Abstract
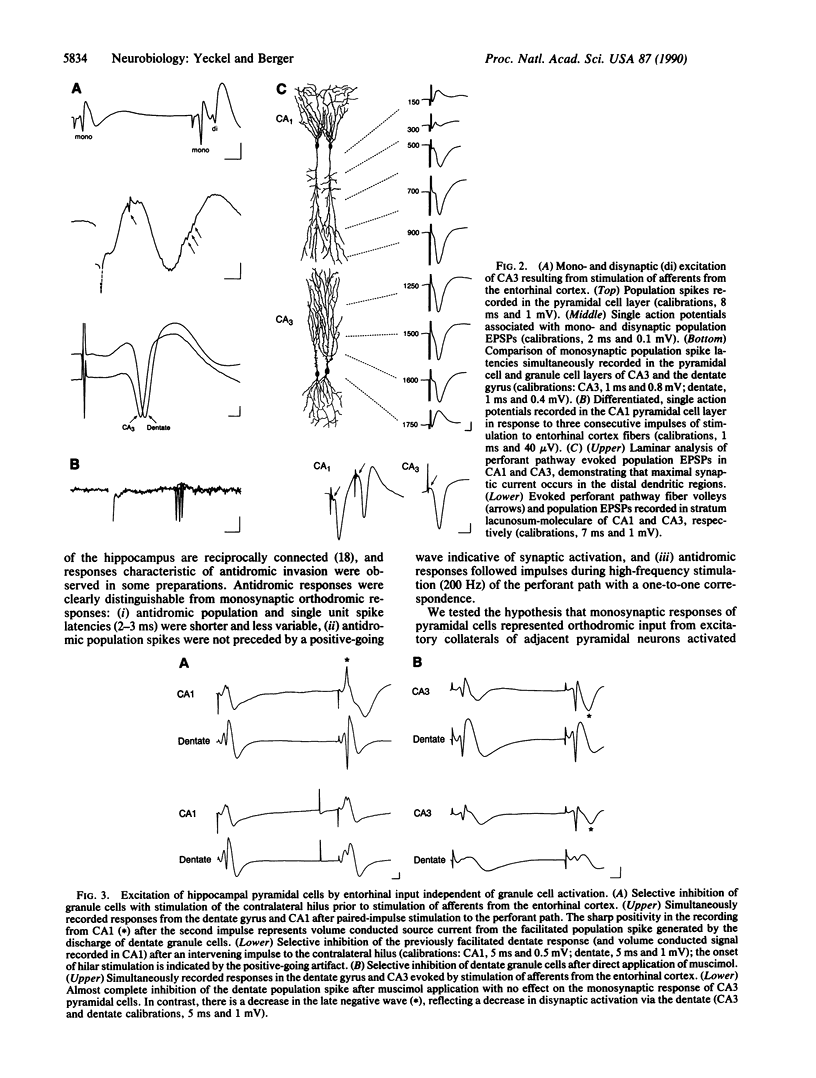
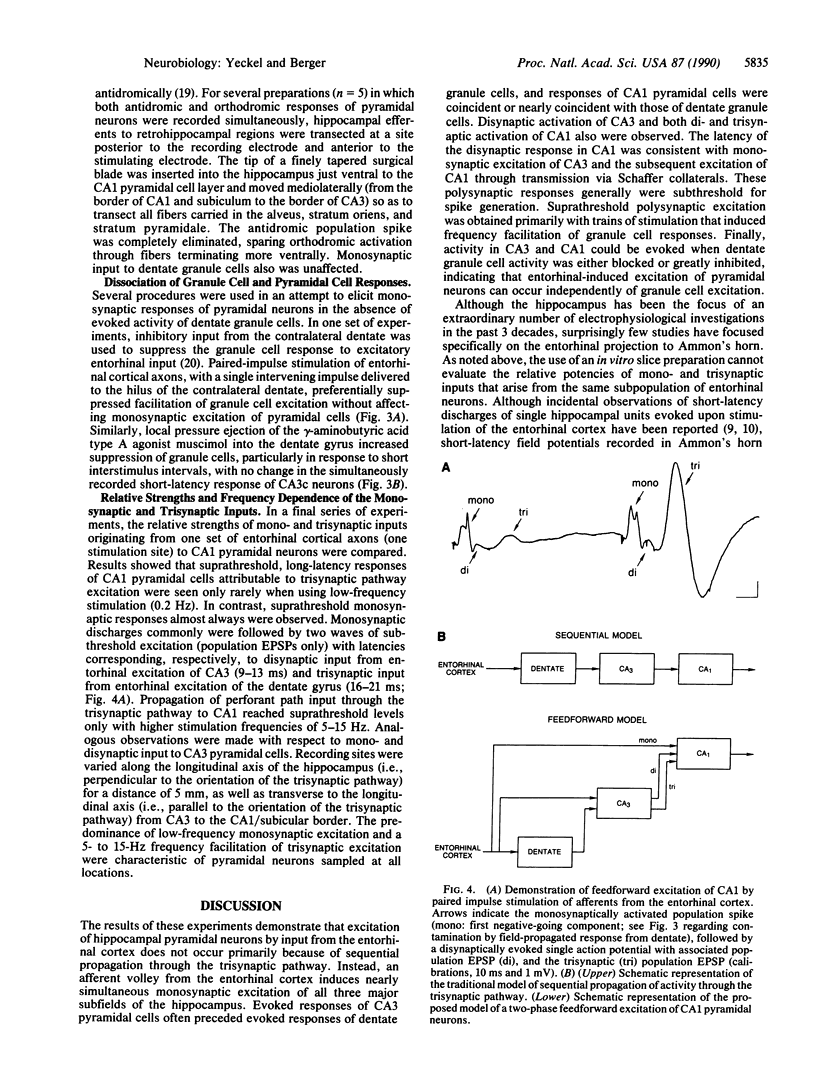
For the past 3 decades, functional characterizations of the hippocampus have emphasized its intrinsic trisynaptic circuitry, which consists of successive excitatory projections from the entorhinal cortex to the dentate gyrus, from granule cells of the dentate to the CA3/4 pyramidal cell region, and from CA3/4 to the CA1/2 pyramidal cell region. Despite unequivocal anatomical evidence for a monosynaptic projection from entorhinal to CA3 and CA1/2, few in vivo electrophysiological studies of the direct pathway have been reported. In the experiments presented here, we stimulated axons of entorhinal cortical neurons in vivo and recorded evoked single unit and population spike responses in the dentate, CA3, and CA1 of hippocampus, to determine if pyramidal cells are driven primarily via the monosynaptic or trisynaptic pathways. Our results show that neurons within the three subfields of the hippocampus discharge simultaneously in response to input from a given subpopulation of entorhinal cortical neurons and that the initial monosynaptic excitation of pyramidal cells then is followed by weaker excitatory volleys transmitted through the trisynaptic pathway. In addition, we found that responses of CA3 pyramidal cells often precede those of dentate granule cells and that excitation of CA3 and CA1 pyramidal cells can occur in the absence of granule cell excitation. In total, these results argue for a different conceptualization of the functional organization of the hippocampus with respect to the propagation of activity through its intrinsic pathways: input from the entorhinal cortex initiates a two-phase feedforward excitation of pyramidal cells, with the dentate gyrus providing feedforward excitation of CA3, and with both the dentate and CA3 providing feedforward excitation of CA1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Excitatory synapses on hippocampal apical dendrites activated by entorhinal stimulation. Acta Physiol Scand. 1966 Apr;66(4):461–472. doi: 10.1111/j.1748-1716.1966.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Rinaldi P. C., Weisz D. J., Thompson R. F. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophysiol. 1983 Nov;50(5):1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Berger T. W., Swanson G. W., Milner T. A., Lynch G. S., Thompson R. F. Reciprocal anatomical connections between hippocampus and subiculum in the rabbit evidence for subicular innervation of regio superior. Brain Res. 1980 Feb 10;183(2):265–276. doi: 10.1016/0006-8993(80)90463-1. [DOI] [PubMed] [Google Scholar]
- Buzsàki G., Eidelberg E. Commissural projection to the dentate gyrus of the rat: evidence for feed-forward inhibition. Brain Res. 1981 Dec 28;230(1-2):346–350. doi: 10.1016/0006-8993(81)90413-3. [DOI] [PubMed] [Google Scholar]
- Christian E. P., Dudek F. E. Electrophysiological evidence from glutamate microapplications for local excitatory circuits in the CA1 area of rat hippocampal slices. J Neurophysiol. 1988 Jan;59(1):110–123. doi: 10.1152/jn.1988.59.1.110. [DOI] [PubMed] [Google Scholar]
- Doller H. J., Weight F. F. Perforant pathway activation of hippocampal CA1 stratum pyramidale neurons: electrophysiological evidence for a direct pathway. Brain Res. 1982 Apr 8;237(1):1–13. doi: 10.1016/0006-8993(82)90553-4. [DOI] [PubMed] [Google Scholar]
- Fox S. E., Ranck J. B., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41(3-4):399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- Herkenham M. The connections of the nucleus reuniens thalami: evidence for a direct thalamo-hippocampal pathway in the rat. J Comp Neurol. 1978 Feb 15;177(4):589–610. doi: 10.1002/cne.901770405. [DOI] [PubMed] [Google Scholar]
- Herreras O., Solis J. M., Martin del Rio R., Lerma J. Characteristics of CA1 activation through the hippocampal trisynaptic pathway in the unanaesthetized rat. Brain Res. 1987 Jun 9;413(1):75–86. doi: 10.1016/0006-8993(87)90155-7. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- Matthews D. A., Salvaterra P. M., Crawford G. D., Houser C. R., Vaughn J. E. An immunocytochemical study of choline acetyltransferase-containing neurons and axon terminals in normal and partially deafferented hippocampal formation. Brain Res. 1987 Jan 27;402(1):30–43. doi: 10.1016/0006-8993(87)91044-4. [DOI] [PubMed] [Google Scholar]
- Miller V. M., Best P. J. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and endorhinal cortex. Brain Res. 1980 Aug 4;194(2):311–323. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- Muller R. U., Kubie J. L., Ranck J. B., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987 Jul;7(7):1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G., Diamond D., Lynch G. S. Dentate granule cells in the rat hippocampal formation have the behavioral characteristics of theta neurons. Brain Res. 1983 Apr 25;266(1):29–37. doi: 10.1016/0006-8993(83)91306-9. [DOI] [PubMed] [Google Scholar]
- Segal M., Disterhoft J. F., Olds J. Hippocampal unit activity during classical aversive and appetitive conditioning. Science. 1972 Feb 18;175(4023):792–794. doi: 10.1126/science.175.4023.792. [DOI] [PubMed] [Google Scholar]
- Segal M. Flow of conditioned responses in limbic telencephalic system of the rat. J Neurophysiol. 1973 Sep;36(5):840–854. doi: 10.1152/jn.1973.36.5.840. [DOI] [PubMed] [Google Scholar]
- Segal M. Hippocampal unit responses to perforant path stimulation. Exp Neurol. 1972 Jun;35(3):541–546. doi: 10.1016/0014-4886(72)90124-0. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976 Jun 1;167(3):285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977 Mar 1;172(1):49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Wyss J. M., Cowan W. M. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978 Oct 15;181(4):681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Winson J., Abzug C. Neuronal transmission through hippocampal pathways dependent on behavior. J Neurophysiol. 1978 May;41(3):716–732. doi: 10.1152/jn.1978.41.3.716. [DOI] [PubMed] [Google Scholar]