Abstract
Insecticides based on Bacillus thuringiensis subsp. israelensis have been used for mosquito and blackfly control for more than 20 years, yet no resistance to this bacterium has been reported. Moreover, in contrast to B. thuringiensis subspecies toxic to coleopteran or lepidopteran larvae, only low levels of resistance to B. thuringiensis subsp. israelensis have been obtained in laboratory experiments where mosquito larvae were placed under heavy selection pressure for more than 30 generations. Selection of Culex quinquefasciatus with mutants of B. thuringiensis subsp. israelensis that contained different combinations of its Cry proteins and Cyt1Aa suggested that the latter protein delayed resistance. This hypothesis, however, has not been tested experimentally. Here we report experiments in which separate C. quinquefasciatus populations were selected for 20 generations to recombinant strains of B. thuringiensis that produced either Cyt1Aa, Cry11Aa, or a 1:3 mixture of these strains. At the end of selection, the resistance ratio was 1,237 in the Cry11Aa-selected population and 242 in the Cyt1Aa-selected population. The resistance ratio, however, was only 8 in the population selected with the 1:3 ratio of Cyt1Aa and Cry11Aa strains. When the resistant mosquito strain developed by selection to the Cyt1Aa-Cry11Aa combination was assayed against Cry11Aa after 48 generations, resistance to this protein was 9.3-fold. This indicates that in the presence of Cyt1Aa, resistance to Cry11Aa evolved, but at a much lower rate than when Cyt1Aa was absent. These results indicate that Cyt1Aa is the principal factor responsible for delaying the evolution and expression of resistance to mosquitocidal Cry proteins.
The mosquitocidal bacterium Bacillus thuringiensis subsp. israelensis is highly effective as a larvicide against a wide range of mosquito (Diptera: Culicidae) and blackfly species (Diptera: Simuliidae) and has been used routinely in many pest and vector control programs for more than 20 years (1, 10, 12, 30). The principal insecticidal component of B. thuringiensis subsp. israelensis is a spherical parasporal body produced during sporulation and composed of four major endotoxin proteins, Cyt1Aa, Cry4Aa, Cry4Ba, and Cry11Aa (6, 11). This parasporal body is one of the most insecticidal known, with a 50% lethal concentration (LC50) in the range of 10 ng/ml against fourth instars of various mosquito species. Numerous studies have shown that the broad spectrum of activity and high toxicity of B. thuringiensis subsp. israelensis are due to synergistic interactions between Cyt1Aa and Cry proteins (5, 6, 11, 27), as well as synergistic interactions that occur among Cry proteins (5, 15). A remarkable property of this bacterium is that despite its intensive use in some geographical regions, such as the Rhine Valley in Germany, no resistance to B. thuringiensis subsp. israelensis has been reported under field conditions after many years of use (1). In contrast to this, high levels of resistance in populations of Culex mosquitoes, well in excess of several-thousand-fold, have been reported to Bacillus sphaericus under field conditions, even though it has been in use for only a few years (13, 16, 18, 20, 21, 31). The primary insecticidal component of the B. sphaericus strains used in these mosquito control operations is a binary toxin, commonly referred to as Bin, that consists of a 42-kDa toxin domain and a 51-kDa binding domain which function as a single toxin (4).
Although no resistance to B. thuringiensis subsp. israelensis has been reported under field conditions, studies in the laboratory have shown that high levels of resistance can be developed when larval populations are selected against recombinant bacilli that produce only one, two, or three of this bacterium's mosquitocidal Cry proteins. For example, when populations of C. quinquefasciatus were selected against a strain producing only Cry11Aa at a concentration that killed 95% (LC95) of the larvae, the level of resistance was 1,000-fold after 28 generations (9). When the same species was selected at the same level with a strain of B. thuringiensis subsp. israelensis that produced Cry11Aa, Cry4Aa, and Cry4Ba, resistance was 217-fold after the same number of generations (9). Interestingly, the level of resistance was only 3.3-fold when C. quinquefasciatus was selected with wild-type B. thuringiensis subsp. israelensis which, in addition to the Cry endotoxins, produces Cyt1Aa (9). The implication of these results is that combinations of Cry endotoxins, and especially combinations of Cry endotoxins and Cyt1Aa, are responsible for delaying resistance.
In addition to its capacity to synergize mosquitocidal Cry proteins, it has been shown that Cyt1Aa can suppress resistance in C. quinquefasciatus populations to Cry proteins (24) and to the B. sphaericus 2362 Bin toxin (26). Suppression was marked, with levels of resistance to Cry11Aa being reduced from greater than 1,000-fold to 8-fold with a mixture consisting of a 3:1 ratio of B. thuringiensis strains that produced, respectively, Cry11Aa and Cyt1Aa (24). In the case of B. sphaericus, resistance of greater than 30,000-fold was completely suppressed with a mixture that contained B. sphaericus sporulated cells and purified crystals of Cyt1Aa at a 10:1 ratio (26).
Data from the various studies summarized above imply that combinations of endotoxins, especially combinations that contain multiple Cry endotoxins and Cyt1Aa, as in wild-type B. thuringiensis subsp. israelensis, are one of the primary factors responsible for the lack of resistance to this mosquito larvicide under field conditions. However, the hypothesis has not been tested experimentally. Thus, in the present study we selected populations of C. quinquefasciatus to individual strains of B. thuringiensis subsp. israelensis that produced either Cry11A, Cyt1Aa, or a 3:1 ratio of these strains for 20 successive generations. In this paper, we report the results of this study, which show that Cyt1Aa suppressed the evolution and phenotypic expression of resistance to Cry11Aa.
MATERIALS AND METHODS
Bacterial strains and toxins.
Toxin preparations used in this study were lyophilized powders of spore and crystal (parasporal body) mixtures of lysed cultures of (i) B. thuringiensis subsp. israelensis 4Q7 that produces Cry11Aa (29), and (ii) a recombinant strain of B. thuringiensis subsp. israelensis 4Q7 that only produces Cyt1Aa (28). Hereafter, these proteins are referred to, respectively, as Cry11A and Cyt1A.
Toxin powder production and storage.
Bacterial strains producing the various toxins were grown on liquid media as described previously (14). Sporulated cells were washed in distilled water and sedimented, and the resultant pellet was lyophilized. For mosquito selections and bioassays, stock suspensions of the crystal and spore powders were prepared in distilled water and homogenized with the aid of approximately 25 glass beads. Stocks were prepared monthly, and 10-fold serial dilutions were prepared weekly. All stocks and dilutions were frozen at −20°C when not in use.
Mosquito strains.
The strains of C. quinquefasciatus used in the study were derived from a synthetic population, Syn-P, that was formed by combining mosquitoes from five separate field populations collected from southern California in 1995. Approximately 100 egg rafts were brought to the laboratory, reared to adults, and sorted to species according to the method of Bohart and Washino (2). Offspring from each collection were pooled and interbred under standard laboratory conditions for a year prior to the initiation of selection. Three colonies were derived from Syn-P by selection with suspensions of powders of the recombinant strains expressing Cry11A, Cyt1A, or a mixture of both Cry11A and Cyt1A (3:1) and named for the toxin(s) used in their selection.
Selection and bioassay procedures.
Selection consisted of exposing groups of 1,000 early fourth instars to suspensions of the lyophilized toxin powders in 1,000 ml of deionized water in enameled metal pans. After 24 h, survivorship was visually estimated and survivors were transferred to clean water and fed and continued their development. On average, more than 10,000 larvae were selected per generation for each selected line. Mortality averaged 56.7% (standard deviation [SD], 15.0%) for the Cry11A-selected line, 55.1% (SD, 15.5%) for the Cyt1A-selected line, and 63.1% (SD, 22.0%) for the Cry11A plus Cyt1Aa-selected line over the first 20 generations. Selection did not occur in generation 15 for the Cyt1A-selected and Cry11A plus Cyt1A-selected lines. Generations were permitted to overlap after generation 20, and selection was continued.
Bioassays consisted of exposing groups of 100 early fourth instars to a range of 10 to 15 different concentrations of toxin suspensions plus an untreated control in 100 ml of deionized water in 8-oz plastic cups. Mortality was evaluated after 24 h, and all data were subjected to probit analysis (8) by using a program for the PC (17). Susceptibility was evaluated around every third generation. A concurrent assay using the unselected parental colony Syn-P was used to estimate changes in susceptibility. Generation 9 of the Cry11A plus Cyt1A-selected line was not bioassayed due to technical problems. Dose-response values with overlapping fiducial limits were not considered to be significantly different. Resistance ratios were calculated by dividing the respective lethal concentration value for the selected strain by that of the Syn-P strain. Synergism was estimated using the method described by Tabashnik (22). The expected toxicity of the 3:1 mixture of Cry11A plus Cyt1A was predicted from the weighted harmonic mean of the toxicity of the individual components. The synergism factor (SF) was calculated by dividing the observed toxicity of the mixture by the predicted value. An SF value of 1 indicates additive toxicity, a value of more than 1 indicates a synergistic interaction, and a value of less than 1 indicates an antagonistic interaction.
RESULTS
Under selection with Cry11A, a significant level of resistance of 3.3-fold at the LC95 was detected in generation 7 (Table 1). Resistance subsequently increased to 238- and 8,862-fold at the LC95 in generations 13 and 17, but it declined slightly to 1,237-fold in generation 20. Similar increases, albeit at overall lower levels, were observed at the LC50. In the line selected with Cyt1A, resistance ratios of 4.9- and 6.1-fold were observed at the LC50 and LC95, respectively, in generation 17 and reached 242-fold at the LC95 in generation 20 (Table 2). Resistance was initially detected sporadically in the mosquitoes selected with the 3:1 mixture of Cry11A plus Cyt1A. Resistance ratios were 2.0 at the LC95 in generation 4 and 5.4 in generation 7, declined in generation 13, and reappeared in generations 17 and 20 with resistance ratios of 4.4 and 8.1 (Table 3).
TABLE 1.
Toxicity of Cry11A toward C. quinquefasciatus selected or nonselected with Cry11A from B. thuringiensis subsp. israelensis for 20 generations
| Mosquito straina | Toxin tested | Generation | LC50 (μg/ml), FLb | LC95 (μg/ml), FL | Resistance ratio at:
|
|
|---|---|---|---|---|---|---|
| LC50 | LC95 | |||||
| SynParent | Cry11A | 4 | 1.65, 0.255-9.68 | 624, 59.4-7,574 | 1.0 | 1.0 |
| Cry11A | Cry11A | 4 | 3.00, 1.63-4.74 | 990, 519-2,431 | 1.8 | 1.6 |
| SynParent | Cry11A | 7 | 1.57, 0.805-3.05 | 129, 38.8-436 | 1.0 | 1.0 |
| Cry11A | Cry11A | 7 | 8.49, 5.25-13.6 | 425, 173-1,103 | 5.4 | 3.3 |
| SynParent | Cry11A | 9 | 7.33, 4.73-11.3 | 332, 136-834 | 1.0 | 1.0 |
| Cry11A | Cry11A | 9 | 15.7, 12.1-20.1 | 2,048, 1,201-3,953 | 2.1 | 6.2 |
| SynParent | Cry11A | 13 | 4.20, 2.32-7.59 | 244, 78.2-789 | 1.0 | 1.0 |
| Cry11A | Cry11A | 13 | 103, 73.6-150 | 58,253, 21,190-∞ | 24.5 | 238 |
| SynParent | Cry11A | 17 | 0.885, 0.329-2.35 | 57.7, 11.9-285 | 1.0 | 1.0 |
| Cry11A | Cry11A | 17 | 91.8, 59.4-152 | 511,705, 101,376-∞ | 103 | 8,862 |
| SynParent | Cry11A | 20 | 3.60, 1.73-7.45 | 3,686, 709-20,488 | 1.0 | 1.0 |
| Cry11A | Cry11A | 20 | 1,358, 664-4,104 | 456,127, 476,570-∞ | 377 | 1,237 |
SynParent, unselected; Cry11A, Cry11A selected.
FL, fiducial limits.
TABLE 2.
Toxicity of Cyt1A toward C. quinquefasciatus selected or nonselected with Cyt1A from B. thuringiensis subsp. israelensis for 20 generations
| Mosquito straina | Toxin tested | Generation | LC50 (μg/ml), FLb | LC95 FL (μg/ml), FL | Resistance ratio at:
|
|
|---|---|---|---|---|---|---|
| LC50 | LC95 | |||||
| SynParent | Cyt1A | 4 | 14.1, 10.5-18.2 | 504, 336-854 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 4 | 31.0, 15.0-64.0 | 289, 70.5-1,206 | 2.2 | 0.6 |
| SynParent | Cyt1A | 7 | 15.9, 5.96-42.4 | 123, 20.7-742 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 7 | 15.1, 12.4-17.9 | 162, 121-234 | 0.95 | 1.3 |
| SynParent | Cyt1A | 9 | 37.5, 15.8-88.9 | 1,412, 206-9,857 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 9 | 28.2, 11.0-72.1 | 668, 100-4,467 | 0.8 | 0.5 |
| SynParent | Cyt1A | 13 | 16.2, 13.8-18.9 | 152, 116-213 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 13 | 15.0, 6.75-33.3 | 181, 40.4-821 | 0.9 | 1.2 |
| SynParent | Cyt1A | 17 | 7.90, 6.52-9.49 | 145, 104-221 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 17 | 38.8, 22.9-65.7 | 883, 328-2,453 | 4.9 | 6.1 |
| SynParent | Cyt1A | 20 | 6.25, 2.96-13.1 | 50.4, 13.9-184 | 1.0 | 1.0 |
| Cyt1A | Cyt1A | 20 | 32.7, 8.47-125 | 12,243, 189-818,272 | 5.2 | 242 |
SynParent, unselected; Cyt1A, Cyt1A selected.
FL, fiducial limits.
TABLE 3.
Toxicity of Cry11A plus Cyt1A (3:1) against C. quinquefasciatus selected or nonselected for 20 generations with Cry11A plus Cyt1A of B. thuringiensis subsp. israelensis
| Mosquito straina | Tested toxins | Generation | LC50 (μg/ml), FLb | LC95 (μg/ml), FL | Resistance ratio at:
|
|||
|---|---|---|---|---|---|---|---|---|
| LC50 | LC95 | SF50 | SF95 | |||||
| SynParent | Cry11A + Cyt1A | 4 | 0.845, 0.746-0.961 | 3.42, 2.68-4.79 | 1.0 | 1.0 | 2.5 | 172 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 4 | 1.08, 0.939-1.24 | 6.76, 5.36-9.03 | 1.3 | 2.0 | ||
| SynParent | Cry11A + Cyt1A | 7 | 0.436, 0.378-0.500 | 2.52, 1.99-3.39 | 1.0 | 1.0 | 4.6 | 50.6 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 7 | 1.14, 0.967-1.34 | 13.7, 10.2-19.5 | 2.6 | 5.4 | ||
| SynParent | Cry11A + Cyt1A | 13 | 0.597, 0.527-0.671 | 2.09, 1.71-2.72 | 1.0 | 1.0 | 8.6 | 101 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 13 | 0.728, 0.488-1.08 | 3.71, 1.79-7.84 | 1.2 | 1.8 | ||
| SynParent | Cry11A + Cyt1A | 17 | 0.247, 0.215-0.283 | 1.09, 0.872-1.45 | 1.0 | 1.0 | 4.6 | 62.3 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 17 | 0.736, 0.640-0.848 | 4.85, 3.79-6.57 | 3.0 | 4.4 | ||
| SynParent | Cry11A + Cyt1A | 20 | 0.610, 0.537-0.693 | 2.99, 2.42-3.90 | 1.0 | 1.0 | 6.6 | 64.8 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 20 | 2.76, 1.76-4.32 | 24.3, 11.0-54.5 | 4.5 | 8.1 | ||
SynParent, nonselected; Cry11A + Cyt1A, Cry11A plus Cyt1A-selected.
FL, fiducial limits.
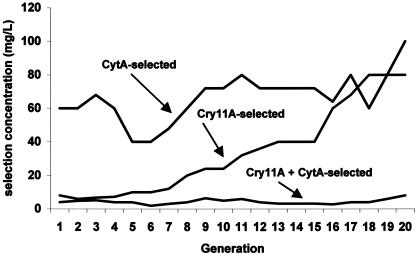
Mosquitoes selected with Cry11A were exposed to steadily increasing concentrations that ranged from 6 μg/ml to a maximum of 80 μg/ml (Fig. 1). Concentrations ranged from 40 to 100 μg/ml in selections with Cyt1A, whereas selection concentrations ranged from 2 to 8 μg/ml with the mixture of Cry11A plus Cyt1A (3:1).
FIG. 1.
Increases in the concentration of B. thuringiensis strains producing Cyt1A, Cry11A, or a combination of these two toxins required to achieve resistance in three corresponding lines of C. quinquefasciatus. Mortality in the line selected for resistance to Cry11A averaged 70% for generations 1 to 10 and then declined in subsequent generations to approximately 50%, regardless of the increase in strain concentration. Mortality in the lines selected with Cyt1A or the Cyt1A plus Cry11A combination remained high and, thus, the concentration of toxin was essentially constant from one generation to the next.
SFs were calculated over the course of the study to estimate synergy between Cry11A and Cyt1A against the Syn-P colony (Table 3) and against the three selected colonies at the end of the study (Table 4). SF values ranged from 5.5 to 101 against Syn-P and were consistently high, from 6.0 to 455 for the selected colonies, confirming the presence of synergy between Cry11A and Cyt1A in the 3:1 mixture throughout the study.
TABLE 4.
Toxicity and cross-resistance of Cry11A, Cyt1A, or Cry11A plus Cyt1A against C. quinquefasciatus after selection for approximately 48 generations with one or two toxins of B. thuringiensis subsp. israelensis
| Mosquito straina | Test toxin(s) | LC50 (μg/ml), FLb | LC95 (μg/ml), FL | Resistance ratio at:
|
|||
|---|---|---|---|---|---|---|---|
| LC50 | LC95 | SF50 | SF95 | ||||
| SynParent | Cyt1A | 25.4, 14.1-45.8 | 280, 93.8-842 | 1.0 | 1.0 | ||
| Cyt1A | Cyt1A | 53.9, 34.0-85.6 | 1,340, 487-3,874 | 2.1 | 4.8 | ||
| Cry11A | Cyt1A | 31.3, 16.3-60.1 | 356, 97.2-1,323 | 1.2 | 1.3 | ||
| Cry11A + Cyt1Aa | Cyt1A | 48.8, 20.9-113 | 692, 132-3,671 | 1.9 | 2.5 | ||
| SynParent | Cry11A | 0.946, 0.419-2.13 | 24.9, 5.57-112 | 1.0 | 1.0 | ||
| Cyt1A | Cry11A | 0.904, 0.424-1.92 | 17.6, 4.44-70.5 | 0.9 | 0.7 | ||
| Cry11A | Cry11A | 18.1, 11.0-29.8 | 1,592, 469-5,723 | 19.1 | 63.9 | ||
| Cry11A + Cyt1A | Cry11A | 4.48, 3.59-5.54 | 231, 155-374 | 4.7 | 9.3 | ||
| SynParent | Cry11A + Cyt1A | 0.209, 0.158-0.278 | 0.832, 0.505-1.42 | 1.0 | 1.0 | 6.0 | 38.8 |
| Cyt1A | Cry11A + Cyt1A | 0.187, 0.107-0.321 | 0.671, 0.252-1.95 | 0.9 | 0.8 | 6.4 | 34.8 |
| Cry11A | Cry11A + Cyt1A | 0.476, 0.422-0.536 | 1.87, 1.53-2.40 | 2.3 | 2.2 | 42.5 | 455 |
| Cry11A + Cyt1A | Cry11A + Cyt1A | 1.07, 0.927-1.24 | 7.30, 5.74-9.84 | 5.1 | 8.8 | 5.4 | 20 |
SynParent, nonselected; Cyt1A, Cyt1A selected; Cry11A, Cry11A selected; Cry11A + Cyt1A, Cry11A plus Cyt1A selected.
FL, fiducial limits.
Generations were allowed to overlap after 20 generations of selection, and selection was continued. The three selected lines and Syn-P were assayed with each of the three different toxin powders around generation 48 to further evaluate resistance and cross-resistance. Resistance to Cyt1A had regressed to 4.8-fold at the LC95 in the Cyt1Aa-selected line, from its previous high of 242-fold (Table 4). No cross-resistance to Cyt1A was evident in the line selected with Cry11A, and no resistance was detected in the line selected with Cry11A plus Cyt1A. Significant resistance to Cry11A was detected in both lines that were exposed to powders containing this toxin. Resistance was 63.9 at the LC95 for the Cry11A-selected line, but it was only 9.3 at the LC95 for the line selected with Cry11A plus Cyt1A. No cross-resistance to Cry11A was detected in the Cyt1A-selected line. With respect to the mixture of Cry11A plus Cyt1A (3:1), no significant resistance was observed in either the Cyt1A-selected line (0.8 at the LC95) or the Cry11A-selected line (2.2 at the LC95). However, resistance was 8.8 at the LC95 in the line selected with Cry11A plus Cyt1A after 48 generations of selection pressure.
DISCUSSION
In this study, we have shown that Cyt1A significantly influenced the evolution of resistance to Cry11A, delaying resistance to this protein in mosquitoes selected with a mixture of these two toxins. In contrast to selection with the individual toxins, which resulted in resistance levels of 1,237-fold for Cry11A alone and 242-fold for Cyt1A alone, mosquitoes selected with the 3:1 mixture of Cry11A plus Cyt1A evolved only 8-fold resistance. The colony selected with the mixture evolved ninefold resistance to Cry11A and no resistance to Cyt1A, clearly demonstrating a slower rate of resistance development to Cry11A in the presence of Cyt1A. In addition to the differences in the levels of resistance by the end of the study, the mosquito strains selected with these mosquitocidal proteins alone or in combination exhibited different patterns of response to long-term selection. The colony selected with Cry11A showed a gradual but steady increase in resistance, and concentrations used to maintain an equivalent level of toxicity increased progressively over the course of the study (Fig. 1). The colony selected with Cyt1A alone developed resistance that was unstable and declined toward the end of the study. Selection concentrations for Cyt1A, which were adjusted to the changes in the susceptibility of the colony, fluctuated within a narrower range than those for Cry11A. The colony selected with the Cyt1A plus Cry11A mixture showed very little change in sensitivity, which was reflected in the very narrow range of selection concentrations.
Synergy between Cry11A and Cyt1A was detected consistently in the 3:1 mixtures throughout the study, and this contributed significantly to the high toxicity of the mixture as well as the slower evolution of resistance in the line selected with Cry11A plus Cyt1A. The capacity of Cyt1A to delay the evolution of resistance to Cry11A is particularly notable, owing to Cyt1A's low toxicity, which in the absence of synergy would be expected to contribute only 10% mortality at the concentrations used against sensitive mosquitoes. For example, synergy increased the toxicity of the 3:1 mixture of Cry11A plus Cyt1A by 455-fold in generation 48 of the Cry11A-resistant mosquito strain, the mostly highly resistant strain.
The differences we observed in the patterns of the evolution of resistance and the final levels of resistance that resulted are likely related to differences between the modes of action of Cry11A and Cyt1A. Cry11A is a homolog of other Cry proteins toxic to coleopterous and lepidopterous insects and probably requires at least one specific microvillar membrane receptor to bind to microvilli and cause toxicity through formation of transmembrane cationic pores (19). Studies of Cry toxins have shown that these receptors, for example, membrane-anchored aminopeptidases, facilitate the development of resistance. In contrast, Cyt1A is unrelated to Cry proteins and has an affinity for lipids, acting by forming large transmembrane lesions in the microvillar membrane as opposed to forming cationic pores (3, 7, 23). The apparent lack of a highly specific glycoprotein receptor or receptors reduces the probability of resistance in mosquitoes selected with Cyt1A.
While differences in the mode of action correlate with the evolution of resistance, they do not provide insight into how Cyt1A delays the development of resistance. Recent preliminary studies in our laboratory using fluorescent dyes showed that Cyt1A forms lesions in the midgut microvillar membrane that allow the Bin toxin from B. sphaericus to enter and cross this membrane in midgut cells of B. sphaericus-resistant mosquitoes and exert toxicity (7). Thus, facilitating membrane entry is also likely to be Cyt1A's mechanism of synergism for Cry toxins. If this were the case, mosquitoes exposed to toxin mixtures containing Cyt1A would be less likely to develop resistance, because toxins would more readily enter the microvillar membrane, even when receptors were absent or modified, as indicated by our preliminary studies of mosquitoes resistant to B. sphaericus.
The experimental demonstration provided in this study, showing that Cyt1A significantly delays the development of resistance to Cry11A, has practical value. Recombinant DNA techniques make it relatively easy to recombine mosquitocidal proteins from a range of different bacterial strains and species to construct bacterial stains of higher potency than occur naturally. The results of the present study show that long-term efficacy, and specifically the evolution of resistance to Cry proteins, will very probably be delayed by including Cyt1A or its homologs in these recombinant bacteria. Additional evidence for this was found in a recent study in which it was reported that only low levels of resistance evolved after long-term selection of C. quinquefasciatus with the mosquitocidal strain of B. thuringiensis subsp. jegathesan, which contains a different cytolytic toxin, Cyt2Bb (25).
Acknowledgments
We thank Jeffrey J. Johnson for his technical assistant during this study.
This research was supported in part by grants from the United States National Institutes of Health (AI45817) and the University of California Mosquito Research Program.
REFERENCES
- 1.Becker, N. 2000. Bacterial control of vector-mosquitoes and black flies, p. 383-398. In J.-F. Charles, A. Delécluse, and C. Nielsen-LeRoux (ed.), Entomopathogenic bacteria: from laboratory to field application. Kluwer, Dordrecht, The Netherlands.
- 2.Bohart, R. N., and R. K. Washino. 1978. Mosquitoes of California. Division of Agricultural Sciences, University of California, Berkeley.
- 3.Butko, P. 2003. Cytolytic toxin Cyt1A and its mechanism of membrane damage: data and hypotheses. Appl. Environ. Microbiol. 69:2415-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles, J.-F., C. Nielsen-LeRoux, and A. Delécluse. 1996. Bacillus sphaericus toxins: molecular biology and mode of action. Annu. Rev. Entomol. 41:451-472. [DOI] [PubMed] [Google Scholar]
- 5.Crickmore, N., E. J. Bone, J. A. Williams, and D. J. Ellar. 1995. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol. Lett. 131:249-254. [Google Scholar]
- 6.Federici, B. A., P. Lüthy, and J. E. Ibarra. 1990. The parasporal body of BTI; structure, protein composition, and toxicity, p. 16-44. In H. de Barjac and D. J. Sutherland (ed.), Bacterial control of mosquitoes and blackflies: biochemistry, genetics, and applications of Bacillus thuringiensis and Bacillus sphaericus. Rutgers University Press, New Brunswick, N.J.
- 7.Federici, B. A., H.-W. Park, D. K. Bideshi, M. C. Wirth, and J. J. Johnson. 2003. Recombinant bacteria for mosquito control. J. Exp. Biol. 206:3877-3885. [DOI] [PubMed] [Google Scholar]
- 8.Finney, D. 1971. Probit analysis. Cambridge University Press, Cambridge, England.
- 9.Georghiou, G. P., and M. C. Wirth. 1997. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 63:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg, L. J., and J. Margalit. 1977. A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata, Culex univitattus, Aedes aegypti, and Culex pipiens. Mosquito News 37:355-358. [Google Scholar]
- 11.Ibarra, J., and B. A. Federici. 1986. Isolation of a relatively nontoxic 65-kilodalton protein's inclusion from the parasporal body of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 165:527-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulla, M. S. 1990. Activity, field efficacy, and use of Bacillus thuringiensis israelensis against mosquitoes, p. 134-160. In H. de Barjac and D. J. Sutherland (ed.), Bacterial control of mosquitoes and blackflies: biochemistry, genetics, and applications of Bacillus thuringiensis and Bacillus sphaericus. Rutgers University Press, New Brunswick, N.J.
- 13.Nielsen-LeRoux, C., J.-F. Charles, I. Thiéry, and G. P. Georghiou. 1995. Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change on midgut brush-border membranes. Eur. J. Biochem. 228:206-210. [DOI] [PubMed] [Google Scholar]
- 14.Park, H.-W., B. Ge, L. S. Bauer, and B. A. Federici. 1998. Optimization of Cry3A yields in Bacillus thuringiensis by use of sporulation-dependent promoters in combination with the STAB-SD mRNA sequence. Appl. Environ. Microbiol. 64:3932-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poncet, S., A. Delécluse, A. Klier, and G. Rapoport. 1995. Evaluation of synergistic interactions among CryIVA, CryIVB, and CryIVD toxic components of Bacillus thuringiensis subsp. israelensis crystals. J. Invertebr. Pathol. 66:131-135. [Google Scholar]
- 16.Rao, D. R., T. R. Mani, R. Rajendran, A. S. Joseph, A. Gajanana, and R. Reuben. 1995. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J. Am. Mosquito Control Assoc. 11:1-5. [PubMed] [Google Scholar]
- 17.Raymond, M., G. Prato, and D. Ratsira. 1993. Probability analysis of mortality assays displaying quantal response, version 3.3. Praxeme, Saint Georges D'Orques, France.
- 18.Rodcharoen, J., and M. S. Mulla. 1994. Resistance development in Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus. J. Econ. Entomol. 87:1133-1140. [Google Scholar]
- 19.Schenpf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal proteins. Microbiol. Mol. Biol. Rev. 68:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva-Filha, M.-H., L. Regis, C. Nielsen-LeRoux, and J.-F. Charles. 1995. Low-level resistance to Bacillus sphaericus in a field-treated population of Culex quinquefasciatus (Diptera: Culicidae). J. Econ. Entomol. 88:525-530. [Google Scholar]
- 21.Sinègre, G., M. Babinot, J. M. Quermal, and B. Gaven. 1994. First field occurrence of Culex pipiens resistance to Bacillus sphaericus in southern France. VII Eur. Meet. Soc. Vector Ecol., p. 17.
- 22.Tabashnik, B. E. 1992. Evaluation of synergism among Bacillus thuringiensis toxins. Appl. Environ. Microbiol. 58:3343-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas, W., and D. J. Ellar. 1983. Mechanism of action of Bacillus thuringiensis var. israelensis insecticidal δ-endotoxin. FEBS Lett. 154:362-368. [DOI] [PubMed] [Google Scholar]
- 24.Wirth, M. C., G. P. Georghiou, and B. A. Federici. 1997. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito Culex quinquefasciatus. Proc. Natl. Acad. Sci. USA 94:10536-10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth, M. C., A. Delécluse, and W. E. Walton. 2004.. Laboratory selection for resistance to Bacillus thuringiensis subsp. jegathesan or a component toxin, Cry11B, in Culex quinquefasciatus Say (Diptera: Culicidae). J. Med. Entomol. 41:435-441. [DOI] [PubMed] [Google Scholar]
- 26.Wirth, M. C., W. E. Walton, and B. A. Federici. 2000. Cyt1Aa from Bacillus thuringiensis restores toxicity of Bacillus sphaericus against Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 37:401-407. [PubMed] [Google Scholar]
- 27.Wu, D., and F. N. Chang. 1985. Synergism in mosquitocidal activity of 26 and 65 kDa proteins from Bacillus thuringiensis subsp. israelensis crystal. FEBS Lett. 190:232-236. [Google Scholar]
- 28.Wu, D., and B. A. Federici. 1993. A 20-kilodalton protein preserves cell viability and promotes CytA crystal formation during sporulation in Bacillus thuringiensis. J. Bacteriol. 175:5276-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu, D., and B. A. Federici. 1995. Improved production of the insecticidal CryIVD protein in Bacillus thuringiensis using cryIA(c) promoters to express the gene for an associated 20-kDa protein. Appl. Microbiol. Biotechnol. 42:697-702. [DOI] [PubMed] [Google Scholar]
- 30.Yap, H.-H. 1990. Field trials of Bacillus sphaericus for mosquito control, p. 307-320. In H. de Barjac and D. J. Sutherland (ed.), Bacterial control of mosquitoes and blackflies: biochemistry, genetics, and applications of Bacillus thuringiensis and Bacillus sphaericus. Rutgers University Press, New Brunswick, N.J.
- 31.Yuan, Z. M., Y. M. Zhang, and E. Y. Liu. 2000. High-level field resistance to Bacillus sphaericus C3-41 in Culex quinquefasciatus from southern China. Biocontrol Sci. Technol. 10:43-51. [Google Scholar]