Abstract
The hypothalamic paraventricular nucleus (PVN) is the major autonomic output area of the hypothalamus and a critical regulatory center for energy homeostasis. The organism's energetic balance is very important for both the regular onset of puberty and regulation of fertility. Several studies have suggested a relationship among neural circuits controlling food intake, energy homeostasis and the kisspeptin peptide. The kisspeptin system is clustered in two main groups of cell bodies [the anterior ventral periventricular region (AVPV) and the arcuate nucleus (ARC)] projecting mainly to gonadotropin‐releasing hormone (GnRH) neurons and to a few other locations, including the PVN. In the present study, we investigated the distribution of the kisspeptin fibers within the PVN of adult CD1 mice. We observed a significant sexual dimorphism for AVPV and ARC, as well as for the PVN innervation. Kisspeptin fibers showed a different density within the PVN, being denser in the medial part than in the lateral one; moreover, in female, the density changed, according to different phases of the estrous cycle (the highest density being in estrus phase). The presence of a profound effect of estrous cycle on the kisspeptin immunoreactivity in AVPV (with a higher signal in estrus) and ARC, and the strong co‐localization between kisspeptin and NkB only in ARC and not in PVN suggested that the majority of the kisspeptin fibers found in the PVN might arise directly from AVPV.
Keywords: anterior ventral periventricular region, arcuate nucleus, diestrus, estrus, Kiss1, PVN
Introduction
The kisspeptin peptide is encoded by the KiSS1 gene, localized on human and murine chromosome 1. The mature peptide is formed by 52 or 54 amino acids. This protein binds specifically to the kisspeptin receptor (Kiss1r), previously known as G‐protein‐coupled receptor‐54 (GPR54; Kotani et al. 2001), whose mutations induce hypogonadotropic hypogonadism (de Roux et al. 2003; Seminara et al. 2003). From a physiological point of view, kisspeptin has been identified as the most powerful regulator of gonadotropin‐releasing hormone (GnRH; Irwig et al. 2004; Pinilla et al. 2012). It is implicated in the timing of puberty onset (Han et al. 2005) and in the mechanism linking energetic status to the reproductive axis (Tena‐Sempere, 2006; Castellano et al. 2010).
The neuroanatomical distribution of kisspeptin‐synthesizing cell populations is conserved across mammalian species. A large population of kisspeptin neurons is described in the arcuate hypothalamic nucleus (ARC) of mice (Gottsch et al. 2004; Smith et al. 2005a; Clarkson & Herbison, 2006), rats (Kauffman et al. 2007), hamsters (Greives et al. 2007), sheep (Franceschini et al. 2006; Goldman et al. 2007; Smith et al. 2007), mares (Decourt et al. 2008), primates (Shahab et al. 2005) and humans (Rometo et al. 2007). In this nucleus kisspeptin is coexpressed with neurokinin B (NkB), endogenous opioid peptide dynorphin A (Dyn) and other signaling molecules. These neurons, abbreviated as the KNDy subpopulation, are critical mediators of pulsatile GnRH neurosecretion (Lehman et al. 2010; Grachev et al. 2014). A second population of kisspeptin‐positive cells is located in the rostral periventricular area of the third ventricle [RP3V, which includes the anterior ventral periventricular region (AVPV) and the periventricular nucleus (PeN)] of mice (Gottsch et al. 2004; Smith et al. 2005b; Clarkson & Herbison, 2006), rats (Irwig et al. 2004; Kauffman et al. 2007), hamsters (Greives et al. 2007), sheep (Franceschini et al. 2006; Goldman et al. 2007; Smith et al. 2007) and humans (Rometo et al. 2007).
These two hypothalamic regions are differentially regulated by testosterone and estradiol, both during development and in adulthood (Smith et al. 2005a,b). Several studies performed in RP3V of adult rodents showed a peculiar dimorphism, with females displaying the highest kisspeptin expression (Clarkson & Herbison, 2006; Kauffman, 2009). Recently, a few studies showed that rodent's kisspeptin system is also dimorphic within the ARC, with the kisspeptin levels significantly higher in females (Knoll et al. 2013; Overgaard et al. 2013).
Kisspeptin fibers branch out from cell bodies in RP3V and ARC, to different hypothalamic areas (Yeo & Herbison, 2011), and, among them, the paraventricular nucleus (PVN) seems to be one of the major targets of the system. In fact, different studies mentioned that in rodents the PVN is highly innervated by kisspeptin fibers (Brailoiu et al. 2005; Clarkson et al. 2009; Yeo & Herbison, 2011). Within the hypothalamus the PVN is the major autonomic output area, with heterogeneous neuronal populations, playing essential roles in neuroendocrine/autonomic regulation (Ferguson et al. 2008). In fact, while the lateral part of the PVN contains magnocellular neurons chiefly projecting to the posterior pituitary [where they release oxytocin (OT) and arginine vasopressin (AVP) into the blood], the medial part of the PVN is characterized by different types of parvocellular neurons that can be identified for the presence of several neurotransmitters, neuropeptides and enzymes involved in the synthesis of neurotransmitters [i.e. tyrosine hydroxylase (TH; Ruggiero et al. 1984), neural nitric oxide synthase (nNOS; Gotti et al. 2004, 2005), but also corticotropin‐releasing hormone (CRH; Wang et al. 2011), thyrotrophin‐releasing hormone (TRH; Kadar et al. 2010), AVP (Caldwell et al. 2008) and somatostatin (Tan et al. 2013)].
In the present study, we describe for the first time the sexual dimorphism of kisspeptin‐immunoreactive (kiss‐ir) system in adult CD1 mice PVN, we analyze the variations of kisspeptin distribution during the estrous cycle, and the coexistence of kisspeptin and other cellular populations within the same nucleus.
Materials and methods
Animals
CD‐1 mice (Mus musculus domesticus) were originally purchased from Charles River Laboratories (Calco, Lecco, Italy) and maintained as an outbreed colony at the University of Torino. The animals were housed in groups of three males or three females in 45 × 25 × 15 cm polypropylene mouse cages at 22 ± 2 °C, under a 12 : 12 h light : dark cycle (light on at 08:00 h). Food and water were provided ad libitum (standard mouse chow 4RF21; Mucedola srl, Settimo Milanese, Italy).
We used three different groups of animals, as detailed below:
experiment 1 (kisspeptin system distribution and male to female comparison): six female in diestrus and six male mice at postnatal day 60 (PND60);
experiment 2 (estrous cycle observation): 10 adult female mice (PND90), four mice in estrus and six mice in diestrus phase.
experiment 3 (interaction between kisspeptin and different neuronal populations of PVN): four adult female mice (PND90) in estrus phase.
Animal care and handling were according to the European Union Council Directive of 22 September 2010 (2010/63/UE); the Italian Ministry of Health and the Ethical Committee of the University of Torino approved all the procedures reported in the present study.
Fixation and tissue sampling
From PND50, female mice were inspected by daily examination of vaginal cytology smears (for details, see Becker et al. 2005; McLean et al. 2012) in order to minimize the potential variations of kisspeptin expression due to the estrous cycle's phase (Adachi et al. 2007). After exhibition of 2 or more consecutive 4‐day estrous cycles, female mice were killed in diestrus for experiment 1; instead, for experiments 2 and 3, at PND90, a group of female mice (n = 4 for experiment 2; and n = 4 for experiment 3) was killed in estrus, and the others (n = 6 for experiment 2) in diestrus.
Male and female mice were deeply anesthetized with a mixture of ketamine–xylazine (respectively, 100 mg mL−1 and 20 mg mL−1) and perfused through the heart with saline solution (0.9%) until vessels were completely blood‐free, and then with the fixative (4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.3). The brains were removed and stored in a freshly prepared paraformaldehyde solution for 2 h at 4 °C, followed by several washings in 0.01 m saline phosphate buffer (PBS). Finally, they were stored in a 30% sucrose solution in PBS at 4 °C, frozen in isopentane pre‐cooled in dry ice at −35 °C, and stored in a deep freezer at −80 °C until sectioning.
Brains were serially cut in the coronal plane at 25 μm thickness with a cryostat, in four series. The plane of sectioning was oriented to match the drawings corresponding to the coronal sections of the mouse brain atlas (Paxinos & Franklin, 2001). Sections were collected in a cryoprotectant solution (Watson et al. 1986) and stored at −20 °C. One series was processed for kisspeptin immunohistochemistry using the free‐floating technique. Brain sections were always stained in groups containing male and females sections, so that between‐assays variance could not cause systematic group differences.
Immunohistochemistry
Single‐label immunohistochemistry
For experiments 1 and 2, the sections collected in the cryoprotectant solution were washed overnight in PBS at pH 7.3. The following day, sections were first washed in PBS containing 0.2% Triton X‐100 (PBS‐T) for 30 min and then treated for blocking endogenous peroxidase activity (PBS solution containing methanol/hydrogen peroxide, 1 : 1, 20 min, at room temperature). Sections were then incubated with normal goat serum (Vector Laboratories, Burlingame, CA, USA) for 30 min and incubated overnight at 4 °C with a polyclonal rabbit anti‐kisspeptin antibody (AC#566, a generous gift of Drs A. Caraty, I. Franceschini and M. Keller, Tours, France; diluted 1 : 10 000 in PBS–Triton X‐100 0.2%). The following day, sections were incubated for 60 min in biotinylated goat anti‐rabbit IgG (Vector Laboratories) at a dilution of 1 : 200 at room temperature. The antigen–antibody reaction was revealed by 60 min incubation with biotin–avidin system (Vectastain ABC Kit Elite, Vector Laboratories). The peroxidase activity was visualized with a solution containing 0.400 mg mL−1 of 3,3′‐diamino‐benzidine (SIGMA‐Aldrich, Milan, Italy) and 0.004% hydrogen peroxide in 0.05 m Tris–HCl buffer pH 7.6. Sections were mounted on chromallum‐coated slides, air‐dried, cleared in xylene and cover‐slipped with Entellan (Merck, Milano, Italy).
The production and characterization of this polyclonal kisspeptin antibody has been described in previous studies (Franceschini et al. 2006; Clarkson et al. 2009).
The AC#566 antibody was raised against the 10 amino acid C‐terminal of murine kisspeptin (amino acid residues 43–52, kp10, YNWNSFGLRY‐NH2, which are required for activation of Gpr54). Mouse kp10 was coupled to bovine serum albumin (BSA) using glutaraldehyde and used as an immunogen in rabbits. Radioimmunoassay analysis and pre‐adsorption controls showed that this antiserum is highly specific to mouse kp10: kisspeptin binding to the antisera is not inhibited by any one of different hypothalamic peptides including other RF‐amide peptides (Franceschini et al. 2006; Clarkson et al. 2009).
We performed the following additional controls in our material: (i) the primary antibody was omitted or replaced with an equivalent concentration of normal serum (negative controls); (ii) the secondary antibody was omitted. In these conditions, cells and fibers were totally unstained.
Double‐label immunofluorescence
For experiment 3, the sections were incubated for 24 h at 4 °C with two primary antibodies, one was always the AC053 antibody (polyclonal sheep anti‐kisspeptin antibody, a generous gift of Drs A. Caraty, I. Franceschini and M. Keller, Tours, France; Franceschini et al. 2013), the second was one of the others listed in Table 1. The primary antibodies were dissolved in a solution of PBS, pH 7.4, and containing 0.5% Triton X‐100 (Merck, Darmstadt, Germany), 1% normal donkey serum (Vector Laboratories) and 1% BSA (Sigma–Aldrich). Sections were washed and incubated, respectively, with solutions of appropriate secondary antibodies (included in Table 1). Sections were then cover‐slipped with antifade mounting medium Mowiol (Sigma–Aldrich).
Table 1.
Primary and secondary antibodies used in the double‐label immunofluorescence assays
| Primary Abs | |||||
| Kisspeptin | AC053 | A. Caraty | Sheep, pc | 1 : 2000 | Poling et al. (2013) |
| NkB | T4450 | Peninsula | Rabbit, pc | 1 : 2000 | Taziaux et al. (2012) |
| AVP | 64717 | ICN | Rabbit, pc | 1 : 8000 | Ferris et al. (1997) |
| nNOS | 24287 | DiaSorin | Rabbit, pc | 1 : 3000 | Gillespie et al. (2005) |
| OT | AB911 | Millipore | Rabbit, pc | 1 : 8000 | Bean et al. (2014) |
| TH | 22941 | Incstar | Mouse, mc | 1 : 8000 | Daadi & Weiss (1999) |
| Secondary Abs | |||||
| Anti‐sheep Alexa Fluor® 555 | A21436 | Invitrogen | Donkey, pc | 1 : 500 | |
| Anti‐rabbit Alexa Fluor® 488 | A21206 | Invitrogen | Donkey, pc | 1 : 500 | |
| Anti‐mouse Alexa Fluor® 488 | A21202 | Invitrogen | Donkey, pc | 1 : 500 | |
AVP, arginine vasopressin; mc, monoclonal antibody; NkB, neurokinin B; nNOS, neural nitric oxide synthase; OT, oxytocin; pc, polyclonal antibody; TH, tyrosine hydroxylase.
Sections were observed and photographed with a laser‐scanning Leica TCS SP5 (Leica Microsystems) confocal microscope. Images were processed using image j (version 1.46r; Wayne Rasband, NIH, Bethesda, MD, USA) and Adobe Photoshop CS4 (Adobe Systems). Only general adjustments to color, contrast and brightness were made.
Quantitative analysis (experiments 1 and 2)
Selected standardized sections of comparable levels covering the ARC (bregma −1.46 to 1.70 mm), the AVPV (bregma 0.50–0.02 mm), the PeN (bregma 0.14–0.22 mm) and the PVN (bregma −0.58 to 0.94 mm) were chosen (Paxinos & Franklin, 2001). For each animal, three (ARC), four (PVN) and six (AVPV, PeN) sections were acquired with a NIKON Digital Sight DS‐Fi1 video camera connected to a NIKON Eclipse 80i microscope (Nikon Italia S.p.S., Firenze, Italy). Digital images were processed and analyzed by imagej (version 1.46r; Wayne Rasband, NIH, Bethesda, MD, USA). Measurements were performed within predetermined fields (region of interest, ROI) as follows:
Experiment 1: kisspeptin system distribution and male to female comparison
Images were digitized by using a 10 × (AVPV and PeN) or a 20 × (ARC and PVN) objective. The ROI was a rectangular box of fixed size and shape covering a large part of each considered nucleus (350 000 μm2 for AVPV; 284 000 μm2 for ARC; 310 000 μm2 for PeN; 380 000 μm2 for PVN).
Experiment 2: estrous cycle observation
Images were digitized by using a 40 × (PVN and ARC) or a 20 × (AVPV) objective. The PVN, in each selected section, was divided into four squares (each of 25 000 μm2) to cover its full extension. These squares did not match with the sub‐nuclei of the PVN, but were chosen in order to have a topographical reference to analyze in more detail the density of immunoreactivity within the PVN by dividing it into four regions: dorso‐medial, dorso‐lateral, ventro‐medial and ventro‐lateral (Fig. 4A). The ROI for ARC (49 000 μm2) as well as that for AVPV (80 000 μm2) was placed within the boundaries of the considered nuclei to fully cover the immunopositive region, using as reference the third ventricle to position the ROI always in the same orientation.
Figure 4.
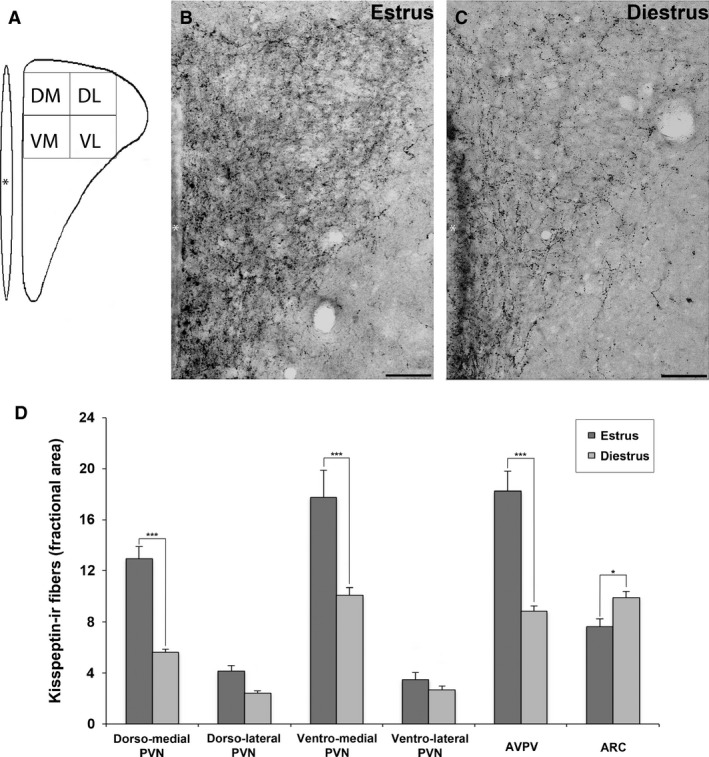
Effect of estrous cycle on the kisspeptin immunoreactivity (kiss‐ir) in adult mice. (A) The representative subdivision of the paraventricular nucleus (PVN) in four quadrants (DM, dorso‐medial; DL, dorso‐lateral; VM, ventro‐medial; VL, ventro‐lateral). (B, C) The comparison of kiss‐ir fibers within the PVN of female CD1 mice in estrus (B) and diestrus (C) phases, respectively. (D) Histograms representing the fractional area covered by kiss‐ir structures (mean ± SEM) in the PVN (DM, DL, VM, VL), anterior ventral periventricular nucleus (AVPV) and arcuate hypothalamic nucleus (ARC) of female adult mice in estrus (dark gray) and diestrus (light gray) phases. *P < 0.05, ***P < 0.001 different from diestrus (P < 0.05, Bonferroni test). Scale bar: 50 μm.
Kisspeptin immureactivity (cell bodies and processes) was measured by calculating in binary transformations (threshold function of the software) the fractional area (percentage of pixels) covered by immunoreactive elements of the images (as previously performed in our laboratory; Viglietti‐Panzica et al. 1994; Plumari et al. 2002; Pierman et al. 2008). Due to differences in the immunostaining, the range of the threshold was individually adjusted for each section, up to cover always the immunoreactivity of smallest fibers. The results obtained from each nucleus were grouped to provide mean (± SEM) values. The statistical analysis was performed, using the spss 22.0 statistic software (SPSS, Chicago, IL, USA), and was undertaken using anova with Student's t‐test to analyze experiment 1 and with post hoc Bonferroni test for experiment 2; values of P ≤ 0.05 were considered significant.
Results
Experiment 1: kisspeptin system distribution and male to female comparison
Qualitative results
In the brain of CD1 adult mice, the distribution of kiss‐ir cell bodies and fibers was similar to that of other previously described strains (Clarkson et al. 2009). As detailed in previous studies, AVPV and PeN (defined together as RP3V; Herbison, 2008), and ARC nuclei show the larger clusters of kisspeptin‐expressing cell bodies in females compared with in males. Accordingly, in CD1 mouse the two most consistent populations of kisspeptin neurons identified across serial brain sections were located in these two regions.
The first group of kisspeptin‐positive neurons was present within the total extension of the RP3V and clustered near the ventricular wall, making it difficult to precisely discern the limits of AVPV and PeN nuclei. Kiss‐ir cells were strongly labeled, and exhibited an oval or circular cell body provided with one or two dendritic processes. The RP3V included also a large number of kiss‐ir fibers. They covered the whole region and extended both dorsally and laterally from the ventricle wall into the adjacent brain regions where kisspeptin cell bodies were not present. A large number of kiss‐ir fibers were located in the ventral aspect of the lateral septum and, in particular, in the anterior portion of the bed nucleus of the stria terminalis; on the contrary, only few kisspeptin fibers were present within the medial septum.
A second large population of kisspeptin‐positive cell bodies was observed caudally, within the ARC. The kiss‐ir positive neurons in ARC had round or oval cell bodies, whereas their processes were difficult to distinguish due to the high density of surrounding immunoreactive processes. In fact, the densest immunostaining of the kisspeptin system was observed within the ARC, where the plexus of immunoreactive fibers clearly outlined each level of the nucleus.
A dense innervation of kiss‐ir fibers was observed within the PVN. These fibers outlined the entire rostro‐caudal extension of the PVN (Fig. 1).
Figure 1.
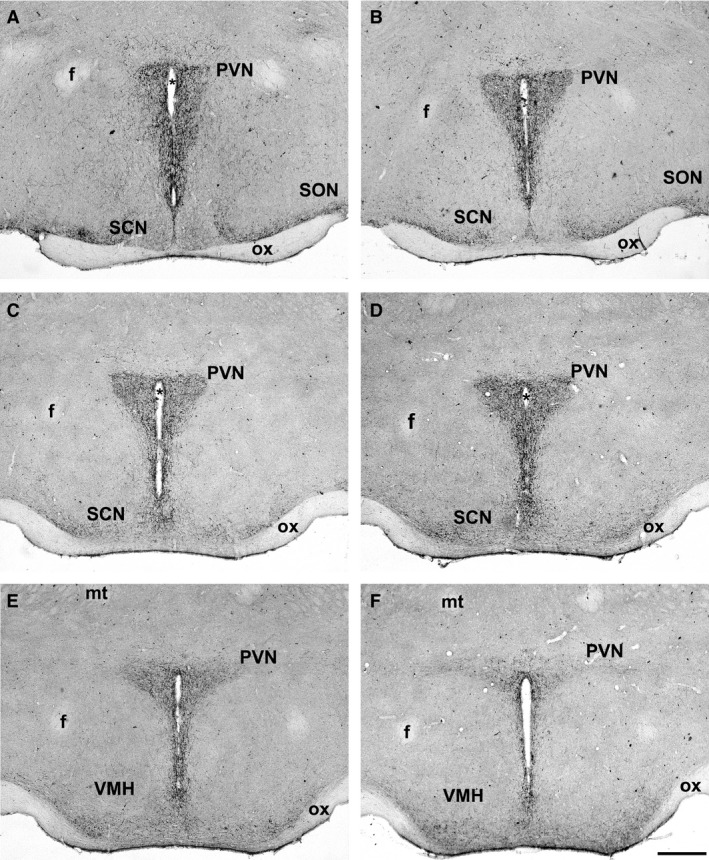
Distribution of kisspeptin‐immunoreactive (kiss‐ir) fibers within the paraventricular nucleus (PVN) of CD1 (diestrus) female mice, from rostral to caudal sections. Kisspeptin fibers outline the boundaries of the PVN and run along the wall of the third ventricle towards the optic chiasm (ox). A moderate innervation is present also within the supraoptic nucleus (SON), while the suprachiasmatic nucleus (SCN) is almost totally empty of immunoreactivity, as well as the ventromedial hypothalamic nucleus (VMH). f, fornix; *, third ventricle; mt, mammillothalamic tract. Scale bar: 100 μm.
Quantitative results
There was a visible difference in the extension of immunoreactivity (including both positive cell bodies and processes), with females (in diestrus) displaying a higher immunoreactivity than male CD1 mice (Fig. 2). The qualitative differences were confirmed by the quantitative analysis showing significant sex differences for each examined nucleus (Fig. 3). This difference was particularly evident for the AVPV and PeN (P < 0.001; Figs 2A,B and 3), but also for the ARC (P < 0.05; Figs 2E,F and 3), and for the amount of immunoreactive fibers in the PVN (P < 0.001; Figs 2C,D and 3).
Figure 2.
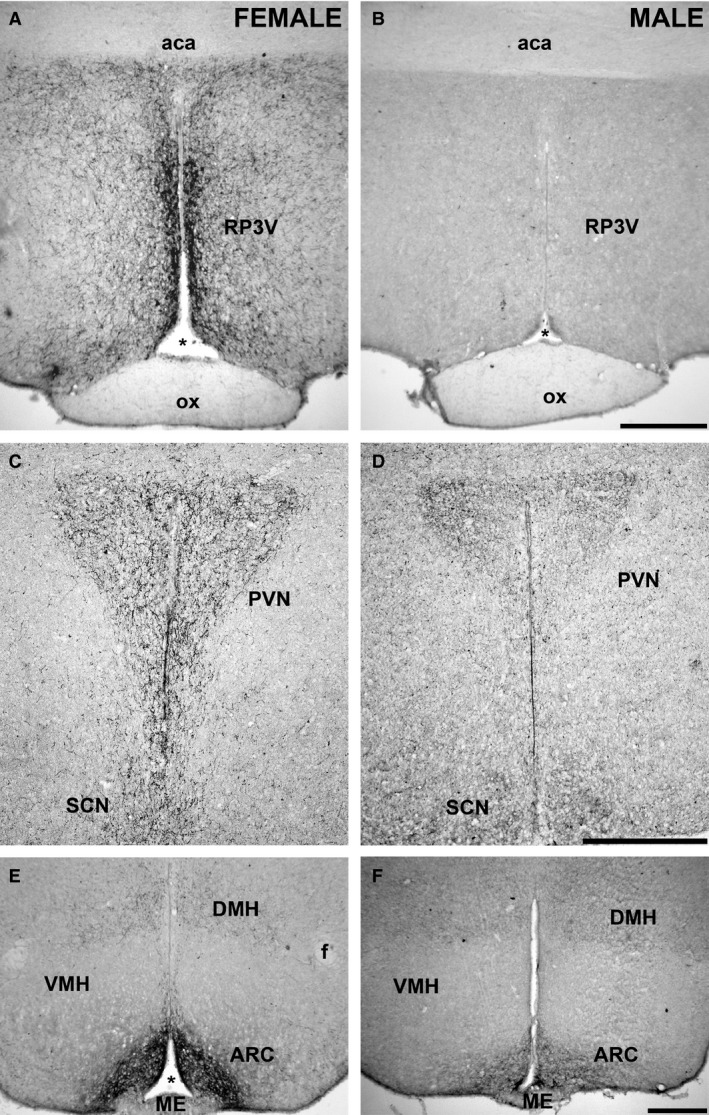
Dimorphic kisspeptin system in the hypothalamic nuclei. Comparison of the distribution of kisspeptin fibers in diestrus female (left panels) and male (right panels) CD1 mice. The expression of kisspeptin is higher in females than in males. (A, B) Rostral periventricular area of the third ventricle (RP3V). (C, D) Paraventricular nucleus (PVN). (E, F) Arcuate hypothalamic nucleus (ARC). ox, optic chiasm; aca, anterior commissure; f, fornix; *, third ventricle; SCN, suprachiasmatic nucleus; VMH, ventromedial hypothalamic nucleus; DMH, dorsomedial hypothalamic nucleus; ME, median eminence. Scale bar: 100 μm.
Figure 3.
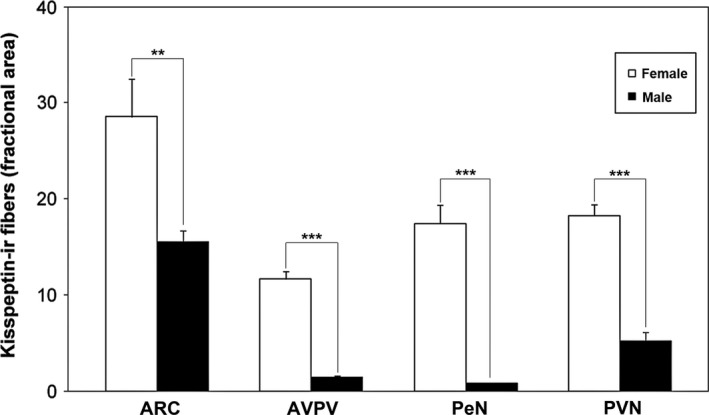
Quantitative study of kisspeptin system in the hypothalamic nuclei. Histograms representing the fractional area covered by kisspeptin‐immunoreactive (kiss‐ir) structures (mean ± SEM) in the arcuate hypothalamic nucleus (ARC), anterior ventral periventricular nucleus (AVPV), periventricular nucleus (PeN) and paraventricular nucleus (PVN) of male (black bars) and female (white bars) CD1 mice. Males showed a lower immunoreactivity in comparison with the female group. **P < 0.01, ***P < 0.001 different from males (P < 0.05; Student's t‐test).
Experiment 2: estrous cycle observation
Whereas at low magnification (Fig. 1) no differences in immunoreactivity for kisspeptin were evident within the female PVN, at higher magnification (Fig. 4B,C) the distribution of PVN kiss‐ir fibers appeared not homogeneous in particular when comparing the medial (corresponding to the parvocellular region) with the lateral (corresponding to the magnocellular one) PVN. The quantitative analysis performed at higher magnification (40 × objective) subdividing the nucleus into four regions (see Materials and methods and Fig. 4A) was subjected to a two‐way anova for repeated‐measures, with the position (lateral vs. medial) and the phase of the estrous cycle (estrus vs. diestrus) as the two independent variables, and the ventral vs. dorsal position as the repeated‐measure. This analysis reported the following F‐values:
position, F 5,48 = 196.384, P < 0.0001;
cycle phase, F 1,48 = 185.551, P < 0.0001;
interaction position/cycle phase, F 5,1,48 = 23.479, P < 0.0001.
In both estrus and diestrus, the two‐by two comparison (Bonferroni test) revealed a significant difference for the medial vs. lateral PVN innervation (estrus, P < 0.001; diestrus, P < 0.001); no significant differences were observed comparing dorso‐lateral vs. ventro‐lateral PVN (estrus P = 0.412; diestrus P = 0.633), whereas the comparison of dorso‐medial vs. ventro‐medial PVN reported significant differences (estrus, P = 0.01; diestrus, P < 0.001).
The comparison of estrus vs. diestrus females revealed a significantly higher immunoreactive fractional area in estrus in comparison to diestrus in all the considered regions: medial PVN (P < 0.001), in particular dorso‐medial PVN (P < 0.001) and ventro‐medial PVN (P < 0.001; Fig. 4D), but also in the lateral PVN (P = 0.021).
The results for ARC and AVPV in the same animals showed a profound effect of estrous cycle on the kiss‐ir. The signal was higher in AVPV (P < 0.001) and lower in ARC (P = 0.026) in estrus in comparison to diestrus (Fig. 4D).
Experiment 3: interaction between kisspeptin and different neuronal populations of the PVN
In adult CD1 estrus female mice the distribution of kisspeptin fluorescence immunoreactivity in PVN and ARC nuclei was similar to that described in experiments 1 and 2. In fact, within the PVN the immunofluorescence appeared not homogeneous with a higher concentration of kiss‐ir fibers in the medial compared with the lateral PVN (Figs 5A and 6, left panels). In the caudal ARC we observed a large population of kisspeptin‐positive cell bodies and fibers (Fig. 5B).
Figure 5.
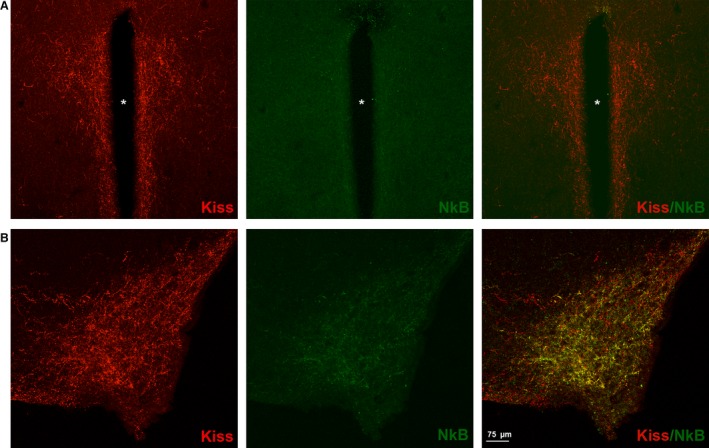
Kisspeptin (Kiss) and neurokinin B (NkB) expression within the paraventricular nucleus (PVN) and arcuate hypothalamic nucleus (ARC) in adult CD1 female mice. Kiss (red) and NkB (green) immunoreactivity in a coronal section of the PVN (A) and ARC (B) of the adult CD1 female mice in estrus phase. A dense Kiss expression is evident in both nuclei, with respect to the NkB signal: is clearly present only in ARC; in PVN the NkB signal is very low. The merge indicates that the Kiss and Nkb immunoreactivity co‐localize with the ARC nucleus.
Figure 6.
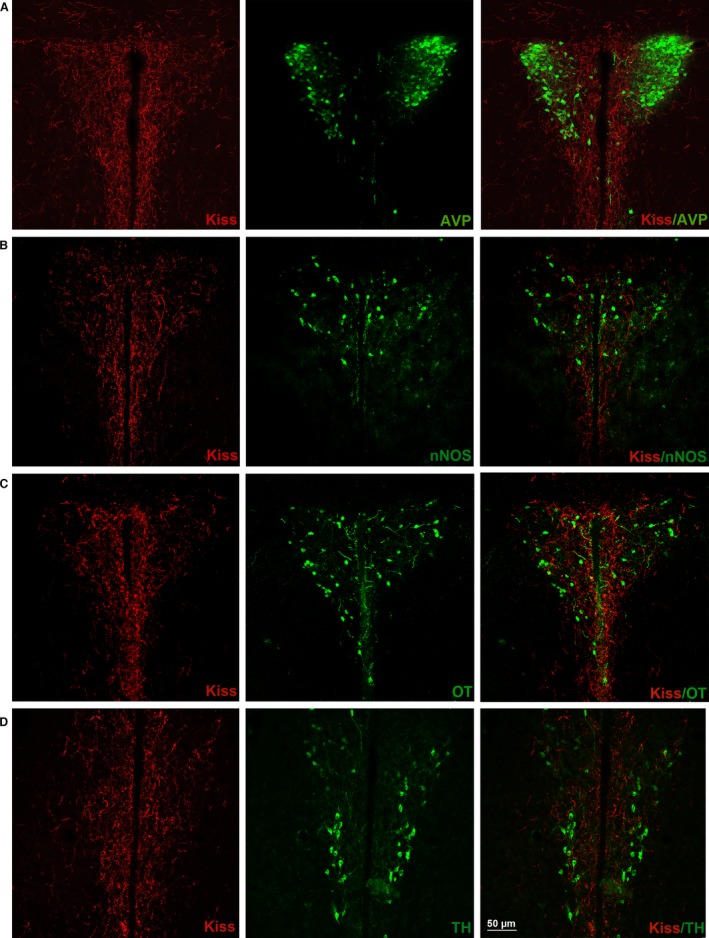
Kisspeptin (Kiss) and paraventricular nucleus (PVN) cellular populations [arginine vasopressin (AVP), neural nitric oxide synthase (nNOS), oxytocin (OT), tyrosine hydroxylase (TH)]. Coronal section of the adult CD1 female mice PVN in estrus phase. It is possible to observe the relations with Kiss (red) and different PVN neuronal populations: (A) AVP (green); (B) nNOS (green); (C) OT (green); (D) TH (green). Note that the total AVP and the majority of nNOS neuronal cell bodies were distributed in lateral PVN, where the concentration of Kiss fibers was lower; in the medial PVN instead, a conspicuous number of OT and TH cell bodies were present.
Coexistence NkB and kisspeptin
The immunoreaction for NkB revealed that there was a high expression of NkB‐ir within the ARC, while in the PVN the NkB signal was very low (Fig. 5, middle panels). Merging the immunofluorescence for kisspeptin and NkB, we saw that kisspeptin strongly co‐localize with NkB in the ARC but not in the PVN (Fig. 5, right panels).
Kisspeptin and AVP, nNOS, OT and TH cell bodies
The four populations that we investigated were differently distributed within the PVN. The AVP‐containing neurons were clustered in the lateral part of the nucleus (magnocellular population) where kisspeptin fibers were very low. Only a few, scattered, small AVP cells were observed in the medial part of the PVN, which was rich in kisspeptin fibers (Fig. 6A). Interactions with kisspeptin fibers were very scarce in both regions. The nNOS‐ir cell bodies, even if present in all parts of the PVN, were mainly distributed in the lateral region of the nucleus (Fig. 6B). Interactions with kisspeptin fibers were very limited. Contrary to the AVP system, the OT cells were observed both in the lateral and medial PVN, including the ventro‐medial part (Fig. 6C). Interactions with kisspeptin fibers were possible both in the medial and lateral part. The TH‐ir cell bodies were also scattered within the PVN; however, they were particularly clustered in the ventro‐medial part, overlapping part of the denser innervation by kisspeptin fibers (Fig. 6D).
Discussion
This study, performed in CD1 mice, confirms previous data indicating that the kisspeptin system in rodents is mainly clustered in a rostral (RP3V) and caudal (ARC) group of neurons. We confirm also the presence of a strong dimorphism (more cells and fibers in females than in males) in the RP3V as well as in the ARC. Previous studies (Clarkson & Herbison, 2006; Clarkson et al. 2009; Lehman et al. 2013) described the presence of kisspeptin fibers in several nuclei, including the PVN, whereas anterograde and retrograde tracing in normal adult female mice or in transgenic female mice showed that these fibers arise from ARC and AVPV (Yeo & Herbison, 2011; Yip et al. 2015). In the present study we demonstrate, for the first time, that the kisspeptin innervation is covering the entire extension of the PVN (suggesting that this nucleus is a major target for the peptide action in addition to the GnRH system), is sexually dimorphic (with females having a denser innervation than males), is not homogenously distributed within the nucleus, and, in females, it changes according to the phases of the estrous cycle.
As described in previous studies in female rat (Smith et al. 2006), kiss‐ir of female CD1 mice changes during the estrous cycle in a different way in the RP3V and the ARC, showing the highest value in RP3V during estrus, when the immunoreactivity is lowest in ARC. Similar changes were observed in the PVN, showing a higher density of positive fibers during estrus. This suggests that the RP3V group could be the major source of the PVN kisspeptin fibers.
Kisspeptin neurons in RP3V and ARC have been directly related to the control of reproduction via the control of GnRH system (Roseweir & Millar, 2009; d'Anglemont de Tassigny & Colledge, 2010; Tsutsui et al. 2010; Navarro & Tena‐Sempere, 2011). In addition, both KISS1 and gonadotropin inhibitory hormone (GnIH) positive cells were described in the ARC, DMH and PVN nuclei of non‐human female primate, suggesting a possible role for kisspeptin in the regulation also of the GnIH system (Smith et al. 2010). It is known that other neuropeptides like Dyn and NkB have been implicated in the regulation of pulsatile GnRH neurosecretion (Lehman et al. 2010; Grachev et al. 2014); moreover, a higher co‐expression of kisspeptin and NkB within the ARC nucleus was previously described in mice both with ISH (Navarro et al. 2009) and immunofluorescence (Pineda et al. 2016). On the contrary, ISH showed that very few Kiss1 neurons in the AVPV co‐expressed NkB (Navarro et al. 2009). Our double‐label immunofluorescence for kisspeptin and NkB confirmed the co‐expression NkB/Kiss1 in ARC nucleus but showed a complete lack of NkB signal associated to kiss‐ir in the PVN. This confirms the hypothesis that the kisspeptin fibers observed in the PVN should arrive chiefly from AVPV and PeN.
Recent tract‐tracing studies in adult female mice demonstrated that only a subset of kisspeptin neurons are contacting the GnRH system (only ~36% of AVPV kisspeptin neurons are connected with GnRH neurons; Kumar et al. 2015; Yip et al. 2015), thus suggesting that a large part of the kisspeptin neuronal population could have other targets. The large, sexually dimorphic innervation of PVN is probably one of the major targets, even if it is not directly related to reproduction, sexual behavior or puberty control. In fact, PVN plays a major role in other neuro‐endocrine functions controlled by distinct neuronal subpopulations (Swanson & Sawchenko, 1980; Maniam & Morris, 2012; Bosch, 2013; Kovács, 2013; Handa & Weiser, 2014; Pyner, 2014; van Swieten et al. 2014; Sladek et al. 2015).
Therefore, the presence of positive fibers along the entire extension of the PVN suggests that kisspeptin could be implicated in the regulation of many of the physiological activities controlled by the PVN.
Our quantitative analysis performed at high enlargement magnification showed that, even if covering the entire nucleus, the innervation of mouse PVN by kisspeptin fibers was heterogeneous. In fact, the density of kisspeptin fibers was higher in the medial than lateral PVN. While the lateral part of the PVN contains magnocellular neurons chiefly projecting to the posterior pituitary (where they release OT and AVP into the blood), the medial part of the PVN is characterized by the presence of different types of parvocellular neurons that can be identified for the presence of several neurotransmitters, neuropeptides and enzymes involved in the synthesis of neurotransmitters (i.e. CRH; Wang et al. 2011; TRH; Kadar et al. 2010; TH; Ruggiero et al. 1984; nNOS; Gotti et al. 2004, 2005; AVP; Caldwell et al. 2008; somatostatin; Tan et al. 2013).
In this study we compared by double‐immunofluorescence the distribution of some of these PVN neuronal populations and that of kisspeptin fibers. On the basis of our results, we can assume that AVP‐ and nNOS‐containing neurons were not strongly related to kisspeptin system. Instead, the presence of several OT‐ and TH‐positive neurons in the medial PVN, where the concentration of kiss‐ir fibers is massive, is suggestive of a possible interrelation between these systems.
On the other hand, kisspeptin could play a role in the regulation of both AVP and OT neurons. In fact, in rat, Rao et al. (2011) showed that kisspeptin significantly increased AVP and OT mRNA expression and, very recently, an in situ hybridization study revealed that in rat diestrus female Kiss1r is co‐expressed in subpopulations of OT neurons of the medial part of the PVN (Higo et al. 2016). Moreover, in AVPV more than half of kiss‐ir neurons express also TH‐ir (Clarkson & Herbison, 2011).
A question about the putative functions of the kisspeptin innervation in the PVN arises from the literature on the Kiss1r distribution within the mammalian brain. To our knowledge only three studies detailed the neuroanatomical distribution of Kiss1r. One was performed with low‐resolution autoradiography for GPR54 mRNA (Lee et al. 1999): the figures are showing the presence of the mRNA in the periventricular hypothalamus, but no details are provided to eventually identify the signal within the PVN. A second study (Herbison et al. 2010) used a transgenic GPR54 LacZ knock‐in mouse model to detail a map of GPR54‐expressing elements within the mouse brain. In this study, in addition to regions where GnRH neurons are located, the authors reported the existence of several nuclei expressing GPR54 LacZ where no kisspeptin fibers have been detected (i.e. hippocampus, supramamillary and pontine nuclei), and regions (as the PVN or the supraoptic nucleus) where the transgene was not expressed even if kisspeptin fibers have been described. Finally, a recent in situ hybridization study detailed the presence of Kiss1r in several hypothalamic and extrahypothalamic regions including the PVN (Higo et al. 2016). Therefore, there is some disagreement on the presence of Kiss1r especially in the PVN that may depend on technical issues, but could also indicate the existence of other ligands for Kiss1r and other receptors for kisspeptin (Herbison et al. 2010).
Kisspeptin belongs to the family of RF‐amide peptides, and it shows high binding activity to neuropeptide FF receptors (FF1 and FF2, also known as GPR74 and 147; Oishi et al. 2011). The distribution of these two receptors has been studied by autoradiography for mRNA in the rat brain (Liu et al. 2001); in particular, FF1 is widely distributed within the hypothalamus, including the PVN. Thus, it is possible that a subpopulation of kisspeptin neurons (mainly from the rostral hypothalamus) project to the PVN to activate the FF1 receptor. This could also be one possible explanation for the anorexigenic effect of centrally injected kisspeptin (Stengel et al. 2011).
In conclusion, we demonstrated that, in CD1 mice, the kisspeptin fibers cover the entire extension of the PVN and that this innervation is sexually dimorphic (with females having a denser innervation than males). Moreover, we confirmed that kisspeptin system in ARC was sexually dimorphic also in CD1 mice. In addition, our data show a heterogeneity in the innervation of the PVN by the kisspeptin, with changes during the estrous cycle (higher density of positive fibers during the estrus) and, finally, they suggest that the source of this innervation may be located in the rostral group of kisspeptin neurons.
Author contributions
MM performed experiments, analyzed data and wrote the paper. AF, DM and GP performed experiments and analyzed data. GCP and SG designed experiments, wrote and supervised the paper.
Acknowledgements
This work has been supported by Fondazione San Paolo (Neuroscience Project), University of Torino and Cavalieri‐Ottolenghi Foundation, Orbassano, Italy. The authors want to acknowledge Drs A. Caraty, I. Franceschini and M. Keller (INRA, Tours, France) that kindly supplied the #566 and AC053 antibody.
References
- Adachi S, Yamada S, Takatsu Y, et al. (2007) Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53, 367–378. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Colledge WH (2010) The role of kisspeptin signaling in reproduction. Physiology (Bethesda) 25, 207–217. [DOI] [PubMed] [Google Scholar]
- Bean JC, Lin TW, Sathyamurthy A, et al. (2014) Genetic labeling reveals novel cellular targets of schizophrenia susceptibility gene: distribution of GABA and non‐GABA ErbB4‐positive cells in adult mouse brain. J Neurosci 34, 13549–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, et al. (2005) Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146, 1650–1673. [DOI] [PubMed] [Google Scholar]
- Bosch OJ (2013) Maternal aggression in rodents: brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc Lond B Biol Sci 368, 20130085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Ohsawa M, et al. (2005) KiSS‐1 expression and metastin‐like immunoreactivity in the rat brain. J Comp Neurol 481, 314–329. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, et al. (2008) Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 84, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano J, Bentsen A, Mikkelsen J, et al. (2010) Kisspeptins: bridging energy homeostasis and reproduction. Brain Res 1364, 129–138. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE (2006) Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin‐releasing hormone neurons. Endocrinology 147, 5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE (2011) Dual phenotype kisspeptin‐dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin‐releasing hormone neurones. J Neuroendocrinol 23, 293–301. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Colledge WH, et al. (2009) Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 21, 673–682. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Weiss S (1999) Generation of tyrosine hydroxylase‐producing neurons from precursors of the embryonic and adult forebrain. J Neurosci 19, 4484–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decourt C, Tillet Y, Caraty A, et al. (2008) Kisspeptin immunoreactive neurons in the equine hypothalamus: interactions with GnRH neuronal system. J Chem Neuroanat 36, 131–137. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ, Samson WK (2008) The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH Jr, Koppel G, et al. (1997) Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci 17, 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, et al. (2006) Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co‐express estrogen receptor alpha. Neurosci Lett 401, 225–230. [DOI] [PubMed] [Google Scholar]
- Franceschini I, Yeo SH, Beltramo M, et al. (2013) Immunohistochemical evidence for the presence of various kisspeptin isoforms in the mammalian brain. J Neuroendocrinol 25, 839–851. [DOI] [PubMed] [Google Scholar]
- Gillespie JI, Markerink‐van Ittersum M, de Vente J (2005) Expression of neuronal nitric oxide synthase (nNOS) and nitric‐oxide‐induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321, 341–351. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL (2007) The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80, 84–97. [DOI] [PubMed] [Google Scholar]
- Gotti S, Chiavegatto S, Sica M, et al. (2004) Alteration of NO‐producing system in the basal forebrain and hypothalamus of Ts65Dn mice: an immunohistochemical and histochemical study of a murine model for Down syndrome. Neurobiol Dis 16, 563–571. [DOI] [PubMed] [Google Scholar]
- Gotti S, Sica M, Viglietti Panzica C, et al. (2005) Distribution of nitric oxide sythase immunoreactivity in the mouse brain. Microsc Res Tech 68, 13–35. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, et al. (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145, 4073–4077. [DOI] [PubMed] [Google Scholar]
- Grachev P, Li XF, Hu MH, et al. (2014) Neurokinin B signaling in the female rat: a novel link between stress and reproduction. Endocrinology 155, 2589–2601. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti MA, et al. (2007) Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, et al. (2005) Activation of gonadotropin‐releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25, 11 349–11 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa R, Weiser M (2014) Gonadal steroid hormones and the hypothalamo‐pituitary‐adrenal axis. Front Neuroendocrinol 35, 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE (2008) Estrogen positive feedback to gonadotropin‐releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, et al. (2010) Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin‐releasing hormone neurons. Endocrinology 151, 312–321. [DOI] [PubMed] [Google Scholar]
- Higo S, Honda S, Iijima N, et al. (2016) Mapping of kisspeptin receptor mRNA in the whole rat brain and its co‐localization with oxytocin in the paraventricular nucleus. J Neuroendocrinol 28, 10.1111/jne.12356. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, et al. (2004) Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS‐1 mRNA in the male rat. Neuroendocrinology 80, 264–272. [DOI] [PubMed] [Google Scholar]
- Kadar A, Sanchez E, Wittmann G, et al. (2010) Distribution of hypophysiotropic thyrotropin‐releasing hormone (TRH)‐synthesizing neurons in the hypothalamic paraventricular nucleus of the mouse. J Comp Neurol 518, 3948–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS (2009) Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides 30, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, et al. (2007) Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148, 1774–1783. [DOI] [PubMed] [Google Scholar]
- Knoll JG, Clay CM, Bouma GJ, et al. (2013) Developmental profile and sexually dimorphic expression of kiss1 and kiss1r in the fetal mouse brain. Front Endocrinol (Lausanne) 4, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, et al. (2001) The metastasis suppressor gene KiSS‐1 encodes kisspeptins, the natural ligands of the orphan G protein‐coupled receptor GPR54. J Biol Chem 276, 34 631–34 636. [DOI] [PubMed] [Google Scholar]
- Kovács K (2013) CRH: the link between hormonal‐, metabolic‐ and behavioral responses to stress. J Chem Neuroanat 54, 25–33. [DOI] [PubMed] [Google Scholar]
- Kumar D, Candlish M, Periasamy V, et al. (2015) Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology 156, 32–38. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O'Neill GP, et al. (1999) Discovery of a receptor related to the galanin receptors. FEBS Lett 446, 103–107. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL (2010) Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin‐releasing hormone secretion. Endocrinology 151, 3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Hileman SM, Goodman RL (2013) Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol 784, 27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guan XM, Martin WJ, et al. (2001) Identification and characterization of novel mammalian neuropeptide FF‐like peptides that attenuate morphine‐induced antinociception. J Biol Chem 276, 36 961–36 969. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ (2012) The link between stress and feeding behaviour. Neuropharmacology 63, 97–110. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, et al. (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp 4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Tena‐Sempere M (2011) Kisspeptins and the neuroendocrine control of reproduction. Front Biosci (Schol Ed) 3, 267–275. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, et al. (2009) Regulation of gonadotropin‐releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29, 11 859–11 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S, Misu R, Tomita K, et al. (2011) Activation of neuropeptide FF receptors by kisspeptin receptor ligands. ACS Med Chem Lett 2, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard A, Tena‐Sempere M, Franceschini I, et al. (2013) Comparative analysis of kisspeptin‐immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides 45, 85–90. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press. [Google Scholar]
- Pierman S, Sica M, Allieri F, et al. (2008) Activational effects of estradiol and dihydrotestosterone on social recognition and the arginine‐vasopressin immunoreactive system in male mice lacking a functional aromatase gene. Horm Behav 54, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Sabatier N, Ludwig M, et al. (2016) A direct neurokinin B projection from the arcuate nucleus regulates magnocellular vasopressin cells of the supraoptic nucleus. J Neuroendocrinol 28, 10.1111/jne.12342. [DOI] [PubMed] [Google Scholar]
- Pinilla L, Aguilar E, Dieguez C, et al. (2012) Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev 92, 1235–1316. [DOI] [PubMed] [Google Scholar]
- Plumari L, Viglietti Panzica C, Allieri F, et al. (2002) Changes in the arginine‐vasopressin immunoreactive systems in male mice lacking a functional aromatase gene. J Neuroendocrinol 14, 971–978. [DOI] [PubMed] [Google Scholar]
- Poling MC, Quennell JH, Anderson GM, et al. (2013) Kisspeptin neurones do not directly signal to RFRP‐3 neurones but RFRP‐3 may directly modulate a subset of hypothalamic kisspeptin cells in mice. J Neuroendocrinol 25, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyner S (2014) The paraventricular nucleus and heart failure. Exp Physiol 99, 332–339. [DOI] [PubMed] [Google Scholar]
- Rao YS, Mott NN, Pak TR (2011) Effects of kisspeptin on parameters of the HPA axis. Endocrine 39, 220–228. [DOI] [PubMed] [Google Scholar]
- Rometo AM, Krajewski SJ, Voytko ML, et al. (2007) Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92, 2744–2750. [DOI] [PubMed] [Google Scholar]
- Roseweir AK, Millar RP (2009) The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update 15, 203–212. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, et al. (2003) Hypogonadotropic hypogonadism due to loss of function of the KiSS1‐derived peptide receptor GPR54. Proc Natl Acad Sci USA 100, 10 972–10 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero DA, Baker H, Joh TH, et al. (1984) Distribution of catecholamine neurons in the hypothalamus and preoptic region of mouse. J Comp Neurol 223, 556–582. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, et al. (2003) The GPR54 gene as a regulator of puberty. N Engl J Med 349, 1614–1627. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, et al. (2005) Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102, 2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek CD, Michelini LC, Stachenfeld NS, et al. (2015) Endocrine‐autonomic linkages. Comp Physiol 5, 1281–1323. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, et al. (2005a) Regulation of KiSS‐1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, et al. (2005b) Differential regulation of KiSS‐1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146, 2976–2984. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, et al. (2006) Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26, 6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, et al. (2007) KiSS‐1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Smith JT, Shahab M, Pereira A, et al. (2010) Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non‐human primate. Biol Reprod 83, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Wang L, Goebel‐Stengel M, et al. (2011) Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. NeuroReport 22, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31, 410–417. [DOI] [PubMed] [Google Scholar]
- van Swieten MM, Pandit R, Adan RA, et al. (2014) The neuroanatomical function of leptin in the hypothalamus. J Chem Neuroanat 61–62, 207–220. [DOI] [PubMed] [Google Scholar]
- Tan HY, Huang L, Simmons D, et al. (2013) Hypothalamic distribution of somatostatin mRNA expressing neurones relative to pubertal and adult changes in pulsatile growth hormone secretion in mice. J Neuroendocrinol 25, 910–919. [DOI] [PubMed] [Google Scholar]
- Taziaux M, Swaab DF, Bakker J (2012) Sex differences in the neurokinin B system in the human infundibular nucleus. J Clin Endocrinol Metab 97, E2210–E2220. [DOI] [PubMed] [Google Scholar]
- Tena‐Sempere M (2006) KiSS‐1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology 83, 275–281. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Kriegsfeld LJ, et al. (2010) Discovery and evolutionary history of gonadotrophin‐inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. J Neuroendocrinol 22, 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietti‐Panzica C, Aste N, Balthazart J, et al. (1994) Vasotocinergic innervation of sexually dimorphic medial preoptic nucleus of the male Japanese quail: influence of testosterone. Brain Res 657, 171–184. [DOI] [PubMed] [Google Scholar]
- Wang L, Goebel‐Stengel M, Stengel A, et al. (2011) Comparison of CRF‐immunoreactive neurons distribution in mouse and rat brains and selective induction of Fos in rat hypothalamic CRF neurons by abdominal surgery. Brain Res 1415, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, et al. (1986) Use of cryoprotectant to maintain long‐term peptide immunoreactivity and tissue morphology. Peptides 7, 155–159. [DOI] [PubMed] [Google Scholar]
- Yeo SH, Herbison AE (2011) Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152, 2387–2399. [DOI] [PubMed] [Google Scholar]
- Yip SH, Boehm U, Herbison AE, et al. (2015) Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin‐releasing hormone (GnRH) neurons in the mouse. Endocrinology 156, 2582–2594. [DOI] [PubMed] [Google Scholar]


