Abstract
The effects of restriction proficiency and premating exposure to toxicants on conjugal transfer of the TOL plasmid between Pseudomonas spp. was investigated by examinations of filter matings. A Pseudomonas putida KT2442-derived strain carrying a gfp-tagged variant of the TOL plasmid was used as a donor, and both restriction-deficient (PAO1162N) and -proficient (PAO2002N) Pseudomonas aeruginosa strains were used as recipients. The in situ enumeration of conjugation events allowed us to obtain frequency estimates that were unbiased by transconjugant growth or plasmid retransfer. We observed a strong dependence of the plasmid transfer frequency on the initial donor-to-recipient ratio of surface matings, which invalidated the use of mass action-based plasmid transfer kinetic estimators. Careful control of the initial parental cell densities permitted evaluations of the true effects of restriction proficiency and toxicant exposure on TOL transfer. At standard donor-to-recipient ratios (10−3 for PAO1162N and 2 × 101 for PAO2002N) and total cell densities (105 cells/mm2 for PAO1162N and 106 cells/mm2 for PAO2002N), plasmid transfer frequencies without toxicant exposure were approximately 10−7 (events/mm2)−1 for PAO1162N and 10−11 (events/mm2)−1 for PAO2002N based on in situ observations of conjugation events. The enumeration of transconjugants via selective plating yielded transfer frequencies that were up to 1 order of magnitude lower. Premating exposure to sodium dodecyl sulfate (1 to 10 mM) significantly increased the transfer frequency for the restriction-proficient strain PAO2002N (P < 0.05) but not for the restriction-deficient strain PAO1162N. On the other hand, premating exposure to ethanol, toluene, or phenol had no positive effect on the plasmid transfer frequency. Clearly, restriction proficiency provides a strong barrier to interspecific transfer of the TOL plasmid, and this barrier was only marginally attenuated by recipient exposure to toxicants within the ranges examined.
Plasmid transfer is considered an important factor in the adaptation of microbial communities to environmental changes and in bacterial evolution (16). The transfer of plasmids has been demonstrated in situations in which it confers a selective advantage (e.g., the transfer of plasmids that carry catabolic genes in pollutant-laden environments [4, 14, 19] and of plasmids that carry metal resistance genes in metal-stressed environments [33]) but also when there is apparently no selective advantage (20, 23). The kinetics of conjugal plasmid transfer are influenced by many factors, including the type of organisms involved, the physiological state of the donor (18, 28), energy or nutrient availability (21, 27), and bacterial distribution and density (2, 20, 30), among others. If plasmid transfer is indeed part of an adaptive response to stress, then stress should increase the ability of donors to transfer or of recipients to acquire a plasmid. For identification of the direct effects of toxicant stress on plasmid transfer rates, experiments conducted in carefully controlled environments and with defined cultures seem preferable before attempts are made to examine these effects in natural settings. Ideally, such experiments would permit a quantification of these effects on intrinsic plasmid transfer rate coefficients (17). One of the boundaries of interspecific horizontal gene transfer is restriction modification systems, by which restriction enzymes cut foreign DNAs at specific sites while resident DNA sites are protected by specific cognate methylases (15). Interspecific DNA transfer can be enhanced when recipients are exposed to stresses such as heat, extreme pH shifts, ethanol, or sodium dodecyl sulfate (SDS) (10, 24), probably because of a temporary repression of restriction. Hence, we set out to evaluate the efficiency of restriction modification for preventing the interspecific plasmid transfer of an archetypal catabolic TOL plasmid among Pseudomonas spp. Furthermore, we examined whether restriction could be attenuated and transfer could be enhanced by exposing recipients to various toxicants before allowing them to mate with donors. A new green fluorescent protein (GFP)-based method that reduces bias from transconjugant growth or plasmid retransfer was developed for plasmid transfer rate estimations and was compared to a selective enumeration method. We documented small effects of recipient premating toxicant exposure but very large effects of restriction proficiency on transfer of the TOL plasmid between Pseudomonas putida and Pseudomonas aeruginosa.
MATERIALS AND METHODS
Strains and growth conditions.
A summary of all bacterial strains and plasmids employed in this study is provided in Table 1. P. putida BBC443 (KT2442 lacIq) (5) was chromosomally tagged with the dsRed gene fused to an Escherichia coli ribosomal promoter via triparental mating with E. coli CM404(pRK2013) and E. coli (pTTN151 [pUTKan::rrnBP1-RBSII-dsRed-T0T1]) (32). A strongly red fluorescent clone, termed P. putida CAP8, was used as a donor in all matings. P. putida CAP8 carries the plasmid TOLgfp, a derivative of the wild-type TOL plasmid tagged with gfpmut3b under the control of the synthetic Lac promoter Pa1/04/03, and hence gfp is only expressed upon transfer to a recipient lacking a Lac repressor (5). P. aeruginosa PAO1162N is a spontaneous naladixic acid-resistant (150 μg/ml) mutant of PAO1162 (Ampr Leu− Res− Mod+; PAO1 derivative) (9). P. aeruginosa PAO2002N is a spontaneous naladixic acid-resistant (200 μg/ml) mutant of PAO2002R, a prototrophic variant of restriction-positive, streptomycin-resistant PAO2002 (1). Strains were grown and enumerated in mineral salts (MS) medium (8) with succinate as a carbon source (17 mM) for SDS matings or in a minimal medium described by Clark and Maaløe with citrate as a carbon source (10 or 40 mM) for all other matings (6) in 100-ml volumes in 500-ml flasks at 30°C with constant agitation (200 rpm). Media were supplemented with rifampin (100 μg/ml), leucine (20 μg/ml), ampicillin (500 μg/ml), streptomycin (300 μg/ml), or naladixic acid (150 or 200 μg/ml) as needed. Solid media for enumeration were prepared by adding 1.5 or 2.0% agar. Transconjugants were enumerated on solid media with appropriate antibiotics and with m-toluic acid (800 μg/ml) as the sole carbon source and were confirmed as Gfp+ DsRed− colonies by epifluorescence microscopy. All enumerations were conducted by drop (20 μl) plating, except transconjugant enumeration for PAO2002N matings, from which very few transconjugants were expected and for which spread plate enumeration (100 to 200 μl) was therefore used.
TABLE 1.
Strains and plasmids used for this study
| Strain or plasmid | Genotype or phenotypea | Reference |
|---|---|---|
| Strains | ||
| P. putida | ||
| BBC443 | lacIq Rifr | 17 |
| CAP8 | lacIqrrnBP1-RBSII-dsRed- T0T1::mini-Tn7 Kmr | This work |
| P. aeruginosa | ||
| PAO2002 | rec Smr R+ M+ | 20 |
| PAO2002N | rec Smr Nalr R+ M+ | This work |
| PAO1162 | leu rmo-11 R− M+ Ampr | 19 |
| PAO1162N | leu rmo-11 R− M+ AmprNalr | This work |
| E. coli | ||
| CM404 | HB101; pro leu thi lacY | 18 |
| Plasmids | ||
| TOLgfp | TOL::Pa1/04/03::gfpmut3b | 17 |
| pTTN151 | pUTKan::rrnBP1-RBSII- dsRed-T0T1 | 18 |
| pRK2013 | Kmr Tra+ | 18 |
Rif, rifampin; Sm, streptomycin; Nal, naladixic acid; Amp, ampicillin; Km, kanamycin; R, restriction; M, modification; rmo, restriction and modification; rec, recombination; leu, leucine; pro, proline; thi, thiamine.
Filter matings.
Filter matings were performed by a 96-well microplate scheme (Multiscreen GV filter plates with a 6-mm internal well diameter and 0.22-μm-pore-size Durapore filters; Millipore Corporation, Bedford, Mass.) that allowed for multiple controlled replicates of several mating conditions within each experiment. Donors were harvested during early stationary phase (optical density at 600 nm [OD600] = 1.00) and recipients were harvested during late exponential phase (OD600 = 0.200 for PAO1162N and 0.300 for PAO2002N) to ensure a consistent average physiological state for the parental cell types. Filtering volumes were calculated from culture densities estimated by measuring the OD600 to obtain desired donor-to-recipient ratios and a total initial cell density of approximately 105 CFU/mm2, unless noted otherwise. An initial surface density of 105 CFU/mm2 ensured complete cell-cell contact and a monolayer of cells within two cell divisions; homogeneity and the degree of surface coverage were easily verified by an inspection of the red fluorescent donor cells. Recipients were filtered into 96-well plates by use of a Multiscreen resistant vacuum manifold (Millipore Corporation) and a vacuum pump (Barnant Company, Barrington, Ill.) under a maximum vacuum pressure of 15 in. of Hg. Recipient cells were thoroughly rinsed by filtering 1 volume of sterile 10 mM MgSO4 or 0.85% NaCl solution before filtering donor suspensions. After the addition of donor suspensions, the filters were subjected to a final rinse with 2 volumes of diluent and then were placed on rectangular plates that contained R2A agar supplemented with 0.45 mM FeSO4 (31) (Difco, Detroit, Mich.). The plates were sealed with Parafilm and incubated at 30°C for 24 or 48 h. At least five replicate filters were prepared for each treatment (three for selective plating enumerations and the others for conjugation event enumerations). After incubation, the filters were punched with ethanol-sterilized punch-out tips (Multiscreen punch tips; Millipore Corporation) with the center tip removed, resuspended in 500 μl of diluent, vortexed for 1 min, and enumerated, or intact filters were transferred cell side up to transconjugant-selective plates for postconjugation selective incubation. Plasmid transfer frequencies were measured by the equation T/(RiDi) or C/(RiDi), expressed in (events/mm2)−1 and derived as follows: number of transconjugants/mm2 × (number of recipients/mm2 × number of donors/mm2)−1 and number of conjugation events/mm2 × (number of recipients/mm2 × number of donors/mm2)−1, respectively. T and C represent the areal densities of transconjugants and conjugation events, respectively, and Ri and Di represent initial mating areal densities of recipients and donors, respectively. The limits on transfer frequencies were therefore set by our ability to detect a single transconjugant cell or conjugation event per examined filter (approximately 1/28 mm2) but varied with experimental conditions due to differences in Ri and Di. Throughout this report, we refer to the expression T/(RiDi) as the transconjugant frequency and to C/(RiDi) as the conjugation frequency and will omit the units whenever possible.
Toxicant exposure matings.
Recipient cultures in late exponential phase were divided into replicate 10-ml aliquots, toxicants were added to the desired concentrations (SDS, 1, 3, 4, 7, and 10 mM; ethanol, 50, 100, 150, 200, and 500 mM; toluene, 1, 2, 5, 8, 10, and 800 mM; phenol, 1, 2, 3, 5, and 10 mM; o-xylene, p-xylene, and m-xylene, 800 mM each), and the cultures were incubated for another hour. Cultures were enumerated after exposure to evaluate the impact on cell density and to obtain initial density estimates, and filter matings were conducted as described above.
Conjugation event enumeration.
At the end of the mating incubation, replicate filters were transferred to transconjugant-selective solid medium (containing m-toluic acid and selective antibiotics) and further incubated for 3 days. These conditions did not permit donor or recipient growth and allowed transconjugant cells, formed during the mating incubation, to develop into microcolonies. Filters were placed cell side down on a microscope slide with a drop of mounting medium (Fluoromount G; Southern Biotechnology Associates Inc., Birmingham, Ala.). Another drop of mounting medium was placed on the filter before a coverslip was laid down to seal the preparation. Slides were observed under an inverted epifluorescence microscope (Nikon TE300, equipped with fluorescein isothiocyanate/Texas Red combination filters, with excitation at 497 and 531 nm and emission at 571 and 627 nm) using a ×20 objective. The entire filter area was screened for green fluorescent microcolonies, which were counted as individual conjugation events, and the production of red fluorescent protein by the donor allowed for verification of homogeneous coverage of the filter surface.
Statistical analyses.
Differences between means were tested by a standard Student t test. All statistical tests were performed at a significance level (α) of 0.05 by the use of commercial software (XLSTAT, version 6.0; Addinsoft, Brooklyn, N.Y.).
RESULTS
Plasmid transfer on solid surfaces, with P. putida CAP8(pTOLgfp) as the donor and P. aeruginosa PAO1162N or PAO2002N as the recipient, was measured as the frequency of conjugation events, C/(DiRi), and the frequency of transconjugants, T/(DiRi). The detection limits varied depending on the initial donor and recipient densities. Conjugation events (detection limit, 0.035 conjugation events/mm2) were often detected in matings for which T was below the detection limit [0.04 CFU/mm2 for PAO2002N(pTOL) and 0.18 CFU/mm2 for PAO1162N(pTOL)]. For matings with PAO1162N, conjugation frequencies were consistently 2 to 40 times higher than the transconjugant frequencies (for toluene, ethanol, and phenol exposure experiments, as described below), except for 48-h matings in the presence of SDS. On the other hand, for matings with PAO2002N, C was consistently within an order of magnitude of T (only 48-h matings were conducted for PAO2002N, as no transconjugants were detected after 24-h matings).
Effect of toxicant exposure on apparent plasmid transfer efficiency.
In initial experiments, the recipient strain PAO2002N was exposed to toxicants at concentrations that would negatively impact cell growth (10% [vol/vol] toluene, p-xylene, m-xylene, and o-xylene, 5 mM phenol, and 200 mM ethanol). Fixed volumes of previously exposed recipient cultures and unexposed donor cultures were then harvested, rinsed, and subjected to 48-h matings. Experiments were conducted with PAO2002N only as the recipient because we postulated that the intrinsically low transfer frequencies with PAO2002N might facilitate observations of any stimulatory effects. Large effects of recipient premating exposures on plasmid transfer were indeed observed (Table 2). Transconjugant frequencies increased upon exposure to ethanol, phenol, toluene, or o-xylene, from a baseline (no toxicant exposure) value of 1.36 × 10−12 ± 0.9 × 10−12 to a maximum value of 7.2 × 10−10 ± 0.59 × 10−10 (for o-xylene). Conjugation events were only detected for matings with recipients that were exposed to ethanol and phenol; the calculated conjugation frequencies also increased up to 300-fold compared to the baseline value. Plasmid transfer was below the detection limit by both methods for recipients that were exposed to p-xylene or m-xylene. Premating exposures of the recipients to toxicants also resulted in a sharp increase in the Di/Ri ratio due to the detrimental effects of solvent exposure on the recipient cell number (the total cell number was not significantly impacted). Donor-to-recipient ratios increased from 147 up to 4.2 × 105. Except for toxicants for which transfer was not detected, higher Di/Ri ratios corresponded to higher inferred transfer frequencies (Table 2).
TABLE 2.
Plasmid transfer frequencies for matings between P. putida CAP8(pTOL::Pa1/04/03 gfpmut3b) and P. aeruginosa PAO2002N that was previously exposed to toxicantsa
| Toxicant | Concn (mM) | Ri (CFU [102]/mm2) | Di/Ri | T/Ri (CFU [10−4]/CFU) | C/Ri (CFU [10−4]/CFU) | T/(RiDi) (10−10) (events/mm2)−1 | C/(RiDi) (10−10) (events/mm2)−1 |
|---|---|---|---|---|---|---|---|
| None | 1,020 ± 145 | 147 | 0.020 ± 0.01 | 0.022 ± 0.012 | 0.0136 ± 0.009 | 0.0147 ± 0.0081 | |
| Ethanol | 200 | 9.7 ± 22 | 1.5 × 104 | 8.5 ± 10.0 | 5.0 ± 0.78 | 0.57 ± 0.68 | 3.3 ± 0.52 |
| Phenol | 5 | 31 ± 30 | 4.9 × 103 | 1.93 ± 1.33 | 0.75 ± 0.41 | 0.129 ± 0.09 | 0.50 ± 0.27 |
| Toluene | 800 | 0.72 ± 0.33 | 2.1 × 105 | 58 ± 79 | ND | 3.9 ± 0.53 | ND |
| o-Xylene | 800 | 0.35 ± 0.25 | 4.2 × 105 | 108 ± 88 | ND | 7.2 ± 0.59 | ND |
| m-Xylene | 800 | 0.50 ± 0.25 | 3.0 × 105 | ND | ND | ND | ND |
| p-Xylene | 800 | 0.35 ± 0.25 | 4.2 × 105 | ND | ND | ND | ND |
Conjugation events and transconjugants were enumerated after 48-h mating incubations. The initial total density was 1.50 × 107 CFU/mm2. ND, not detected. Values are means±standard deviations.
A similar set of 48-h matings was conducted, with both recipients being exposed to SDS (0 to 10 mM). When PAO2002N was the recipient (Table 3), transconjugants were only detected at the highest SDS concentration; conjugation events were detected at all exposure concentrations, and inferred conjugation frequencies were significantly higher for cells exposed to SDS concentrations of 1, 7, and 10 mM than for unexposed cells. The Di/Ri ratios were fairly constant for PAO2002N, differing no more than twofold.
TABLE 3.
Plasmid transfer frequencies for matings between P. putida CAP8(pTOL::Pa1/04/03 gfpmut3b) and P. aeruginosa PAO1162N or PAO2002N that was previously exposed to SDSa
| Recipient | Concn (mM) of SDS | Di/Ri | Di + Ri (CFU [103]/mm2) | Df + Rf (CFU [103]/mm2) | T/(DiRi) (10−10) (events/mm2)−1 | C/(DiRi) (10−10) (events/mm2)−1 |
|---|---|---|---|---|---|---|
| PAO2002N | 0 | 0.08 | 400 ± 56 | 5,800 ± 1,400 | ND | 0.078 ± 0.0062 |
| 1 | 0.12 | 5,900 ± 3,326 | ND | 0.154 ± 0.029 | ||
| 2 | 0.09 | 5,600 ± 1,170 | ND | 0.140 ± 0.094 | ||
| 4 | 0.06 | 5,900 ± 3,200 | ND | 0.089 ± 0.063 | ||
| 7 | 0.06 | 6,500 ± 1,990 | ND | 0.51 ± 0.043 | ||
| 10 | 0.05 | 6,100 ± 3,400 | 0.104 ± 0.036 | 0.116 ± 0.029 | ||
| PAO1162N | 0 | 13 | 146 ± 2.1 | 400 ± 150 | 2,700 ± 640 | |
| 1 | 43 | 580 ± 113 | 17,600 ± 12,200 | |||
| 4 | 378 | 2,300 ± 149 | 92,000 ± 44,000 | |||
| 7 | 378 | 2,800 ± 490 | 240,000 ± 65,000 | 0.37 ± 0.051 | ||
| 10 | >1,970 | 2,800 ± 690 | 600,000 ± 480,000 | 0.78 ± 0.163 | ||
| 0 | 20 | 41.9 ± 4.7 | 347 ± 21 | 88 ± 42 | 1.36 ± 0.73 | |
| 1 | 3.5 | 66.5 ± 6.2 | 15.8 ± 4.8 | 0.154 ± 0.044 | ||
| 4 | 31 | 199 ± 9.8 | 148 ± 69 | 0.96 ± 0.034 | ||
| 7 | 9.3 | 326 ± 63 | 86 ± 16.2 | 0.43 ± 0.157 | ||
| 10 | 3.3 | 383 ± 38 | 21.0 ± 5.1 | 0.154 ± 0.018 |
C, T, Df, and Rf were enumerated after 48-h mating incubations. The initial total density was 1.50 × 107 CFU/mm2; all values are means±standard deviations; values shown in bold are statistically different from mating results with unexposed recipients. ND, not detected.
An initial set of experiments with PAO1162N as the recipient (Table 3) revealed significantly higher transconjugant frequencies after SDS exposures than those observed for baseline mating (significant differences at 4 and 7 mM [α = 0.05]). Increased SDS exposures also resulted in large increases in the Di/Ri ratio (up to 1,000-fold). In a second experiment, with PAO1162N exposed to incremental doses of SDS, the Di/Ri ratio was more closely controlled by adjusting the volume of the recipient culture employed. Increases in transfer frequency were not observed, and much lower overall frequencies were noted. This set of observations led us to evaluate how the applied cell densities, especially the Di/Ri ratios, affected the computed plasmid transfer frequencies.
Effect of initial Di/Ri ratio on transfer frequency.
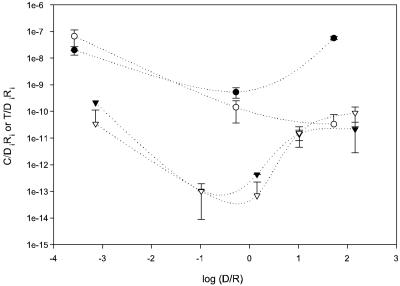
The transfer frequency observed at initial donor-to-recipient ratios near unity and in the absence of any premating toxicant exposure was on the order of 10−10 (events/mm2)−1 for PAO1162N, measured as the conjugation or transconjugant frequency after 24 h (Fig. 1). For PAO2002N, the observed conjugation frequency was 10−14 (events/mm2)−1 but could only be detected after 48 h based on conjugation events: transconjugant densities at this and any lower Di/Ri ratio were below the detection limits, resulting in a calculated upper bound on the transconjugant frequency (Fig. 1). When Di/Ri ratios varied from 10−3 to 102 while maintaining total surface densities, different transfer frequencies were measured, with frequencies increasing at Di/Ri ratios of 1 or less (Fig. 1). For example, at a Di/Ri ratio of 10−3, conjugation frequencies increased to 3 × 10−11 (1,000-fold) and 3 × 10−8 (events/mm2)−1 (100-fold) for PAO2002N and PAO1162N, respectively. The trend in calculated transconjugant frequencies tracked the experimentally confirmed conjugation frequencies for PAO2002N. Transfer frequencies for PAO1162N were consistently 4 orders of magnitude higher than those for PAO2002N.
FIG. 1.
Conjugation (open symbols) and transconjugant (filled symbols) frequencies at different initial donor-to-recipient ratios for matings between P. putida CAP8(pTOL::gfp) and P. aeruginosa PAO1162N (circles) or PAO2002N (triangles). Initial total surface densities were 1.62 × 106 ± 1.81 × 106 and 2.8 × 107 ± 1.21 ×107 CFU/mm2 for PAO2002N and PAO1162N, respectively. Mating times were 48 and 24 h for PAO2002N and PAO1162N, respectively. For PAO2002N matings at Di/Ri ratios of ≤1, transconjugant densities were below the detection limit, and a maximum value for the transconjugant frequency was calculated.
Interestingly, for PAO2002N matings, the efficiency of transfer expressed relative to the recipient (C/Ri) was constant over a Di/Ri range of 0.001 to 1 (C/Ri = 10−8) but increased at Di/Ri ratios of >1 (up to 10−5 for a Di/Ri ratio of 100), while the donor efficiency (C/Di) increased when the Di/Ri ratio was <1 (from 10−8 at a Di/Ri ratio of 1 to 10−5 at a Di/Ri ratio of 10−3) and was relatively constant for Di/Ri ratios of >1. These results indicate that the mating outcome (conjugation events) was determined in large part by donor or recipient limitation at Di/Ri ratios below and above 1, respectively. This analysis was not possible for strain PAO1162N because matings were conducted with a smaller range of ratios.
The strong effects of the initial Di/Ri ratios on computed plasmid transfer frequencies demanded careful control of these ratios in subsequent experiments in which the effects of toxicant exposures were examined. Furthermore, the ratios had to be chosen to enable the detection of transconjugant and conjugation events. Di/Ri ratios of 10−3 and 20 were applied for further PAO1162N and PAO2002N matings, respectively.
Effect of toxicant exposure on recipient density.
The previously employed toxicant concentrations would be impractical for conducting matings at controlled Di/Ri ratios, given that the reduction in recipient cell density led to as high as 103-fold increases in the Di/Ri ratio (Table 2). Hence, the effect of toxicant exposure on cell survival was examined over a smaller concentration range, i.e., from 1 to 10 mM for toluene, phenol, and SDS and from 50 to 500 mM for ethanol, to enable subsequent matings. Small but negative effects on cell numbers were observed in PAO2002N cultures exposed to toluene or phenol for 1 h (maximal reductions of 37% ± 9% and 27% ± 24%, respectively), while PAO1162N cell numbers decreased insignificantly when cells were exposed to toluene or even increased in the presence of phenol for the range examined (Table 4). Ethanol and SDS, on the other hand, negatively affected the cell numbers of both PAO2002N and PAO1162N at incremental concentrations, with some evidence of a higher sensitivity by PAO1162N.
TABLE 4.
Effects of toxicant exposure on P. aeruginosa PAO1162N and PAO2002N cell numbers
| Toxicant | Concn (mM) | Cell no. (mean ± SD)a
|
|
|---|---|---|---|
| PAO1162N | PAO 2002N | ||
| Ethanol | 50 | 0.90 ± 0.29 | 1.22 ± 0.35 |
| 100 | 0.88 ± 0.22 | 1.42 ± 0.63 | |
| 150 | 0.70 ± 0.11 | 1.22 ± 0.34 | |
| 200 | 0.68 ± 0.09 | 0.98 ± 0.50 | |
| 500 | 0.39 ± 0.24 | 0.52 ± 0.28 | |
| Toluene | 1 | 0.72 ± 0.30 | 0.89 ± 0.18 |
| 2 | 0.84 ± 0.37 | 0.91 ± 0.29 | |
| 5 | 0.88 ± 0.46 | 0.69 ± 0.11 | |
| 8 | 0.90 ± 0.42 | 0.69 ± 0.27 | |
| 10 | 0.89 ± 0.45 | 0.63 ± 0.09 | |
| Phenol | 1 | 1.19 ± 0.36 | 0.86 ± 0.07 |
| 2 | 0.96 ± 0.15 | 0.80 ± 0.13 | |
| 3 | 1.36 ± 0.37 | 0.84 ± 0.14 | |
| 5 | 1.47 ± 0.91 | 0.74 ± 0.14 | |
| 10 | 1.12 ± 0.55 | 0.73 ± 0.24 | |
| SDS | 1 | 0.40 ± 0.43 | 0.92 ± 0.38 |
| 2 | 0.16 ± 0.12 | 0.73 ± 0.23 | |
| 4 | 0.14 ± 0.08 | 0.43 ± 0.12 | |
| 7 | 0.05 ± 0.04 | 0.22 ± 0.04 | |
| 10 | 0.05 ± 0.05 | 0.20 ± 0.06 | |
Values reflect cell densities after 1 h of toxicant exposure normalized to cell densities without exposure (n ≥ 4). Bold values are significantly different from values for unexposed cultures.
In experiments conducted at controlled initial Di/Ri ratios and total surface cell densities, recipient cultures were exposed to a range of concentrations of each toxicant up to a maximum value, at which negative effects on at least one recipient were observed (Table 4). Furthermore, the amount of recipient cell culture employed in matings was adjusted to maintain a fairly constant initial recipient cell density and Di/Ri ratio. Under these well-controlled conditions, no effect on transfer frequencies was observed when PAO2002N was exposed to toluene, phenol, or ethanol (Table 5). In a repeat of the ethanol treatment with lower initial surface coverage but a consistent Di/Ri ratio, the lack of an effect on plasmid transfer frequencies was confirmed. A premating exposure of PAO1162N to phenol, toluene, or ethanol similarly did not yield statistically significant or consistent effects on transfer frequencies: a premating exposure to ethanol (at 50 and 100 mM) or toluene (at 8 mM) decreased the transfer frequency, while 10 mM phenol increased the transfer frequency (Table 6).
TABLE 5.
Plasmid transfer frequencies for matings between P. putida CAP8(pTOL::Pa1/04/03 gfpmut3b) and P. aeruginosa PAO2002N that was previously exposed to toxicantsa
| Toxicant | Di/Ri | Di + Ri (CFU [103]/ mm2) | Concn (mM) | Df + Rf (CFU [103]/ mm2) | T/(DiRi) (10−10) (events/mm2)−1 | C/(DiRi) (10−10) (events/mm2)−1 |
|---|---|---|---|---|---|---|
| Ethanol | 28 | 1,020 ± 260 | 0 | 8,300 ± 440 | 0.047 ± 0.032 | 0.132 ± 0.070 |
| 50 | 9,400 ± 5,300 | 0.127 ± 0.091 | 0.071 ± 0.065 | |||
| 100 | 11,500 ± 1,550 | 0.120 ± 0.150 | 0.151 ± 0.119 | |||
| 150 | 10,000 ± 3,400 | 0.050 ± 0.034 | 0.38 ± 0.24 | |||
| 200 | 8,200 ± 3,900 | 0.095 ± 0.072 | 0.32 ± 0.27 | |||
| 26 | 24 ± 0.149 | 0 | 1,790 ± 382 | 240 ± 171 | 24 ± 8.3 | |
| 100 | 901 ± 576 | 137 ± 102 | 45 ± 46 | |||
| 200 | 1,170 ± 789 | 89 ± 31 | 30 ± 10.1 | |||
| Toluene | 20 | 580 ± 34 | 0 | 9,610 ± 1,530 | 0.176 ± 0.13 | 0.173 ± 0.136 |
| 1 | 8,600 ± 5,100 | 0.21 ± 0.25 | 0.033 ± 0.047 | |||
| 2 | 4,800 ± 2,700 | 0.24 ± 0.27 | 0.173 ± 0.157 | |||
| 5 | 10,100 ± 2,500 | 0.048 ± 0.021 | 0.196 ± 0.085 | |||
| 8 | 7,400 ± 4,200 | 0.190 ± 0.13 | 0.149 ± 0.187 | |||
| 10 | 9,500 ± 6,500 | 0.22 ± 0.192 | 0.21 ± 0.28 | |||
| Phenol | 25 | 930 ± 27 | 0 | 5,800 ± 3,700 | 0.0184 ± 0.008 | 0.073 ± 0.081 |
| 1 | 4,400 ± 1,410 | ND | 0.024 ± 0.033 | |||
| 2 | 3,400 ± 1,790 | 0.048 ± 0.042 | 0.073 ± 0.065 | |||
| 3 | 4,700 ± 3,900 | 0.022 ± 0.0095 | 0.126 ± 0.066 | |||
| 5 | 3,800 ± 2,000 | ND | 0.088 ± 0.140 | |||
| 10 | 3,500 ± 1,400 | 0.060 ± 0.039 | 0.070 ± 0.133 |
C, T, Df, and Rf were enumerated after 48-h mating incubations. The initial total density was 1.50 × 107 CFU/mm2; all values are means±standard deviations. ND, not detected.
TABLE 6.
Plasmid transfer frequencies for matings between P. putida CAP8(pTOL::Pa1/04/03 gfpmut3b) and P. aeruginosa PAO1162N that was previously exposed to toxicantsa
| Toxicant | Di/Ri | Di + Ri (CFU [103]/mm2) | Concn (mM) | Df + Rf (CFU [103]/mm2) | T/DiRi) (10−10) (events/mm2)−1 | C/(DiRi) (10−10) (events/mm2)−1 |
|---|---|---|---|---|---|---|
| Ethanol | 0.0013 | 220 ± 17 | 0 | 5,800 ± 850 | 139 ± 48 | 2,400 ± 670 |
| 50 | 6,400 ± 1,380 | 152 ± 123 | 1,400 ± 250 | |||
| 100 | 8,000 ± 670 | 99 ± 85 | 1,610 ± 320 | |||
| 150 | 6,900 ± 3,300 | 106 ± 114 | 2,700 ± 680 | |||
| 200 | 3,800 ± 1,560 | ND | 2,300 ± 350 | |||
| 0.0003 | 760 ± 70 | 500 | 5,600 ± 44 | ND | 163 ± 29 | |
| Toluene | 0.0013 | 700 ± 47 | 0 | 8,000 ± 3,000 | 590 ± 260 | 2,800 ± 580 |
| 1 | 3,700 ± 910 | 126 ± 38 | 840 ± 450 | |||
| 2 | 7,900 ± 640 | 380 ± 54 | 1,470 ± 770 | |||
| 5 | 5,600 ± 1,240 | 131 ± 66 | 1,540 ± 350 | |||
| 8 | 4,800 ± 1,310 | 74 ± 64 | 280 ± 270 | |||
| 10 | 7,000 ± 1,310 | 193 ± 76 | 560 ± 192 | |||
| Phenol | 0.0030 | 194 ± 3 | 0 | 5,400 ± 1,290 | 140 ± 49 | 260 ± 116 |
| 1 | 4,200 ± 1,470 | 23 ± 10.1 | 480 ± 460 | |||
| 2 | 3,000 ± 570 | ND | 600 ± 390 | |||
| 3 | 3,700 ± 1,200 | ND | 330 ± 420 | |||
| 5 | 3,200 ± 720 | 23 ± 8.0 | 420 ± 350 | |||
| 10 | 6,200 ± 1,600 | 48 ± 0.0 | 1,930 ± 980 |
C, T, Df, and Rf were enumerated after 24-h mating incubation. All values are means ± standard deviations; values shown in bold are statistically different from mating results for unexposed recipients. ND, not detected.
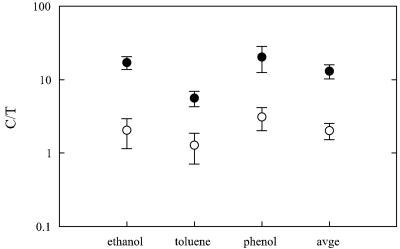
For both PAO1162N and PAO2002N matings, high variabilities in transconjugant densities made transconjugant-based transfer frequencies less precise estimators than conjugation event-based frequencies. The measured transconjugant frequency values were consistently lower than the conjugation frequencies for 24-h matings with PAO1162N, but they were of the same magnitude for 48-h matings with PAO2002N (Fig. 2).
FIG. 2.
Ratios of conjugation densities to transconjugant densities for matings between P. putida CAP8(pTOL::gfp) and P. aeruginosa PAO1162N (closed symbols) or PAO2002N (open symbols). Mean values for simultaneously executed mating experiments with controlled total initial densities and Di/Ri ratios (Tables 5 and 6) are presented. Error bars reflect standard deviations of the means.
DISCUSSION
We developed a method that allowed the direct quantification of conjugation events and contrasted it with inferences of conjugation events from enumerations of transconjugants on selective media. Conjugation events were typically more numerous than transconjugants (Tables 5 and 6; Fig. 2), with a notable exception being the PAO1162N matings with SDS exposure, for which transconjugant counts were several orders of magnitude higher than conjugation event counts (Table 3). The direct quantification of conjugation events should yield a more accurate estimate of donor-to-recipient transfer kinetics because it is unbiased by transconjugant growth and plasmid retransfer, which would increase transconjugant counts. Conversely, the method presented here does not permit a direct estimation of plasmid retransfer (transconjugant-to-recipient) kinetics, although such a factor may be of extreme ecological relevance. Plasmid instability and the incomplete expression of plasmid-carried genes, on the other hand, would decrease transconjugant counts and result in an underestimation of the number of conjugation events. The proposed method, which relies on in situ detection of GFP expression from a universally strong promoter, can further minimize this bias, especially if the postmating selective incubation can be reduced or eliminated. Since the TOL plasmid appears to be quite stable and the xyl pathway is readily expressed in PAO1162 (29), the higher transconjugant counts during matings in the presence of SDS were likely caused by plasmid retransfer or transconjugant growth during these 48-h matings (7), while the higher frequencies inferred from conjugation events for all other matings are consistent with other reports (12).
Comparisons of the effects of various environmental and biological factors on plasmid transfer kinetics would be facilitated if a system-independent intrinsic kinetic parameter were available. Such a parameter, termed plasmid fertility, based on an underlying mass action model, has been successfully proposed and tested for conjugal plasmid transfer in well-mixed homogenous systems (17, 26, 29). The adequacy of this parameter to describe plasmid transfer kinetics in surface matings has been ambiguous (25, 30). Our results (Fig. 1) clearly indicate that the plasmid transfer efficiency [measured as either T/(RiDi) or C/(RiDi)], which should provide a rough estimate of the proposed plasmid fertility parameter, is clearly a function of the Di/Ri ratio and hence is not independent of the system as was observed for liquid matings. The mass action model therefore fails to describe plasmid transfer kinetics in surface-immobilized cell mixtures. As a result, the apparent effect of the Di/Ri ratio on the plasmid transfer frequency cannot be uncoupled from any direct effect of toxicant exposure on the plasmid transfer frequency (Tables 2 and 3), and experiments with carefully controlled and consistent Di/Ri ratios were mandatory to examine the direct effects of toxicants.
Although interspecies transfer of a modified TOL plasmid from P. putida to some other rRNA group I Pseudomonas strains (P. fluorescens EEZ20, P. aeruginosa 7NSK2, and P. stutzeri EEZ22) has been reported to occur with similar frequencies to those of intraspecific transfer (10−1 to 10−2 T/R after overnight filter mating of high-density donor and recipient mixtures) (22), such frequencies were only approached in this study with the restriction-deficient strain PAO1162N at the highest initial Di/Ri ratios (approximately 5.5 × 10−2 ± 6.7 × 10−3 T/R). Plasmid transfer from P. putida to the restriction-proficient strain P. aeruginosa PAO2002N was typically 3 to 4 orders of magnitude less frequent than that to the restriction-deficient strain PAO1162N (approximately 10−11 versus 10−7). Similar quantitative effects of restriction proficiency on plasmid transfer efficiency by conjugation and transformation have been observed (3, 11, 24). Clearly, the restriction-modification system can represent a significant barrier to interspecific horizontal transfer for the PAO strains, contributing to their sexual isolation (15). The reason that another P. aeruginosa strain (the rhizosphere isolate 7NSK2) is an apparently more permissive recipient of plasmids from P. putida has yet to be determined (13, 22).
For all experiments, the plasmid donor culture was grown to early stationary phase to minimize the effects of its antecedent growth conditions on the observed plasmid transfer kinetics (28), while any effects of solvent exposure on donor properties were avoided by repeated washing of the exposed recipient suspension prior to mating.
Initial observations suggested that toxicant exposure increased the transfer frequency of the TOL plasmid to the restriction-proficient recipient strain PAO2002N as much as 500-fold (Table 2). However, a concomitant increase in the Di/Ri ratio made it impossible to attribute the increase in transfer frequency to a direct toxicant exposure effect. In subsequent experiments in which the Di/Ri ratios and total initial surface densities were constant and/or the toxic stress was lower, smaller effects were observed. A premating exposure to toluene or phenol at the concentrations tested did not have significant effects on transfer frequencies for PAO2002N. In contrast, a premating exposure of PAO1162N to toluene or phenol was associated with a marginal decrease and increase, respectively, in transfer frequencies (although this was statistically significant only at a few concentrations). Ethanol, which was tested at similar concentrations as before, had no statistically significant effect on plasmid transfer frequencies to PAO2002N and had a negative effect on PAO1162N. Finally, SDS exposure had a definite positive effect on plasmid transfer frequency to the restriction-proficient recipient PAO2002N, and it was previously observed to repress restriction in Corynebacterium glutamicum (24). Although alleviation of the restriction system by heat exposure has been noted for several bacteria (34), the effect of solvent (ethanol) and surfactant (SDS) exposure has only been noted for C. glutamicum so far (24). Clearly, SDS exposure increased conjugal transfer to the restriction-proficient strain PAO2002N, but the suite of tested solvents did not stimulate such an effect within the examined range. The effects of the tested solvents at increasingly higher concentrations warrant further study, while the exact mechanism by which SDS enhances conjugal transfer remains to be elucidated.
Acknowledgments
This research was made possible through grants from the National Science Foundation (BES 9702361) and the U.S. Department of Energy (NABIR-DE-FG02-97ER62476).
We thank Bjarke Bak Christensen for the gift of P. putida BBC443 and Tim Tolker-Nielsen for the pUTKan::rrnBP1-RBS T0T1 construct.
REFERENCES
- 1.Bale, M. J., J. C. Fry, and M. J. Day. 1987. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J. Gen. Microbiol. 133:3099-3107. [DOI] [PubMed] [Google Scholar]
- 2.Barkay, T., N. Kroer, L. D. Rasmussen, and S. J. Sørensen. 1995. Conjugal transfer at natural population densities in a microcosm simulating an estuarine environment. FEMS Microbiol. Ecol. 16:43-53. [Google Scholar]
- 3.Berndt, C., P. Meier, and W. Wackernagel. 2003. DNA restriction is a barrier to natural transformation in Pseudomonas stutzeri JM300. Microbiology 149:895-901. [DOI] [PubMed] [Google Scholar]
- 4.Christensen, B. B., C. Sternberg, J. B. Andersen, L. Eberl, S. Møller, M. Givskov, and S. Molin. 1998. Establishment of new genetic trait in a microbial biofilm community. Appl. Environ. Microbiol. 64:2247-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, B. B., C. Sternberg, and S. Molin. 1996. Bacterial plasmid conjugation on semi-solid surfaces monitored with the green fluorescent protein (Gfp) from Aequorea victoria as a marker. Gene 173:59-65. [DOI] [PubMed] [Google Scholar]
- 6.Clark, J. D., and O. Maaløe. 1967. DNA replication and the cell cycle in Escherichia coli cells. J. Mol. Biol. 23:99-112. [Google Scholar]
- 7.Dahlberg, C., M. Bergstrom, and M. Hermansson. 1998. In situ detection of high levels of horizontal plasmid transfer in marine microbial communities. Appl. Environ. Microbiol. 64:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daigger, G. T., and C. P. L. G. J. Grady. 1982. An assessment of the role of physiological adaptation in the transient response of bacterial cultures. Biotechnol. Bioeng. 24:1427-1444. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, N. W., and B. W. Holloway. 1971. Pleiotropy of p-fluorophenylalanine-resistant and antibiotic hypersensitive mutants of Pseudomonas aeruginosa. Genet. Res. (Cambridge) 18:185-197. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, R. A., R. A. Helm, and S. R. Maloy. 1999. Increasing DNA transfer efficiency by temporary inactivation of host restriction. BioTechniques 26:891-900. [DOI] [PubMed] [Google Scholar]
- 11.Geisenberger, O., A. Ammendola, B. B. Christensen, S. Molin, K.-H. Schleifer, and L. Eberl. 1999. Monitoring the conjugal transfer of plasmid RP4 in activated sludge and in situ identification of the transconjugants. FEMS Microbiol. Lett. 174:9-17. [DOI] [PubMed] [Google Scholar]
- 12.Hausner, M., and S. Wuertz. 1999. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 65:3710-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höfte, M. 2004. Personal communication.
- 14.Hohnstock, A. M., K. G. Stuart-Keil, E. E. Kull, and E. L. Madsen. 2000. Naphthalene and donor cell density influence field conjugation of naphthalene catabolism plasmids. Appl. Environ. Microbiol. 66:3088-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeltsch, A. 2003. Maintenance of species identity and controlling speciation of bacteria: a new function for restriction/modification systems? Gene 317:13-16. [DOI] [PubMed] [Google Scholar]
- 16.Levin, B. R., and C. T. Bergstrom. 2000. Bacteria are different: observations, interpretations, speculations, and opinions about the mechanisms of adaptive evolution in prokaryotes. Proc. Natl. Acad. Sci. USA 97:6981-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin, B. R., F. M. Stewart, and V. E. Rice. 1979. The kinetics of conjugative plasmid transmission: fit of a single mass action model. Plasmid 2:247-260. [DOI] [PubMed] [Google Scholar]
- 18.Muela, A., M. Pocino, I. Arana, J. I. Justo, J. Iriberri, and I. Barcina. 1994. Effect of growth phase and parental cell survival in river water on plasmid transfer between Escherichia coli strains. Appl. Environ. Microbiol. 60:4273-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newby, D. T., T. J. Gentry, and I. L. Pepper. 2000. Comparison of 2,4-dichlorophenoxyacetic acid degradation and plasmid transfer in soil resulting from bioaugmentation with two different pJP4 donors. Appl. Environ. Microbiol. 66:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Normander, B., B. B. Christensen, S. Molin, and N. Kroer. 1998. Effect of bacterial distribution and activity on conjugal gene transfer on the phylloplane of the bush bean (Phaseolus vulgaris). Appl. Environ. Microbiol. 64:1902-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce, D. A., M. J. Bazin, and J. M. Lynch. 2000. Substrate concentration and plasmid transfer frequency between bacteria in a model rhizosphere. Microb. Ecol. 40:57-63. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Gonzalez, M.-I., E. Duque, and J. L. Ramos. 1991. Conjugational transfer of recombinant DNA in cultures and in soils: host range of Pseudomonas putida TOL plasmids. Appl. Environ. Microbiol. 57:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandaa, R. A., and Ο. Enger. 1994. Transfer in marine sediments of the naturally occurring plasmid pRAS1 encoding multiple antibiotic resistance. Appl. Environ. Microbiol. 60:4234-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer, A., J. Kalinowski, and A. Puhler. 1994. Increased fertility of Corynebacterium glutamicum recipients in intergeneric matings with Escherichia coli after stress exposure. Appl. Environ. Microbiol. 60:756-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen, L. 1990. Dynamics of plasmid transfer on surfaces. J. Gen. Microbiol. 136:1001-1007. [DOI] [PubMed] [Google Scholar]
- 26.Simonsen, L., D. M. Gordon, F. M. Stewart, and B. R. Levin. 1990. Estimating the rate of plasmid transfer: an end-point method. J. Gen. Microbiol. 136:2319-2325. [DOI] [PubMed] [Google Scholar]
- 27.Smets, B. F., B. E. Rittmann, and D. A. Stahl. 1995. Quantification of the effect of substrate concentration on the conjugal transfer rate of the TOL plasmid in short-term batch mating experiments. Lett. Appl. Microbiol. 21:167-172. [DOI] [PubMed] [Google Scholar]
- 28.Smets, B. F., B. E. Rittmann, and D. A. Stahl. 1993. The specific growth rate of Pseudomonas putida PAW1 influences the conjugal transfer rate of the TOL plasmid. Appl. Environ. Microbiol. 59:3430-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smets, B. F., B. E. Rittmann, and D. A. Stahl. 1994. Stability and conjugal transfer kinetics of a TOL plasmid in Pseudomonas aeruginosa PAO 1162. FEMS Microbiol. Ecol. 15:337-350. [Google Scholar]
- 30.Sudarshana, P., and G. Knudsen. 1995. Effect of parental growth on dynamics of conjugative plasmid transfer in the pea spermosphere. Appl. Environ. Microbiol. 61:3136-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timms-Wilson, T. M., and M. J. Bailey. 2001. Reliable use of green fluorescent protein in fluorescent pseudomonads. J. Microb. Methods 46:77-80. [DOI] [PubMed] [Google Scholar]
- 32.Tolker-Nielsen, T., U. C. Brinch, P. C. Ragas, J. B. Andersen, C. S. Jacobsen, and S. Molin. 2000. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182:6482-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Top, E. M., H. De Rore, J.-M. Collard, V. Gellens, G. Slobodkina, W. Verstraete, and M. Mergeay. 1995. Retromobilization of heavy metal resistance genes in unpolluted and heavy metal polluted soil. FEMS Microbiol. Ecol. 18:191-203. [Google Scholar]
- 34.Velkov, V. V. 1999. How environmental factors regulate mutagenesis and gene transfer in microorganisms. J. Biosci. 24:529-559. [Google Scholar]