYFP controls. While various subsets of dendritic cells, monocytes and macrophages are CD11cYFP+ using flow cytometry based detection, we previously demonstrated that in CD11cYFP mice, CD11b+CD11c+ myeloid cells are primarily labeled with Yellow fluorescent protein (YFP) at fluorescence intensities sufficient for detection by 2 photon microscopy9.
First, we assayed endogenous localization of APC and CD4+ T cells in early atherosclerotic lesions. We administered anti-CD4 PE antibody into live Apoe −/− Il27ra +/− CD11cYFP or Apoe −/− Il27ra −/− CD11cYFP mice to label endogenous CD4+ T cells. Aortas were isolated 30 min after antibody administration and the localization of APC (labeled by CD11cYFP) or CD4+ T cells (labeled by PE) was assessed by 2 photon microscopy. In agreement with our flow cytometry data, we found higher numbers of both YFP+ cells and CD4-PE+ cells in aortas of Apoe −/− Il27ra −/− mice (Fig. 5A,B). We also found higher number APC and CD4+ T cells, which co-localized with each other, implying their possible local interactions in the atherosclerotic tissue (Fig. 5C).
Figure 5.

IL-27R deficiency promotes CD4+ T cells-APC interactions in the aortas. CD4+ T cells were labeled by administration of anti-CD4-PE antibody into live Apoe −/− and Apoe −/− Il27ra −/− mice after 5 weeks of WD feeding and localization of CD11cYFP+ APC and CD4-PE+ T cells were imaged by 2 photon and confocal microscopy in aortas of Apoe −/− Il27ra +/− CD11c YFP (A) and Apoe −/− Il27ra −/− CD11c YFP (B) mice. (C) Quantification of co-localizing cells in Apoe −/− Il27ra +/− CD11c YFP and Apoe −/− Il27ra −/− CD11c YFP aortas. (D,E) Two photon microscopy imaging revealed increased number of interacting cells in explanted aortas of Apoe −/− Il27ra −/− CD11c YFP mice (E) compared to control Apoe −/− CD11c YFP (D) fed with WD for 15 weeks. Isolated aortas were co-cultured with sorted from spleens CD4+ T cells obtained from atherosclerotic Apoe −/− Il27ra −/− mice. (F) Percent of CD4+ T cells interacting with CD11cYFP+ APC. (G) Reduced velocity of CD4+ T cells in Apoe −/− Il27ra −/− CD11c YFP aortas compared to Apoe −/− CD11c YFP aortas. Data are mean ± SEM from 4 independent experiments.
To assay the role of IL-27R signaling in regulation of antigen presentation, we utilized a method of explanted aorta live imaging, which we have previously developed9. We sorted CD4+ T cells from the spleens and paLNs of Apoe −/− Il27ra −/− mice fed with WD for 16 weeks and labeled them with SNARF dye. These labeled CD4+ T cells derived from atherosclerotic mice were co-cultured with explanted aortas from Apoe −/− Il27ra −/−CD11cYFP and Apoe −/−CD11cYFP mice. The behavior and APC-CD4+ T cells interactions were imaged by 2 photon microscopy 12 h later. In agreement with our observations of increased MHCII expression (Fig. 4E–G) and increased numbers of co-localizing APC and CD4+ T cells (Figs 4I and 5A,B), we found a higher percentage of APC-CD4+ T cells interactions in Apoe −/− Il27ra −/− CD11cYFP aortas compared to Apoe −/−CD11cYFP controls (Fig. 5D–F and Supplementary movies S3 and 4). Our earlier work revealed that only activated memory T cells are capable of migrating to aortas and interacting there with APC. We also previously proved that MHCII blockade completely abrogate CD4+ T cells-APC interactions, underlying the importance of MHCII expression in local intra-aortic APC- T cells interactions9.
One important characteristic of the productive antigen presentation is the reduction of CD4+ T cells velocity and their prolonged co-localization with APC8, 9. Indeed, we found a reduction of CD4+ T cells speed (Fig. 5F) as well as higher percentage of APC-CD4+ T cells interactions in Apoe −/− Il27ra −/− aortas (Fig. 5G).
Overall our data demonstrate a suppressive role of IL-27R signaling on myeloid cells activation, antigen presentation and interaction with CD4+ T cells, thus positioning IL-27R signaling as an important regulator of immune response in atherosclerosis.
Elevated expression of pro-inflammatory cytokines in IL-27R deficient mice
Local T cell re-activation promotes T cell-derived cytokine production9. We compared cytokines produced by T cells in aortas of Apoe −/− Il27ra −/− and Apoe −/− Il27ra +/− mice with early and advanced atherosclerotic lesions. We found upregulated production of several pro-inflammatory cytokines including IFNγ, TNF-α, IL-17A in aortas of Apoe −/− Il27ra −/− mice compared to Apoe −/− Il27ra +/− control mice with early lesions (Fig. 6A,B). The production of these cytokines was further elevated in aortas with advanced lesions (Fig. 6C,D). Similar changes of pro-inflammatory cytokine production were also found in the spleen and paLN both at early and advanced stages of the disease (Supplementary Fig. S5).
Figure 6.
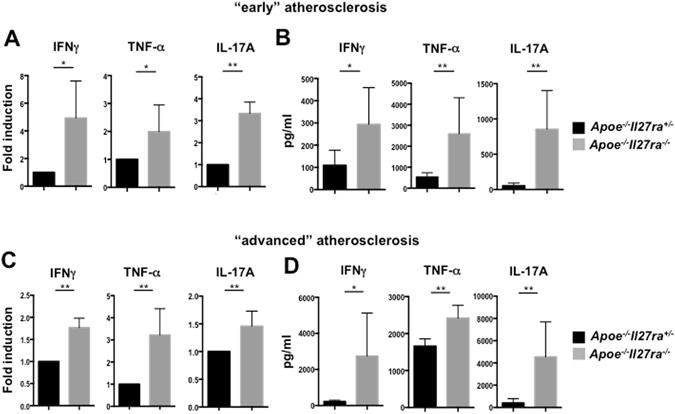
Increased expression of pro-inflammatory cytokines in aortas of Apoe −/− Il27ra −/− mice. (A,C) Relative gene expression of IFNγ, TNF-α and IL-17A in aortas of Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 weeks (A) or 18 weeks (C) were normalized to L-32 gene expression and fold induction was calculated based on the gene expression in aortas of control Apoe −/− Il27ra +/− (n = 5). (B,D) Production of pro-inflammatory cytokines IFNγ, TNF-α and IL-17A in aortas of Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice at different stages of atherosclerosis was measured by multiplex cytokines array in supernatants of aortic cell suspension obtained from Apoe −/− Il27ra +/− (n = 5) or Apoe −/− Il27ra −/− (n = 5) mice fed with WD for 7 (B) or 18 weeks (D), stimulated with anti-CD3/anti-CD28 for 48 hours. Data are mean ± SEM from 3 independent experiments.
These data demonstrate enhanced T cell derived cytokine production, elevated at least partially due to enhanced interactions with APC. IL-27R signaling, therefore, was found to have an essential immunoregulatory role, reducing expression of myeloid- (Fig. 4A–D) and T cell-derived cytokines (Fig. 6) both in early and in advanced stages of atherosclerosis. Some of these pro-inflammatory cytokines are also known regulators of chemokines, which in turn drive immune cell recruitment, providing positive feedback loop in atherosclerosis progression.
Taken together, our data extend the previously suggested anti-inflammatory role of IL-27R signaling in atherosclerosis to the Apoe −/− model, demonstrate its important contribution both at early and advanced stages of the disease and show a previously unrecognized mechanism of IL-27R signaling in regulating antigen presentation in the aortic wall.
Discussion
The role of IL-27 in various models of infections was extensively investigated in the last decade17, 18, 24, 25. However, the role of IL-27/IL-27R signaling axis in chronic inflammatory diseases is still poorly understood. The mechanisms of immunoregulatory IL-27R signaling in atherosclerosis are largely unknown with only two publications attempted to address it in animal models.
It was suggested by previous work in Ldlr −/− model that IL-27R signaling serves as an important anti-inflammatory stimulus in atherosclerosis, as demonstrated in mice with global or hematopoietic IL-27R deficiency19, 31. IL-27R signaling was implicated in the regulation of foam cell formation since an enhanced oxLDL uptake was observed in macrophages obtained from Ldlr −/− atherosclerosis-prone IL-27R deficient mice19, 31. Moreover, IL-27R deficiency in hematopoietic cells led to accelerated disease progression in Ldlr −/− mice, in particular via upregulated IL-17A production by CD4+ T cells19, thereby illuminating an important role of IL-27R signaling in controlling of adaptive immunity in atherosclerosis.
Here we evaluated the role of IL-27R signaling in the vessel wall inflammation and assayed atherogenesis in another atherosclerosis model (Apoe −/− mice). We found that IL-27R signaling is required to suppress inflammation and atherosclerosis in Apoe −/− mice both at early (7 weeks of WD) and advanced (18 weeks of WD) stages of the disease. We showed that IL-27R signaling controls endothelial cells activation, chemokine production as well as suppresses myeloid cells and T cells accumulation and activation. Moreover, we found increased level of maturation and enhanced MHCII expression on APC from Apoe −/− Il27ra −/− mice, which led in enhanced antigen presentation and interaction with CD4+ T cells in the vessel wall as determined by 2 photon microscopy.
IL-27R is expressed by intestinal epithelial cells37 and aortic endothelial cells19, suggesting its potential role in regulation of these cell types. Activation of endothelial cells is an important step facilitating immune cells recruitment to the aortic wall in atherosclerosis. IFNγ, TNF-α and IL-4 cytokines were shown to regulate endothelial cells activation and adhesion molecules expression, therefore favoring the recruitment of specific subsets of effector T cells38, 39. Here we found that IL-27R signaling during the early phase of atherosclerosis development is implicated into the regulation of the adhesion molecules VCAM-1, P-selectin, E-selectin and PECAM-1 expression by vascular endothelial cells. We showed that IL-27R deficient Apoe −/− mice expressed significantly higher level of VCAM-1 and ICAM-1 in the aortas already at 7 weeks of WD feeding. Elevated level of adhesion molecules expression persists also at advanced stages of the disease suggesting continuous effect of IL-27R signaling on the suppression of endothelial cells activation in atherosclerosis. Even though previous study did not find any effect of recombinant IL-27 on VCAM-1 expression by HUVEC31, our data suggest that IL-27R signaling in vivo is important suppressor of endothelial cells activation. Moreover, our in vitro studies support our in vivo observations, since rIL-27 treatment significantly reduced the expression of several adhesion molecule genes including VCAM-1, P-selectin, E-selectin and PECAM-1 elevated in response to acLDL treatment of cultured endothelial cells. Possible discrepancies between two studies could be explained by variations in experimental system used: human venular endothelial cells31 or mouse lung endothelial cells, examined in our study. The analysis of chemokine production revealed upregulated CCL2 and CCL5 chemokines production in Apoe −/− Il27ra −/− mice compared to controls. Overall these data suggest an important role for IL-27R signaling in controlling cell recruitment in atherosclerosis.
Enhanced adhesion molecules expression and chemokine production in the absence of IL-27R signaling therefore conspire in increased accumulation of immune cells in the aortic wall. Similar to previously described in Ldlr −/− mice19, 31, here we also found that Il27ra −/− deficiency in Apoe −/− mice led to higher numbers of CD45+ cells recruited to the aorta, among them TCRβ+ T cells as well as several subsets of myeloid cells; namely CD11b+CD11c−, CD11b+CD11c+ and CD11b−CD11c+ cells.
Notably, here we showed that IL-27R deficient Apoe −/− mice were not only characterized by higher numbers, but also increased activation of myeloid cells subsets in the aortas. The expression of IL-27R on myeloid cells was previously reported, but its possible direct signaling role is still unclear. Previous studies demonstrated that stimulation of macrophages with recombinant IL-27 in vitro promotes pro-inflammatory gene expression29. However, in vivo the opposite was noted, i.e. IL-27R deficient DC were shown to be more activated and produced more pro-inflammatory cytokines upon LPS stimulation30. Similar findings were reported for peritoneal macrophages isolated from IL-27R deficient Ldlr −/− mice, where increased CCL2 and IL-6 production was detected31. Our data in Apoe −/− model of atherosclerosis argues for an important anti-inflammatory role of IL-27R signaling in myeloid cell compartment as demonstrated by the analysis of myeloid subsets composition and activation status in atherosclerotic Apoe −/− Il27ra −/− mice. The analysis of the cytokine spectrum produced in the aorta revealed increased levels of IL-1α, IL-1β and IL-6. These cytokines are produced primarily by myeloid cells in a variety of physiological settings40, including atherosclerosis41, and were previously shown to be pro-atherogenic42–44.
Most importantly, CD11b+CD11c+ and CD11b−CD11c+ myeloid cells from Apoe −/− Il27ra −/− mice exhibited significantly higher surface expression of MHCII both in aortas and secondary lymphoid organs, indicating their enhanced maturation. Importantly, an up-regulation of MHCII expression on myeloid cell subsets was detected already at 7 weeks of WD feeding, i.e. during early stage of the disease development.
Antigen presentation is a key step in activation of CD4+ T cells in inflammatory settings, including atherosclerosis9, 45. Typically antigen-presentation occurs in specialized lymphoid organs, however, local interactions and their role in the maintenance of T cells activation and local inflammation have been described9, 10, 46. We have previously demonstrated that antigen presenting cells can interact in the aortic wall with CD4+ T cells, resulting in their local re-activation and enhanced cytokine production9. Analysis of APC function in Apoe −/− Il27ra −/− mice revealed increased frequencies and longevities of interactions between CD4+ T cells and APC in aortas of Apoe −/− Il27ra −/− mice compared to controls at early and advanced stages of the disease, implicating heightened level of local antigen presentation. These data allow us to propose previously unexplored role of IL-27R signaling in regulation of APC function in atherosclerosis.
Previous work demonstrated that cytokines produced by re-activated T cells promote macrophage activation, scavenger receptor expression and foam cell formation, therefore promoting atherosclerosis9. Here, in Apoe −/− model, we detected elevated level of T cell-derived, pro-inflammatory cytokines including IL-17A, IFNγ and TNF-α in the aortas of Apoe −/− Il27ra −/− mice compared to Apoe −/− Il27r +/− controls. The elevated level of T cell derived cytokines indicates heightened activation of T cells, which is at least partially driven by their increasingly productive interactions with APC in the absence of regulatory IL-27R signaling.
Because of the broad IL-27R expression, most likely multiple mechanisms and cell subsets are contributing to the disease pathogenesis in an IL-27R dependent manner. Earlier well-documented studies demonstrated the direct role of IL-27R signaling on multiple T helper cell subsets17. Our previous work elucidated suppressive role of IL-27R signaling in the regulation of T cell function in atherosclerosis in Ldlr −/− mice with hematopoietic deficiency of IL-27R. Hirase at al. further demonstrated immunosuppressive role of IL-27 (Ebi3) and IL-27R signaling within the hematopoietic system and implicated IL-27R signaling into the regulation of foam cell formation31. Here we propose another mechanism, which can act in parallel with the already described mechanisms, by which IL-27R signaling directly controls inflammation within the vessel wall. Our data suggest that due to the immunoregulatory role of IL-27R in antigen presenting cell in the aorta, IL-27R therefore may indirectly suppress CD4+ T cells activation and production of pro-atherogenic T cell derived cytokines via controlling efficiency of antigen presentation.
Overall our data suggest that IL-27R signaling in atherosclerosis is an important regulator of both innate and adaptive immunity at early and late stages of the disease. Functional IL-27R signaling is involved in the regulation of endothelial cells activation and adhesion molecules expression. IL-27R signaling also controls myeloid cells activation, and myeloid cell-derived cytokine production and antigen presentation, which are essential for the induction and maintenance of T cell activation. This enhances our understanding of direct effects of IL-27R signaling on lymphocytes. Further studies using cell specific deletion of IL-27R will be required to address cell specific role of IL-27R cytokine signaling in inflammation in atherosclerosis.
Materials and Methods
Mice, Diet and cell lines
Il27ra −/− mice (JAX #018078) were crossed to Apoe −/− mice (JAX #002052) or to previously generated in the lab Apoe −/−CD11cYFP mice. Apoe −/− Il27ra +/−CD11cYFP mice were bred to Apoe −/− Il27ra +/−CD11cYFP to generate Apoe −/− Il27ra −/−CD11cYFP, Apoe −/− Il27r +/−CD11cYFP and Apoe −/−CD11cYFP mice for imaging experiments. Apoe −/− Il27ra +/− were bred to Apoe −/− Il27ra −/− mice for all other studies.
CD45.1 (JAX #002014) mice were from Jackson Labs and bred in house. Mice were bred and housed under specific pathogen-free conditions in an AAALAC-approved barrier facility at Fox Chase Cancer Center (FCCC). The genotyping was performed by standard polymerase chain reaction protocols. Both male and female mice were used in the study. Animal numbers for each experiment are given in the Figure legends. Apoe −/− Il27ra +/− and Apoe −/− Il27ra −/− mice were fed with “Western Diet” (Teklad TD 88137) for 7 or 18 weeks beginning at 8 weeks after birth. Animal experiments were approved by the Animal Care Committee (IACUC) at FCCC.
Mice for live imaging experiments were bred and housed under specific pathogen-free conditions and fed with WD for various time points beginning at 8 weeks after birth in an AAALAC-approved barrier facility at La Jolla Institute for Allergy and Immunology (LJI). All imaging experiment procedures were approved by the Animal Care Committee at LJI.
Mouse lung endothelial cell line (mLEC) was kindly provided by Drs. Jonathan Chernoff and Maria Radu (FCCC). Cell were cultured in DMEM containing 20% FBS, supplemented with Endothelial Cell Growth Factor (75 μg/ml) (Sigma). To assess changes on gene expression cells were pretreated with acLDL (100 μg/ml) (Invitrogen) for 6 hours at 37 °C in serum free media, followed by for 24 h incubation with rIL-27 (25ng/ml) (eBioscience).
All methods were performed in accordance with the relevant institutional guidelines.
Quantification of Aortic Atherosclerotic Lesions
To quantify aortic atherosclerosis lesions, aortic roots were isolated from the hearts and frozen on dry ice in O.C.T. (Optimal Cutting Temperature) compound Tissue Tek (Sakura) and stored at −80 °C. 5 μm sections were taken starting at the aortic valve plane and covering 400 μm in intervals of 50 μm. Sections were stained with Oil Red O/hematoxylin/light green staining. Images were acquired by Nikon Eclipse 80i microscope with a 4× 0.2 NA objective. Atherosclerotic plaque sizes were quantified using Fiji software (NIH) and represented as average of all sections in each mouse.
Immunofluorescence
Immunofluorescent staining of 5 μm aortic root section was performed as previously described19. Briefly, aortic sections were stained overnight at 4 °C with primary antibodies specific to mouse antigens: hamster anti-CD11c (HL3, BD Bioscience), rat anti-CD11b-FITC (M1/70, BD Bioscience), rat anti-CD3 (17A2, Biolegend) followed by staining with secondary antibodies for 1 hour at room temperature (RT): goat anti-FITC Alexa Fluor 488 (Molecular Probes), goat anti-rat IgG Alexa Fluor 568 (Molecular Probes), and goat anti-hamster IgG DyLight 649 (Jackson Immunoresearch). MHCII was stained by anti-MHCII-PE (M5/114.15.2; eBioscience) Sections were counterstained with DAPI and embedded in Prolong Gold. Images were acquired on a Leica SP8 DM6000 inverted confocal microscope using HCX PLAPO 20x and 40x oil-immersion objectives at 405 nm, 488 nm, 563 nm and 633 nm excitation wavelength. Imaris Software was employed to adjust brightness and one-step smoothing on all images in parallel.
RNA Isolation and Gene Expression
The tissues (aorta, spleen or paraaortic lymph node (paLN)) were homogenized with RNAse/DNase free 2.8mm Ceramic Beads using Omni Bead Ruptor 24 in PureZOL RNA Isolation Reagent (Bio-Rad Laboratories) followed by RNA isolation using Aurum Total RNA Fatty and Fibrous Tissue kit (Bio-Rad Laboratories) according to manufacturer’s protocol. First strand cDNA was synthetized using the iScript Reverse Transcription Supermix (Bio-Rad Laboratories). Gene expression was analyzed by SYBR green real-time polymerase chain reaction (Bio-Rad Laboratories) using primers for L-32, VCAM-1, ICAM-1, F4-80, MHCII, IFNγ, TNF-α, IL-17A, IL-6, IL-1α, IL-1β, CCL2, CCL5, P-selectin, E-selectin, PECAM-1. For each gene the reaction was performed in duplicate and mean value was used for calculations. Gene expression was normalized to L-32 expression. Data were analyzed using Prism statistical software (GraphPad).
Flow Cytometry Analysis
Cells from the aorta, spleen and paLN were isolated as described before19. Briefly, Apoe −/− Il27ra +/− or Apoe −/− Il27ra −/− mice were euthanized by CO2 inhalation. The aortas were perfused with 30 ml of PBS containing 2% heparin and isolated under the dissection microscope. Collected aortas, spleens or paLNs were cut into small pieces followed by digesting in 2 ml of enzymatic cocktail, containing 450 U/ml collagenase type I, 250 U/ml collagenase type XI, 120 U/ml hyaluronidase type I, 120 U/ml DNAse I (all enzymes from Sigma) in 1x HBSS and incubated in a shaker at 37 °C for 55 min. Obtained cell suspension was stained with following antibodies: CD45-PerCP (30-F11; BioLegend), CD11b-eFluor 450 (M1/70; eBioscience), CD11c-APC (N418; eBioscience), MHCII-Alexa Fluor 700 (M5/114.15.2; eBioscience), TCRβ-eFluor 780 (H57-597; eBioscience), CD69-PE-Cy7 (H1.2F3; eBioscience), B220-FITC (RA3-6B2; eBioscience), CD4-APC (GK1.5; eBioscience), CD8-PE (53-6.7; eBioscience) and LIVE/DEAD Yellow fixable dye (Invitrogen) and analyzed by flow cytometry (LSRII, BD Biosciences). Obtained data were analyzed using FlowJo software.
Adoptive transfer
CD45.1+ monocytes were isolated from spleen and peripheral blood of B6/CD45.1 mice using EasySep negative selection mouse monocyte Enrichment Kit (StemCell Tech) according to manufacturer’s protocol. 1.5 × 106 cells were injected i.v. into Apoe −/− Il27ra −/− or Apoe −/− Il27 +/− mice fed with WD for 7 weeks. Monocyte accumulation to the aortas was assessed by flow cytometry 48 hours after cell transfer.
Cell sorting, labeling and antigen presentation in explanted aorta
Isolation of aortas and T cells were performed as previously described9. Briefly, CD4+ T cells were purified from spleen and paLN using Robosep negative selection kit (StemCell Technology) and labeled for 10 min at 37 °C with 2.5 µM SNARF (Molecular Probes). Aortas were surgically removed from Apoe −/− or Apoe −/− Il27ra −/− mice fed with WD for 16 weeks and incubated with 5 × 105 CD4+ T cells obtained from spleens and paLNs of Apoe −/− Il27ra −/− mice fed WD for 16 weeks. T cells were incubated with the explanted aorta for 12 hours in 750 μl of complete RPMI 1640 media without any additional stimulation. In preparation of live image acquisition, the ends of each aorta were glued to a coverslip with Histoacryl glue (TissueSeal LLC), put in a Petri dish, maintained at 37 °C and superfused with RPMI medium 1640 without phenol red (Invitrogen) bubbled with a gas mixture containing 95% O2 and 5% CO2.
Two-photon microscopy
Two-photon imaging was performed using a DM 6000 upright microscope with 4 non-descanned detectors (Leica Microsystems) and a Chameleon Ultra Ti: Sapphire laser (Coherent) tuned at 900 to 1000 nm for acquisition using a water-dipping objective Olympus XLUMPLFL 20XW, NA0.95. Emitted fluorescence was split with 2 dichroic mirrors (560 nm and 593 nm) and passed through filters (Semrock) 535/22 nm, 585/40 nm and 624/40 nm. To label CD4 T cells in vivo 10 μg of CD4-PE (RM4-5) antibody was administered to anesthetized mouse. CD4-PE was excited using confocal 543 nm laser. Typically, 10 to 20 z-planes spaced 10 to 15 μm apart were acquired at 512 × 512 pixels every 1 minute. The sequential acquisition with combination of 2 photon and confocal lasers was used to visualize YFP+ and PE+ cells.
Cell tracking
The Imaris software was used to process 3D video data by detecting cells in each fluorescence channel and creating tracks by linking the detected cells over time. Tracks were manually edited to improve accuracy. The Imaris software was used to calculate interaction duration and cell velocities.
Cytokine and chemokine protein analysis
Cell suspensions obtained from digested aortas were incubated with plate bound anti-CD3 and anti-CD28 antibodies to stimulate T cells activation for 48 h in complete RPMI 1640 media. Supernatants were collected and cytokine secretion was measured by mouse Procarta 17-Plex cytokine array (eBioscience) on MagPix instrument (Luminex) according to manufacturer’s instructions. Because 17-plex also included myeloid cell cytokines and chemokines, their production was also detected from the same supernatant.
Statistical analysis
Data were analyzed using Prism software (GraphPad). Student’s 2-tailed T-test and Wilcoxon signed-rank test was used to compare fold induction of gene expression by real-time PCR. Data are expressed as mean ± SEM. *p < 0.05, ** p < 0.01, *** p < 0.001. P values < 0.05 was considered significant.
Electronic supplementary material
Acknowledgements
We acknowledge the help of Laboratory Animal Facility, Flow cytometry, Cell culture and Microscopy facilities at FCCC and LJI. We thank Dr. Sergei Grivennikov (FCCC) for critical reading of the manuscript. This work was supported NCI P30 Cancer Center Grant to FCCC; and by AHA SDG 13SDG14490059, WW Smith Charitable Trust, NIH R21 CA202396, and NIH R56 HL133669 grants to E.K.K.
Author Contributions
I.O.P. and E.K.K. designed the study and planned the experiments; I.O.P. and A.R.F. performed the experiments. I.O.P. and E.K.K. wrote the manuscript. K.L. provided assistance with data interpretation and manuscript writing. Z.M. provided expert help with live microscopy experiments and critical reading of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01828-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robbins CS, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ensan S, et al. Self-renewing resident arterial macrophages arise from embryonic CX3CR1(+) precursors and circulating monocytes immediately after birth. Nat Immunol. 2016;17:159–168. doi: 10.1038/ni.3343. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 4.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mallat Z, Taleb S, Ait-Oufella H, Tedgui A. The role of adaptive T cell immunity in atherosclerosis. J Lipid Res. 2009;50(Suppl):S364–369. doi: 10.1194/jlr.R800092-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koltsova EK, Hedrick CC, Ley K. Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms. Curr Opin Lipidol. 2013;24:371–380. doi: 10.1097/MOL.0b013e328363d298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 8.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 9.Koltsova EK, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 11.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 12.Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015;26:673–685. doi: 10.1016/j.cytogfr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mallat Z, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.RES.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 14.Pinderski Oslund LJ, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.ATV.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 15.Mallat Z, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 16.Robertson AK, et al. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–969. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 19.Koltsova EK, et al. Interleukin-27 receptor limits atherosclerosis in Ldlr-/- mice. Circ Res. 2012;111:1274–1285. doi: 10.1161/CIRCRESAHA.112.277525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/S1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 21.Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stumhofer JS, et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat Immunol. 2010;11:1119–1126. doi: 10.1038/ni.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabe S, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–7702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 24.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 25.Tang SC, Fan XH, Pan QM, Sun QS, Liu Y. Decreased expression of IL-27 and its correlation with Th1 and Th17 cells in progressive multiple sclerosis. J Neurol Sci. 2015;348:174–180. doi: 10.1016/j.jns.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/S1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 27.Cox JH, et al. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL-27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol. 2013;190:1510–1518. doi: 10.4049/jimmunol.1201017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflanz S, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 31.Hirase T, et al. Interleukin 27 inhibits atherosclerosis via immunoregulation of macrophages in mice. Am J Physiol Heart Circ Physiol. 2013;305:H420–429. doi: 10.1152/ajpheart.00198.2013. [DOI] [PubMed] [Google Scholar]
- 32.Lusis AJ. Genetics of atherosclerosis. Trends Genet. 2012;28:267–275. doi: 10.1016/j.tig.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregory JC, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilhan F, Kalkanli ST. Atherosclerosis and the role of immune cells. World J Clin Cases. 2015;3:345–352. doi: 10.12998/wjcc.v3.i4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diegelmann J, Olszak T, Goke B, Blumberg RS, Brand S. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J Biol Chem. 2012;287:286–298. doi: 10.1074/jbc.M111.294355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 39.Briscoe DM, Cotran RS, Pober JS. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992;149:2954–2960. [PubMed] [Google Scholar]
- 40.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 43.Kamari Y, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1alpha. Biochem Biophys Res Commun. 2011;405:197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19:2364–2367. doi: 10.1161/01.ATV.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 45.Weber C, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bedoui S, Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: a role for antigen presentation beyond lymphoid organs? Curr Opin Immunol. 2011;23:124–130. doi: 10.1016/j.coi.2010.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


