Abstract
The plantar calcaneal spur (PCS) is a bony outgrowth from the calcaneal tuberosity and has been studied using various methods including cadavers, radiography, histology and surgery. However, there are currently a number of discrepancies in the literature regarding the anatomical relations, histological descriptions and clinical associations of PCS. Historically, authors have described the intrinsic muscles of the foot and/or the plantar fascia as attaching to the PCS. In this article we review the relationship between the PCS and surrounding soft tissues as well as examining the histology of the PCS. We identify a number of key associations with PCS, including age, weight, gender, arthritides, plantar fasciitis and foot position; these factors may function as risk factors in PCS formation. The etiology of these spurs is a contentious issue and it has been explained through a number of theories including the degenerative, inflammatory, traction, repetitive trauma, bone‐formers and vertical compression theories. We review these and finish by looking clinically at the evidence that PCS causes heel pain.
Keywords: bony outgrowth, calcaneal tuberosity, plantar calcaneal spur
Introduction
The plantar calcaneal spurs (PCS) are a bony outgrowth from the calcaneal tuberosity and has been studied via numerous methods including cadavers, radiography, histology and during surgery (Abreu et al. 2003) (Fig. 1). PCS are typically described as bony outgrowths arising just anterior to the medial process of the calcaneal tuberosity, but there is no consistent definition in the literature (Chang & Miltner, 1934; Gerster et al. 1977; Tountas & Fornasier, 1996; Abreu et al. 2003; Franson, 2008). Some authors define them as projections of larger than 1 or 2 mm, while others use microscopy or subjective assessment (Resnick et al. 1977; Prichasuk & Subhadrabandhu, 1994; Kumai & Benjamin, 2002; Abreu et al. 2003). In the young to middle‐aged population PCS prevalence is 11–21%. This is constant across various ethnicities with rates of 11% in India, 13% in Ireland, 15% in Zimbabwe, 16% in Thailand, 17% in Europe and 21% in America (Banadda et al. 1992; Prichasuk & Subhadrabandhu, 1994; Barrett et al. 1995; Riepert et al. 1995; Kullar et al. 2014; Moroney et al. 2014, 1995). This rate increases with age to 55% in those over 62, to 59–78% in those with current or previous heel pain, and up to 81% in those with osteoarthritis (Table 1; Tanz, 1963; Bassiouni, 1965; Gerster et al. 1977; Prichasuk & Subhadrabandhu, 1994; Barrett et al. 1995; Riepert et al. 1995; Wainwright et al. 1995; Li & Muehleman, 2007; Menz et al. 2008). In the past, gonorrhea, syphilis, hereditary factors, metabolic disturbances and gout were all proposed to be related to the development of PCS; these have subsequently been discredited (Winthrop, 1909; Griffith, 1910; Roland, 1912; Waechter & Sonnenschein, 1915; Paul & Henry, 1916; Von Lackum & Palomeque, 1930; Chang & Miltner, 1934; Steindler & Smith, 1938; Gould, 1942). A number of robust associations do exist, however; these are potential risk factors for spur development and will be dealt with in this review along with how they may relate to PCS etiology.
Figure 1.
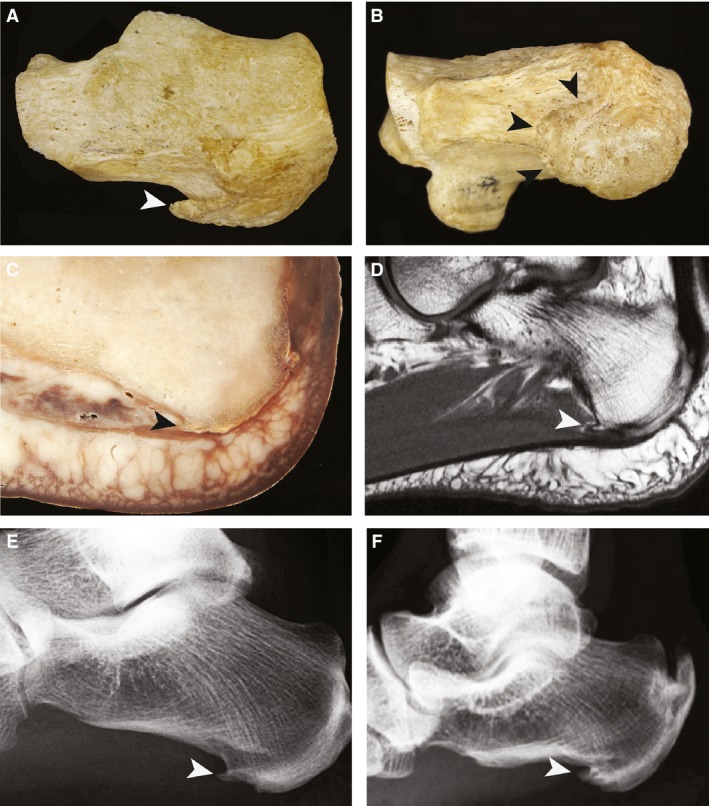
The black and white arrowheads indicate the PCS spur, (A) skeleton lateral view, (B) skeleton inferior view, (C) sagittal plastinated medial cadaver transection (E12 technique), (D) sagittal medial MRI, (E) lateral foot X‐ray of simple spur, (F) lateral foot X‐ray of irregular spur.
Table 1.
Prevalence of PCS in various populations
| Prevalence of PCS | |
|---|---|
| Normal Population | 16% (n = 400; Prichasuk & Subhadrabandhu, 1994), 16% (n = 1027; Riepert et al. 1995), 21% (n = 200; Barrett et al. 1995), 27% (n = 461; Rubin & Witten, 1963) |
| Heel pain | 59% (n = 148; Lapidus & Guidotti, 1965), 66% (n = 82; Prichasuk & Subhadrabandhu, 1994) |
| Age | 55% (> 62, n = 216; Menz et al. 2008), 98% with PCS > 40 (n = 425; Rubin & Witten, 1963), 89.5% aged 41–50 vs. 60% aged 21–40 (OA, n = 168; Bassiouni, 1965) |
| Weight | 46% with PCS overweight (n = 425; Rubin & Witten, 1963), 70% XY, most XX with PCS overweight (n = 37; Duvries, 1957) |
| Foot pronation | 62% with PCS and 81% with symptomatic PCS (n = 1000; Shama et al. 1983) |
| Arthritides | 92% with PCS had osteophytes vs. 23% without PCS (n = 425; Rubin & Witten, 1963) |
| Ankylosing spondylitis | 20% (n = 80; Resnick et al. 1977), 28% (n = 35; Gerster et al. 1977), 29% (n = 38; Mason et al. 1959) |
| Osteoarthritis | 56% (n = 70; Gerster et al. 1977), 80% (n = n = 119; Menz et al. 2008), 81% (n = 168; Bassiouni, 1965) |
| Psoriatic arthritis | 25% (n = 52; Resnick et al. 1977) |
| Rheumatoid arthritis | 22% (n = 282; Bassiouni, 1965), 23% (n = 128; Resnick et al. 1977), 28% (n = 100; Gerster et al. 1977), 43% (n = 81; Mason et al. 1959) |
| Plantar fasciitis | 67% with PCS vs. 2.5% in controls (n = 77; Wainwright et al. 1995), 89% with PCS vs. 32% in controls (n = 19; Johal & Milner, 2012) |
Anatomy of PCS
PCS originate from the calcaneal tuberosity, located on the posterior plantar surface of the calcaneus. The majority of PCS arise from the medial process of the tuberosity, but they can also originate from the lateral processes and the sulcus (Roland, 1912; Duvries, 1957; Rubin & Witten, 1963; Tanz, 1963). The anatomical appearance of PCS is highly variable but can be broadly categorized as simple or irregular (Mason et al. 1959; Rubin & Witten, 1963; McCarthy & Gorecki, 1979). Simple PCS are triangular structures that taper to a sharp point from a broad base (Duvries, 1957; Brody, 1962; McCarthy & Gorecki, 1979). They have defined smooth sclerotic cortical borders and well developed trabeculae (Rubin & Witten, 1963; Resnick et al. 1977). In contrast, irregular spurs have poorly defined borders and no clear trabeculae (Rubin & Witten, 1963).
It is important to understand the anatomy of the foot to understand PCS. The plantar fascia (PF) is a critical structure in this regard; it consists of a dense connective tissue arising from the medial process of the calcaneal tuberosity and inserting into the digits of the foot (Barrett et al. 1995; Wearing et al. 2006). The PF functions to maintain the medial longitudinal arch of the foot as well as absorbing forces placed on the foot across the mid‐tarsal joints (Schepsis et al. 1991). The PF contains fibrocytes and is known not only to transmit energy passively but also to respond to the requirements placed on it by changing the extracellular matrix composition (Wearing et al. 2006). The PF is significantly thicker in patients with PCS than in those without, 4.95–6.1 mm vs. 3.22–4.0 mm (Berkowitz et al. 1991; Kumai & Benjamin, 2002; Abreu et al. 2003). The relationship between the PCS and the PF is highly variable and the PF may be associate with none, part or all of the spur (Barrett et al. 1995; Li & Muehleman, 2007). Some authors describe the PCS as embedded within the PF, whereas other authors maintain the PF does not attach to the PCS, but rather remains plantar to it (Duvries, 1957; McCarthy & Gorecki, 1979; Forman & Green, 1990; Kumai & Benjamin, 2002; Smith et al. 2007). One large study showed histologically that the PF inserted into the periosteum of the spur in 46% of cases and that this could occur anywhere along the length of the spur (Li & Muehleman, 2007).
The first layer of the intrinsic muscles of the foot consists of the abductor hallucis (AH), flexor digitorum brevis (FDB) and abductor digiti minimi (ADM); the PCS may be closely associated with each of these (Forman & Green, 1990; Li & Muehleman, 2007). The second layer consists of quadratus plantae and it attaches deep to the PCS (Forman & Green, 1990). Tanz (1963) was one of the earliest authors to show that PCS can arise from muscle rather than from the PF itself. The FDB attaches to the medial process of the calcaneal tuberosity or the apex of the PCS if present, anterior and superior to the attachment of the PF (Forman & Green, 1990). The FDB inserts into the periosteum of the PCS along with the PF in 22% of cases, most commonly at the spurs distal tip and inferior aspects (14%), but also at the superior aspect (8%; Li & Muehleman, 2007). The PCS is oriented along the axis of the PF and FDB approximately half of the time (Abreu et al. 2003). The ADM also attaches directly to the PCS, although not as extensively as the FDB does; the PCS is aligned with the ADM 20–50% of the time (Bassiouni, 1965; Abreu et al. 2003; Smith et al. 2007). The AH arises just medial to the FDB origin and has been hypothesized to contribute to PCS formation (Forman & Green, 1990). PCS have also been shown to arise from around the calcaneal insertion point of the long plantar ligament (Bassiouni, 1965; Sebes, 1989).
Histology of PCS
Histological analysis shows that the PCS consists of a core of mature lamellar bone and demonstrates evidence of degeneration and fibro‐cartilaginous proliferation, along with one or more of intramembranous, chondroidal and endochondral ossification occurring at the surface (Tountas & Fornasier, 1996; Kumai & Benjamin, 2002; Smith et al. 2007). The histological specimens are extremely heterogeneous, however, and there is no robust correlation between histology and clinical outcomes (Tountas & Fornasier, 1996). The irregular surface of PCS and the presence of osteoclasts suggest continuous active bone turnover; however, there is disagreement in studies regarding the type of bone‐forming activity occurring, as well as its location (Tountas & Fornasier, 1996; Kumai & Benjamin, 2002; Smith et al. 2007). There is some evidence that larger spurs show a greater level of cortical thickening compared with smaller spurs; however, this is disputed (Kumai & Benjamin, 2002; Smith et al. 2007). Variable parts of the spur are covered with a fibrous connective tissue layer which is richly innervated and vascularized (Kumai & Benjamin, 2002; Abreu et al. 2003; Li & Muehleman, 2007; Smith et al. 2007). One large study found this fibrocartilage was present on all spurs, at the inferior aspect in 38%, the inferior aspect and distal tip in 30%, and at the inferior aspect, distal tip and superior aspect in 32% (Li & Muehleman, 2007).
Associations with PCS
PCS and age
The prevalence of PCS increases in older age groups (Rubin & Witten, 1963; Bassiouni, 1965; Gerster et al. 1977; Banadda et al. 1992; Prichasuk & Subhadrabandhu, 1994; Riepert et al. 1995, 1963). One study estimated the prevalence of PCS in individuals over 62 at 55%, whereas in another study, 98.4% of those with a PCS were over 40 (Rubin & Witten, 1963; Menz et al. 2008). Older people also have changes to their gait pattern which may play a role in PCS development. These include a greater relative contact time of both the heel and mid foot, along with a reduced step length (Scott et al. 2007).
PCS and weight
The prevalence of PCS increases with weight (Duvries, 1957; Rubin & Witten, 1963; Smith et al. 2007; Menz et al. 2008). Rubin & Witten (1963) found that 46% of those with PCS were overweight compared with 27% in the control group, and Moroney et al. (2014) found 82% of those with PCS were overweight or obese. Furthermore, after adjusting for age and gender, those with PCS were 6.9 times more likely to be obese compared with those without PCS (Menz et al. 2008). One study found that those with PCS were four times as likely to have diabetes than those without PCS; however, it is unclear if this is an independent risk factor (Moroney et al. 2014).
PCS and foot position
There is a statistically significant correlation between foot pronation and the development of PCS, with 62% of patients with a spur and 81% with a painful spur having a pronated foot radiographically (Shama et al. 1983). One study showed that individuals with plantar heel pain had a higher incidence of PCS compared with controls (65.9 vs. 15.5%) and that they also had a lower calcaneal pitch, 15.9° vs. 20.5° (Prichasuk & Subhadrabandhu, 1994). This is consistent with observational data that patients with PCS generally have flat feet (Duvries, 1957). Other studies, however, have found no association between foot posture and PCS (Lapidus & Guidotti, 1965; Menz et al. 2008).
PCS and gender
There is discrepancy in the literature about whether females have a higher incidence of PCS. Papers which focus on older populations generally find no significant difference in incidence (Bassiouni, 1965; Menz et al. 2008). However, papers with a younger population demonstrate that females have higher incidence than men. Moroney et al. (2014) found, at an average age of 37.9, incidences of 17 vs. 9%. Similarly, Riepert et al. (1995) reported incidences of 16.3 vs. 6.5% and Banada et al. (1992) incidences of 18 vs. 13%. In one study that broke the population down into nine age cohorts, PCS was reported to occur more commonly in women under 49 years of age than in men. They proposed that this age‐dependent difference may be due to the altered foot biomechanics of wearing high‐heeled shoes (Toumi et al. 2014; Yung‐Hui & Wie‐Hsien, 2005).
PCS and arthritides
Spurs occur commonly in all arthritides, with estimates as high as 80% in osteoarthritis and 72% in rheumatology patients over 61 years (Davis & Blair, 1950; Mason et al. 1959; Bassiouni, 1965; Gerster et al. 1977; Resnick et al. 1977; Menz et al. 2008; Weiss, 2012). Individuals with a PCS are 3–10 times more likely to have at least one area of osteoarthritis compared with the normal population and 92% of those with PCS have osteophytes at other points in their body vs. 23% in those without PCS (Rubin & Witten, 1963; Moroney et al. 2014). It has been hypothesized that the gross appearance of PCS may represent the underlying pathological condition, but these relationships are not exclusive or diagnostic (Roland, 1912; Rubin & Witten, 1963; Sebes, 1989).
PCS and plantar fasciitis
PCS are present in 45–85% of those with plantar fasciitis; they also share a number of risk factors such as obesity and advancing age, suggesting that the two may be linked etiologically (Sadat‐Ali, 1998; Gibbon & Long, 1999; Ozdemir et al. 2004; Irving et al. 2006; Levy et al. 2006; Osborne et al. 2006;Menz et al. 2008). Plantar fasciitis is hypothesized to be due to mechanical overload of the PF resulting in microtears; the repeated trauma from heel strike inhibits the reparative response and results in a chronic fascial inflammatory condition characterized by remodeling, fibrosis and even ossification at the fascial insertion point on the calcaneus (Kosmahl & Kosmahl, 1987; Schepsis et al. 1991; Wearing et al. 2006). However, histologically there is little evidence of an active inflammatory infiltrate being present, but studies have found an increase in mucoid ground substance, collagen degeneration, fibrogenic hyperplasia and calcification, suggesting that this condition is more of a degenerative fasciosis rather than an inflammatory fasciitis (Schepsis et al. 1991; Delmi et al. 1995; Mosier et al. 1999; Lemont et al. 2003).
Etiology of PCS
A growing number of cases report both symptomatic and asymptomatic posteriorly directed pediatric calcaneal spurs, suggesting that at least in a minority of cases PCS may represent variations in the normal development of the calcaneus (Robinson, 1976; Wiechen, 1987; Carlı et al. 2013). The etiology of the remaining acquired PCS is still not completely clear; however, several theories have been proposed. Traditionally it was hypothesized that PCS occur via repetitive stress/traction of the PF or the intrinsic musculature at their insertion into the calcaneus. This then results in subsequent inflammation and spur development (Roland, 1912; Duvries, 1957; McCarthy & Gorecki, 1979; Forman & Green, 1990; Prichasuk & Subhadrabandhu, 1994; Abreu et al. 2003). The PF tension is increased when the medial longitudinal arch is lowered; furthermore, an increase in bodyweight correlates with greater pressures during walking (determination coefficient 0.66) with the largest changes seen in the longitudinal arch pressure (Kogler et al. 1996; Onwuanyi, 2000; Hills et al. 2001). This is consistent with PCS being associated with obesity. Electron microscopy of two PCS revealed that PCS fibers, which form as a result of stress, loosely align along the line of traction that the PF and intrinsic foot musculature exert (McCarthy & Gorecki, 1979). PCS have also been proposed to be part of the normal process of aging, with a general tendency toward ossification of ligaments, such as the long plantar ligament, and tendons where they insert into bone (Mason et al. 1959; Lapidus & Guidotti, 1965; McCarthy & Gorecki, 1979). In opposition to this theory, Achilles spurs, which are known to occur as the result of traction, are positioned within the Achilles tendon; however, over half of PCS are not within the PF or the intrinsic muscles of the foot but rather are surrounded by loose connective tissue (Kumai & Benjamin, 2002; Li & Muehleman, 2007). Also, despite PF release and spur excision, recurrence rates of 31–50% were found within 9 months, suggesting a different etiology (Chang & Miltner, 1934; Tountas & Fornasier, 1996).
A number of authors believe inflammation is important in PCS formation, either occurring secondary to a stress injury or as the natural progression of plantar fasciitis (Mason et al. 1959; Gerster et al. 1977; Sebes, 1989; Abreu et al. 2003). It has been suggested that the inflammation process results in a localized proliferation around the PF origin and irregular spur formation (Duvries, 1957; Mason et al. 1959). Irregular spurs also occur more commonly on the plantar calcaneal aspect than on the posterior aspect, consistent with a natural evolution of fasciitis (Gerster et al. 1977). PCS occurred in 89% of patients with plantar fasciitis compared with 32% in age‐ and gender‐matched controls (Johal & Milner, 2012). There is also histological evidence of degenerative changes in the plantar fascia enthesis which may contribute to PCS formation (Kumai & Benjamin, 2002; Abreu et al. 2003). MRI analysis, however, showed inflammatory changes in only 8% of cases and histological analysis of both cadaveric and surgical specimens revealed no evidence of concurrent inflammation (Tountas & Fornasier, 1996; Abreu et al. 2003).
More recently, several authors have suggested that PCS may be an adaptive response to repetitive, vertically orientated forces (Kumai & Benjamin, 2002; Li & Muehleman, 2007; Menz et al. 2008). Larger studies analyzing the trabecular pattern present in PCS show it to be predominantly perpendicular to the long axis of the spur and the weight‐bearing surface (Kumai & Benjamin, 2002; Li & Muehleman, 2007). The calcaneus transmits the majority of the bodyweight from the talus to the ground. Wolf's law states that bone growth is dynamic and adapts to loads placed on it and this trabecular pattern of growth in spurs is consistent with a vertically oriented force vector (Li & Muehleman, 2007). The previously mentioned fibrocartilage associated with the spur is thought to serve the same function as articular or fibrocartilage in a weight‐bearing joint, buffering the forces transmitted to the surrounding tissue and muscle (Kumai & Benjamin, 2002; Li & Muehleman, 2007). In the same way that osteophytes develop to alter the load placed on synovial joints when they have been compromised by disease or injury, PCS may represent a comparable adaptation to mechanical stressors to reduce the risk of failure at the PF calcaneal insertion site or to protect the calcaneus against microfractures (Kumai & Benjamin, 2002; Benjamin et al. 2006). The association of PCS with arthritides and obesity further supports this theory (Rubin & Witten, 1963; Rogers et al. 1997). An increase in bodyweight may also accelerate the natural degenerative processes of age‐related stiffening on the heel pad, inhibiting its ability to effectively dampen the force of impacts (Hsu et al. 1998; Onwuanyi, 2000; Menz et al. 2008). Patients with less soft tissue under the heel develop greater plantar pressures while walking (Morag & Cavanagh, 1999). The increased rate of PCS with age also supports this theory, as there is a relationship between age and increased heel pressure, independent of structural and functional variables (Morag & Cavanagh, 1999). PCS development may be due to repetitive ground reactive forces generating stress and microtrauma, resulting ultimately in pathophysiological bone development (Moretti et al. 2006; Li & Muehleman, 2007). Historically, Roland hypothesized that the epiphysis of the calcaneus extends down to the plantar aspect and that repetitive trauma results in PCS formation (Roland, 1912). Studies have shown that a single traumatic injury is insufficient to initiate PCS formation, but repetitive trauma with low forces can cause histological changes and as the impact force is increased, fewer repetitions are required (Tanz, 1963; Brand, 2006). A number of both structural and functional factors determine the load exerted on the calcaneal process during walking and may serve as risk factors in developing PCS. These factors include gait abnormalities such as excessive medial loading of the calcaneus during foot pronation, the area, thickness and elasticity of the plantar heel fat pad, the longitudinal arch structure, linear kinematics, the individual's weight and age, as well as the shoes worn (Perry, 1983; Hsu et al. 1998; Morag and Cavanagh, 1999; Ozdemir et al. 2004; Brand, 2006; Li & Muehleman, 2007). Finally, the bone‐formers theory suggests that certain people have a genetic predisposition to form new bone in response to mechanical stressors (Rogers et al. 1997). This explains why some individuals will develop spurs whereas others, when subjected to the same or even greater stress, will not (Li & Muehleman, 2007). Thus whether a spur is formed may be a result of both the stress experienced, based on a number of functional and structural factors, and the individual's genetic predisposition to forming bone.
PCS and heel pain
Historically, a causative relationship was assumed between PCS and heel pain (Waechter & Sonnenschein, 1915; Chang & Miltner, 1934; Duvries, 1957; Lapidus & Guidotti, 1965; McCarthy & Gorecki, 1979). Observational studies showed that the point of maximal tenderness was directly underneath the spur and that those with PCS were 4.6 times more likely to have a current or previous history of heel pain (Brody, 1962; Menz et al. 2008). The relationship is not this simple, however, as PCS are present in 10–63% of asymptomatic controls (Tanz, 1963; Lapidus & Guidotti, 1965; Barrett et al. 1995; Ozdemir et al. 2004; Osborne et al. 2006). One study showed that 45% of the heels analyzed were either painful with no PCS or had a PCS but were not painful (Lapidus & Guidotti, 1965). Furthermore, the outcome of PF release and spur excision is not based solely on PCS recurrence and some patients have ongoing pain, suggesting that PCS are not always the primary cause of pain (Hertzler, 1926; Tountas & Fornasier, 1996).
There is, however, a correlation between heel pain and PCS, and although PCS do occur in asymptomatic people, they occur at higher rates in those who are symptomatic (Vyce et al. 2010; Moroney et al. 2014). In those who do experience pain with PCS, this has been hypothesized to be due to a wide range of factors, including the size, shape, nerve compression and associated inflammation, as well as whether the PCS is weight‐bearing or there is a micro fracture present (Duvries, 1957; Tanz, 1963; Wainwright et al. 1995; Sadat‐Ali, 1998; Smith et al. 2007). Two meta‐analyses have revealed body mass indext (BMI) and PCS are two factors associated with chronic plantar heel pain. There is also some evidence of an association with age, decreased dorsiflexion at the ankle and prolonged periods of standing (Irving et al. 2006; McMillan et al. 2009). PCS has also been hypothesized to decrease the elasticity of the heel fat pad, an important factor in the etiology of heel pain (Baxter & Zingas, 1995; Ozdemir et al. 2004). The inferior calcaneal nerve, a branch of the lateral plantar nerve, is susceptible to compression from PCS, as it passes between the intrinsic muscles of the foot and the calcaneal tuberosity to innervate the ADM muscle (Tanz, 1963; Forman & Green, 1990; Delfaut et al. 2003; Chundru et al. 2008). Nerve entrapment (Baxter's neuropathy) results in ADM atrophy and heel pain (Tanz, 1963; Louisia & Masquelet, 1999). There is a significant association between ADM atrophy and calcaneal spurs, with an odds ratio of 3.6 after multivariate logistic regression (Chundru et al. 2008). Branchinng off from the inferior calcaneal nerve there is also a calcaneal branch which supplies the calcaneal tuberosity and this has also been shown to be susceptible to PCS compression and may result in heel pain (Rondhuis & Huson, 1986; Louisia & Masquelet, 1999). Inflammation in the soft tissue surrounding the spur may be important in determining whether the spur is painful or not (Li & Muehleman, 2007). In one study, individuals with a painful heel who had both a PCS and plantar fasciitis underwent shock wave therapy (Moretti et al. 2006). During the study there were no significant changes in spur size; however, in 61% inflammation disappeared, and this was strongly correlated with a decrease in pain (Moretti et al. 2006). Low dose tissue irradiation, which is well known for its anti‐inflammatory effects, has also proven to be an effective method of decreasing heel pain associated with PCS, with marked improvement seen in 61–97% of patients and benefits lasting up to several years (Micke & Seegenschmiedt, 2004; Muecke et al. 2007; Hermann et al. 2013; Uysal et al. 2015). Randomized control trials comparing individual doses of 0.1–1.0 Gy and total doses of 0.6–6.0 Gy have shown the long‐term effectiveness of a single dose at 0.5 Gy, given twice a week up to a total dose of 3.0 Gy (Heyd et al. 2007; Niewald et al. 2008, 2015; Ott et al. 2013, 2014). The current hypothesized mechanism is via altered cytokine and chemokine release, as well as changes to adhesion molecules and decreased oxidative burst formation (Voll et al. 1997; Kern et al. 2000; Schaue et al. 2002; Rödel et al. 2008). An MRI study found edema at the plantar fascia enthesis (suggestive of plantar fasciitis) in approximately 50% of PCS cases (Ali et al. 2006). Furthermore, the pain associated with PCS in those with plantar fasciitis is more likely to be moderate (60%), compared with controls (2.5%) matched for length of spur, age and sex (Wainwright et al. 1995). Duvries (1957) postulated that both inflammatory changes and whether the spur was weight‐bearing are important in determining whether PCS cause pain. PCS may become weight‐bearing due to altered foot anatomy such as a depression in the longitudinal arch. PCS shape may also be important, with sharp spurs being associated with symptoms lasting less than 6 months and blunt spurs with symptoms lasting more than 6 months (Sadat‐Ali, 1998).
Although PCS are associated with heel pain in over 50% of cases, there are also many other causes of heel pain, such as tears in the plantar fascia, plantar fasciitis, calcaneal fractures and fat pad atrophy (Furey, 1975; Burks & Buk, 2003; Li & Muehleman, 2007; Smith et al. 2007; Alshami et al. 2008; Johal & Milner, 2012). These factors have all been found in both the presence and absence of PCS, suggesting that the spur is only one of a number of factors which contributes to heel pain.
Conclusion
The PCS is bony outgrowth of the calcaneal tuberosity and occurs in the general population in around 15% of people and in higher proportions in the elderly, the overweight, those with heel pain, plantar fasciitis, arthritides and abnormal foot biomechanics. It was initially thought that PCS were the result of traction from the plantar fascia or intrinsic muscles of the foot; however, it is now known that the majority are found deep to the PF, surrounded by fibrocartilage. They are hypothesized to function as an adaptive skeletal response to redistribute the impact forces away from the calcaneal insertion site to the surrounding tissues via a buttressing effect. The PCS trabecular pattern is consistent with a vertically oriented ground force vector and is in agreement with key associations listed above, namely, increasing weight, age, and alterations in foot structure, all factors that determine the heel pressure generated. There is also evidence that certain individuals have a genetic predisposition to generate new bone and undergo osteogenesis at levels of mechanical stress which are not sufficient to initiate bone growth in others. This is further supported by the association of PCS and osteoarthritis.
The relationship between PCS and painful heels has been confirmed via multiple meta‐analyses. However, a subpopulation of those with PCS are completely asymptomatic. Factors which may be important in determining whether a given PCS will cause an individual heel pain include the size of the spur, whether there is compression of the inferior calcaneal nerve, spur factures, concurrent inflammation, fat pad abnormalities or degeneration, and the individual's occupational environment. A number of other potential causes of heel pain appear prominently in the etiology of PCS, including tears in the plantar fascia, plantar fasciitis, calcaneal fractures and fat pad atrophy.
A consistent definition of what constitutes a calcaneal spur would benefit future research in this area. According to this literature review, a bony growth larger than 2 mm should constitute a spur, clearly distinguishing them from simple cortical irregularities. Further research on the normal anatomy and histology of the calcaneal tuberosity along with the variation present within the population would provide a valuable comparison for those with symptomatic heels both with and without PCS. A number of papers on shock wave therapy and low dose tissue irradiation are demonstrating effective interventions for those experiencing heel pain associated with PCS. However, current research regarding PCS would predict that it is going to become a worsening problem in the future with an aging population, a corresponding increase in degenerative bone conditions, and a global rise in obesity.
Author contributions
Conceived study: SAM. Literature search: OY. Data analysis: JK. Drafted manuscript: JK. Critically revised manuscript and approved the final draft: SAM.
References
- Abreu MR, Chung CB, Mendes L, et al. (2003) Plantar calcaneal enthesophytes: new observations regarding sites of origin based on radiographic, MR imaging, anatomic, and paleopathologic analysis. Skeletal Radiol 32, 13–21. [DOI] [PubMed] [Google Scholar]
- Ali M, Chen T, Crues J (2006) MRI of the foot. 12, 10.
- Alshami AM, Souvlis T, Coppieters MW (2008) A review of plantar heel pain of neural origin: differential diagnosis and management. Man Ther 13, 103–111. [DOI] [PubMed] [Google Scholar]
- Barrett SL, Day SV, Pignetti TT, et al. (1995) Endoscopic heel anatomy: analysis of 200 fresh frozen specimens. J Foot Ankle Surg 34, 51–56. [DOI] [PubMed] [Google Scholar]
- Banadda BM, Gona O, Vaz R, Ndlovu DM (1992) Calcaneal spurs in a black African population. Foot Ankle Int 13, 352–354. [DOI] [PubMed] [Google Scholar]
- Bassiouni M (1965) Incidence of calcaneal spurs in osteo‐arthrosis and rheumatoid arthritis, and in control patients. Ann Rheum Dis 24, 490–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, et al. (2006) Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 208, 471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz JF, Kier R, Rudicel S (1991) Plantar fasciitis: MR imaging. Radiology 179, 665–667. [DOI] [PubMed] [Google Scholar]
- Brand PW (2006) Pressure sores – the problem. J Tissue Viability 16, 9–11. [DOI] [PubMed] [Google Scholar]
- Brody B (1962) Progressive changes in the pathology of a heel spur. J Am Podiatry Assoc 52, 754–755. [PubMed] [Google Scholar]
- Burks JB, Buk A (2003) Bilateral fractures of the infracalcaneal exostosis. J Foot Ankle Surg 42, 43–44. [DOI] [PubMed] [Google Scholar]
- Carlı AB, Tekin L, Akarsu S, et al. (2013) Calcaneal spur in an 18‐month‐old boy. Scand J Rheumatol 42, 83–84. [DOI] [PubMed] [Google Scholar]
- Chang C, Miltner L (1934) Periostitis of the os calcis. J Bone Joint Surg 16, 355–364. [Google Scholar]
- Chundru U, Liebeskind A, Seidelmann F, et al. (2008) Plantar fasciitis and calcaneal spur formation are associated with abductor digiti minimi atrophy on MRI of the foot. Skeletal Radiol 37, 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Blair HC (1950) Spurs of the calcaneus in Strümpell‐Marie disease; report of fifteen cases. J Bone Joint Surg Am 32A, 838–840. [PubMed] [Google Scholar]
- Delfaut EM, Demondion X, Bieganski A, et al. (2003) Imaging of foot and ankle nerve entrapment syndromes: from well‐demonstrated to unfamiliar sites. Radiographics 23, 613–623. [DOI] [PubMed] [Google Scholar]
- Delmi M, Kurt AM, Meyer JM, Hoffmeyer P (1995) Calcification of the tibialis posterior tendon: a case report and literature review. Foot Ankle Int 16, 792–795. [DOI] [PubMed] [Google Scholar]
- Donald EB, Christopher Z (1995) The foot in running. J Am Acad Orthop Surg 3, 136–145. [DOI] [PubMed] [Google Scholar]
- Duvries HL (1957) Heel spur (calcaneal spur). AMA Arch Surg 74, 536–542. [DOI] [PubMed] [Google Scholar]
- Forman WM, Green MA (1990) The role of intrinsic musculature in the formation of inferior calcaneal exostoses. Clin Podiatr Med Surg 7, 217–223. [PubMed] [Google Scholar]
- Franson J (2008) Some new ideas in the treatment of retrocalcaneal exostosis. Foot Ankle Spec 1, 309–311. [DOI] [PubMed] [Google Scholar]
- Furey JG (1975) Plantar fasciitis. The painful heel syndrome. J Bone Joint Surg Am 57, 672–673. [PubMed] [Google Scholar]
- Gerster JC, Vischer TL, Bennani A, et al. (1977) The painful heel. Comparative study in rheumatoid arthritis, ankylosing spondylitis, Reiter's syndrome, and generalized osteoarthrosis. Ann Rheum Dis 36, 343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon WW, Long G (1999) Ultrasound of the plantar aponeurosis (fascia). Skeletal Radiol 28, 21–26. [DOI] [PubMed] [Google Scholar]
- Gould E (1942) Three generations of exostoses of the heel. J Hered 33, 228. [Google Scholar]
- Griffith H (1910) Osteophytes of the os calcis. J Bone Joint Surg 2, 501–506. [Google Scholar]
- Hermann RM, Meyer A, Becker A, et al. (2013) Effect of field size and length of plantar spur on treatment outcome in radiation therapy of plantar fasciitis: The bigger the better?. Int J Radiat Oncol Biol Phys 87, 1122–1128. [DOI] [PubMed] [Google Scholar]
- Hertzler AE (1926) Bursitides of the plantar surface of the foot. Am J Surg 1, 117–126. [Google Scholar]
- Heyd R, Tselis N, Ackermann H, et al. (2007) Radiation therapy for painful heel spurs: results of a prospective randomized study. Strahlenther Onkol 183, 3–9. [DOI] [PubMed] [Google Scholar]
- Hills A, Hennig E, McDonald M, et al. (2001) Plantar pressure differences between obese and non‐obese adults: a biomechanical analysis. International journal of obesity. 25, 1674–1679. [DOI] [PubMed] [Google Scholar]
- Hsu T‐C, Wang C‐L, Tsai W‐C, et al. (1998) Comparison of the mechanical properties of the heel pad between young and elderly adults. Arch Phys Med Rehabil 79, 1101–1104. [DOI] [PubMed] [Google Scholar]
- Irving DB, Cook JL, Menz HB (2006) Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport 9, 11–22, discussion 23–24. [DOI] [PubMed] [Google Scholar]
- Johal KS, Milner SA (2012) Plantar fasciitis and the calcaneal spur: Fact or fiction? Foot Ankle Surg 18, 39–41. [DOI] [PubMed] [Google Scholar]
- Kern PM, Keilholz L, Forster C, et al. (2000) Low‐dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiotherapy and Oncology 54, 273–282. [DOI] [PubMed] [Google Scholar]
- Kogler GF, Solomonidis SE, Paul JP (1996) Biomechanics of longitudinal arch support mechanisms in foot orthoses and their effect on plantar aponeurosis strain. Clin Biomech (Bristol, Avon) 11, 243–252. [DOI] [PubMed] [Google Scholar]
- Kosmahl E, Kosmahl H (1987) Painful plantar heel, plantar fasciitis, and calcaneal spur: etiology and treatment. J Orthop Sports Phys Ther 9, 17–24. [DOI] [PubMed] [Google Scholar]
- Kullar JS, Randhawa GK, Kullar KK (2014) A study of calcaneal enthesophytes (spurs) in Indian population. Int J Appl Basic Med Res 4, S13–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumai T, Benjamin M (2002) Heel spur formation and the subcalcaneal enthesis of the plantar fascia. J Rheumatol 29, 1957–1964. [PubMed] [Google Scholar]
- Lapidus PW, Guidotti FP (1965) Painful heel: report of 323 patients with 364 painful heels. Clin Orthop 39, 178–186. [PubMed] [Google Scholar]
- Lemont H, Ammirati KM, Usen N (2003) Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc 93, 234–237. [DOI] [PubMed] [Google Scholar]
- Levy JC, Mizel MS, Clifford PD, Temple HT (2006) Value of radiographs in the initial evaluation of nontraumatic adult heel pain. Foot Ankle Int 27, 427–430. [DOI] [PubMed] [Google Scholar]
- Li J, Muehleman C (2007) Anatomic relationship of heel spur to surrounding soft tissues: greater variability than previously reported. Clin Anat 20, 950–955. [DOI] [PubMed] [Google Scholar]
- Louisia S, Masquelet AC (1999) The medial and inferior calcaneal nerves: an anatomic study. Surg Radiol Anat 21, 169–173. [DOI] [PubMed] [Google Scholar]
- Mason RM, Murray RS, Oates JK, et al. (1959) A comparative radiological study of Reiter's disease, rheumatoid arthritis and ankylosing spondylitis. J Bone Joint Surg Br 41‐B, 137–148. [DOI] [PubMed] [Google Scholar]
- McCarthy DJ, Gorecki GE (1979) The anatomical basis of inferior calcaneal lesions. A cryomicrotomy study. J Am Podiatry Assoc 69, 527–536. [DOI] [PubMed] [Google Scholar]
- McMillan AM, Landorf KB, Barrett JT, Menz HB, Bird AR (2009) Diagnostic imaging for chronic plantar heel pain: a systematic review and meta‐analysis. J Foot Ankle Res 2, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz HB, Zammit GV, Landorf KB, et al. (2008) Plantar calcaneal spurs in older people: longitudinal traction or vertical compression? J Foot Ankle Res 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micke O, Seegenschmiedt MH, German Cooperative Group on Radiotherapy for Benign Diseases (2004) Radiotherapy in painful heel spurs (plantar fasciitis) – results of a national patterns of care study. Int J Radiat Oncol Biol Phys 58, 828–843. [DOI] [PubMed] [Google Scholar]
- Morag E, Cavanagh PR (1999) Structural and functional predictors of regional peak pressures under the foot during walking. J Biomech 32, 359–370. [DOI] [PubMed] [Google Scholar]
- Moretti B, Garofalo R, Patella V, et al. (2006) Extracorporeal shock wave therapy in runners with a symptomatic heel spur. Knee Surg Sports Traumatol Arthrosc 14, 1029–1032. [DOI] [PubMed] [Google Scholar]
- Moroney PJ, O'Neill BJ, Khan‐Bhambro K, et al. (2014) The conundrum of calcaneal spurs: do they matter? Foot Ankle Spec 7, 95–101. [DOI] [PubMed] [Google Scholar]
- Mosier SM, Pomeroy G, Manoli A (1999) Pathoanatomy and etiology of posterior tibial tendon dysfunction. Clin Orthop Relat Res 1, 12–22. [DOI] [PubMed] [Google Scholar]
- Muecke R, Micke O, Reichl B, et al. (2007) Demographic, clinical and treatment related predictors for event‐free probability following low‐dose radiotherapy for painful heel spurs–a retrospective multicenter study of 502 patients. Acta Oncol 46, 239–246. [DOI] [PubMed] [Google Scholar]
- Niewald M, Seegenschmiedt MH, Micke O, et al. (2008) Randomized multicenter trial on the effect of radiotherapy for plantar Fasciitis (painful heel spur) using very low doses – a study protocol. Radiat Oncol 3, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewald M, Holtmann H, Prokein B, et al. (2015) Randomized multicenter follow‐up trial on the effect of radiotherapy on painful heel spur (plantar fasciitis) comparing two fractionation schedules with uniform total dose: first results after three months' follow‐up. Radiat Oncol 10, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwuanyi ON (2000) Calcaneal spurs and plantar heel pad pain. Foot 10, 182–185. [Google Scholar]
- Osborne HR, Breidahl WH, Allison GT (2006) Critical differences in lateral X‐rays with and without a diagnosis of plantar fasciitis. J Sci Med Sport 9, 231–237. [DOI] [PubMed] [Google Scholar]
- Ott OJ, Jeremias C, Gaipl US, et al. (2013) Radiotherapy for calcaneodynia. Results of a single center prospective randomized dose optimization trial. Strahlenther Onkol 189, 329–334. [DOI] [PubMed] [Google Scholar]
- Ott OJ, Jeremias C, Gaipl US, et al. (2014) Radiotherapy for benign calcaneodynia: long‐term results of the Erlangen Dose Optimization (EDO) trial. Strahlenther Onkol 190, 671–675. [DOI] [PubMed] [Google Scholar]
- Ozdemir H, Yilmaz E, Murat A, et al. (2005) Sonographic evaluation of plantar fasciitis and relation to body mass index. Eur J Radiol 54, 443–447. [DOI] [PubMed] [Google Scholar]
- Ozdemir H, Söyüncü Y, Ozgörgen M, et al. (2004) Effects of changes in heel fat pad thickness and elasticity on heel pain. J Am Podiatr Med Assoc 94, 47–52. [DOI] [PubMed] [Google Scholar]
- Paul S, Henry F (1916) Hereditary Syphilis as an etiological factor in spurs on the os calcis. Surg Gynecol Obstet. 22, 674–678. [Google Scholar]
- Perry J (1983) Anatomy and biomechanics of the hindfoot. Clin Orthop 9–15. [PubMed] [Google Scholar]
- Prichasuk S, Subhadrabandhu T (1994) The relationship of pes planus and calcaneal spur to plantar heel pain. Clin Orthop 192–196. [PubMed] [Google Scholar]
- Resnick D, Feingold ML, Curd J, et al. (1977) Calcaneal abnormalities in articular disorders. Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and Reiter syndrome. Radiology 125, 355–366. [DOI] [PubMed] [Google Scholar]
- Riepert T, Drechsler T, Urban R, et al. (1995) The incidence, age dependence and sex distribution of the calcaneal spur. An analysis of its x‐ray morphology in 1027 patients of the central European population. RöFo 162, 502–505. [DOI] [PubMed] [Google Scholar]
- Robinson HM (1976) Symmetrical Reversed Plantar Calcaneal Spurs in Children: A Normal Variant? 1. Radiology 119, 187–188. [DOI] [PubMed] [Google Scholar]
- Rödel F, Hofmann D, Auer J, et al. (2008) The anti‐inflammatory effect of low‐dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther Onkol 184, 41–47. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shepstone L, Dieppe P (1997) Bone formers: osteophyte and enthesophyte formation are positively associated. Ann Rheum Dis 56, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland O (1912) Pathogenesis of spur formation on the os calcis. J Bone Joint Surg 2, 257–274. [Google Scholar]
- Rondhuis JJ, Huson A (1986) The first branch of the lateral plantar nerve and heel pain. Acta Morphol Neerl Scand 24, 269–279. [PubMed] [Google Scholar]
- Rubin G, Witten M (1963) Plantar calcaneal spurs. Am J Orthop 5, 38–41. [PubMed] [Google Scholar]
- Sadat‐Ali M (1998) Plantar fasciitis/calcaneal spur among security forces personnel. Mil Med 163, 56–57. [PubMed] [Google Scholar]
- Schaue D, Marples B, Trott KR (2002) The effects of low‐dose X‐irradiation on the oxidative burst in stimulated macrophages. Int J Radiat Biol 78, 567–576. [DOI] [PubMed] [Google Scholar]
- Schepsis AA, Leach RE, Gorzyca J (1991) Plantar fasciitis. Etiology, treatment, surgical results, and review of the literature. Clin Orthop 185–196. [PubMed] [Google Scholar]
- Scott G, Menz HB, Newcombe L (2007) Age‐related differences in foot structure and function. Gait Posture 26, 68–75. [DOI] [PubMed] [Google Scholar]
- Sebes JI (1989) The significance of calcaneal spurs in rheumatic diseases. Arthritis Rheum 32, 338–340. [DOI] [PubMed] [Google Scholar]
- Shama SS, Kominsky SJ, Lemont H (1983) Prevalence of non‐painful heel spur and its relation to postural foot position. J Am Podiatry Assoc 73, 122–123. [DOI] [PubMed] [Google Scholar]
- Smith S, Tinley P, Gilheany M, et al. (2007) The inferior calcaneal spur — Anatomical and histological considerations. Foot 17, 25–31. [Google Scholar]
- Steindler A, Smith A (1938) Spurs of the os calcis. Surg Gynecol Obstet 66, 663–665. [Google Scholar]
- Tanz SS (1963) Heel pain. Clin Orthop 28, 169–178. [PubMed] [Google Scholar]
- Toumi H, Davies R, Mazor M, et al. (2014) Changes in prevalence of calcaneal spurs in men & women: a random population from a trauma clinic. BMC Musculoskelet Disord 15, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tountas AA, Fornasier VL (1996) Operative treatment of subcalcaneal pain. Clin Orthop. and related research 332, 170–178. [DOI] [PubMed] [Google Scholar]
- Uysal B, Beyzadeoglu M, Sager O, et al. (2015) Role of radiotherapy in the management of heel spur. Eur J Orthop Surg Traumatol 25, 387–389. [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, et al. (1997) Immunosuppressive effects of apoptotic cells. Nature 390, 350–351. [DOI] [PubMed] [Google Scholar]
- Von Lackum W, Palomeque E (1930) Gonorrheal spurs a misnomer. J Am Med Assoc 95, 472–473. [Google Scholar]
- Vyce SD, Addis‐Thomas E, Mathews EE, Perez SL (2010) Painful prominences of the heel. Clin Podiatr Med Surg 27, 443–462. [DOI] [PubMed] [Google Scholar]
- Waechter A, Sonnenschein HD. (1915) Painful heels due to exostosis of the os calcis. NY State J Med 102, 190–191. [Google Scholar]
- Wainwright A, Kelly A, Winson I (1995) Calcaneal spurs and plantar fasciitis. Foot 5, 123–126. [Google Scholar]
- Weiss E (2012) Calcaneal spurs: examining etiology using prehistoric skeletal remains to understand present day heel pain. Foot (Edinb) 22, 125–129. [DOI] [PubMed] [Google Scholar]
- Wiechen PJ (1987) Reversed calcaneal spurs in children. Skeletal Radiol 16, 17–18. [DOI] [PubMed] [Google Scholar]
- Wearing SC, Smeathers JE, Urry SR, et al. (2006) The pathomechanics of plantar fasciitis. Sports Med 36, 585–611. [DOI] [PubMed] [Google Scholar]
- Winthrop G (1909) Gonorrheal exostosis of the os calcis: report of cases. J Am Med Assoc LIII, 715–716. [Google Scholar]
- Yung‐Hui L, Wei‐Hsien H (2005) Effects of shoe inserts and heel height on foot pressure, impact force, and perceived comfort during walking. Appl Ergon 36, 355–362. [DOI] [PubMed] [Google Scholar]


