Abstract
Campylobacter incidence in England and Wales between 1990 and 1999 was examined in conjunction with weather conditions. Over the 10-year interval, the average annual rate was determined to be 78.4 ± 15.0 cases per 100,000, with an upward trend. Rates were higher in males than in females, regardless of age, and highest in children less than 5 years old. Major regional differences were detected, with the highest rates in Wales and the southwest and the lowest in the southeast. The disease displayed a seasonal pattern, and increased campylobacter rates were found to be correlated with temperature. The most marked seasonal effect was observed for children under the age of 5. The seasonal pattern of campylobacter infections indicated a linkage with environmental factors rather than food sources. Therefore, public health interventions should not be restricted to food-borne approaches, and the epidemiology of the seasonal peak in human campylobacter infections may best be understood through studies in young children.
Nearly 30 years ago, campylobacter infection emerged as a leading bacterial cause of gastroenteritis in developed countries (47). Two species, Campylobacter jejuni and Campylobacter coli, are responsible for over 99% of human campylobacter infections (6, 23). Major infection sources include undercooked poultry, contaminated milk, untreated water, and animal contact (24). The public health consequences of human campylobacter infection are large, in part because of its high incidence (61). In developed countries, campylobacter causes more illnesses than Shigella spp. and Salmonella spp. combined (55). Only a fraction of cases are reported (25), and some estimates suggest as much as 1% of the population in the United States and Europe is affected by campylobacter each year (57). The annual cost in the United States in 1996 alone was estimated at US$4.3 billion (5), and £69.6 million was the estimated cost in the United Kingdom in 1994 (43). In addition to acute gastroenteritis, campylobacter infections may be complicated by neurological (35, 53), rheumatological (26, 34), and renal (10) problems. Effective prevention and control strategies to reduce the population burden of campylobacter infections require a robust understanding of the epidemiology of this disease. Case-control studies to investigate the origins of human infection showed that the majority of cases of campylobacter infection were not explained by the commonly recognized risk factors (1, 13, 14, 30, 37, 44). For example, despite public health interventions focused on reducing food-borne transmission (16, 17), campylobacter incidence remains high (15). A striking phenomenon is the remarkably pronounced and consistent seasonal pattern, for which the explanation is unclear (29, 38, 41). This study investigated the relationship between seasonal variation in human campylobacter infection in England and Wales and environmental conditions to obtain new insights with respect to disease transmission.
MATERIALS AND METHODS
Epidemiological data.
Campylobacter data were obtained from the Health Protection Agency, London, United Kingdom, and included all laboratory-confirmed cases reported in England and Wales between 1990 and 1999 (n = 440,012). Cases associated with foreign travel or known to be part of community or family outbreaks were excluded (22) on the basis of their clinical history, leaving 401,270 cases for analysis. Records included the date when stool specimens were received for analysis at the primary laboratory, as well as gender and age of each patient (with 97.7 and 95.0% completeness, respectively).
Demographic data.
Demographic data for England and Wales, obtained from the Office for National Statistics, London, United Kingdom, provided denominators for calculation of incidence rates. Mid-year populations categorized by age, gender, and Health Authority (HA) were obtained from 1990 to 1999, using revised figures generated by the 2001 national census (40).
Regional partitioning.
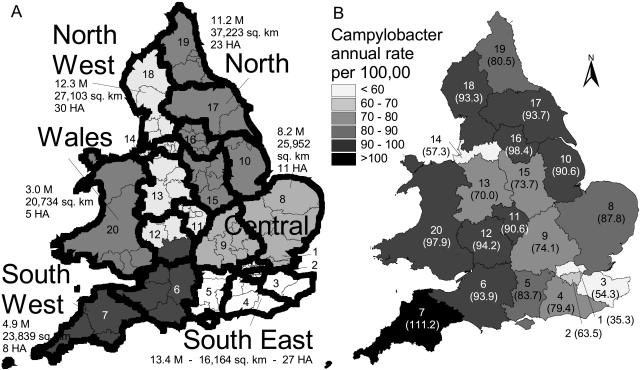
Epidemiological data were obtained for 104 HAs. Two types of spatial partitioning were performed (Fig. 1A) to facilitate analysis and avoid small-scale spatial artifacts. Data were aggregated into 20 districts chosen to ensure relative homogeneity within each district. The data were also aggregated into six regions (Wales, Northwest, Southwest, North, Central, and Southeast, including 16 HAs in London), to further summarize the data. Demographic data were reported over the corresponding areas.
FIG. 1.
(A) Map of England and Wales showing 104 HAs (thin outlines), 20 numbered districts (thick outline), and 6 regions (gray shading), with corresponding populations in millions for 2000 (40), surface area (in square kilometers), and the number of HAs. Districts: 1, North London; 2, South London; 3, Kent; 4, Surrey and Sussex; 5, Berkshire, Hampshire, and Isle of Wight; 6, Avon, Somerset, Dorset, and Wiltshire; 7, Devon and Cornwall; 8, Essex and East Anglia; 9, central counties; 10, South Humber and Lincolnshire; 11, central Midlands; 12, southwest Midlands and Gloucestershire; 13, northwest Midlands; 14, Greater Liverpool and Manchester; 15, East Midlands; 16, West and South Yorkshire, North Yorkshire, and Lincolnshire; 17, Cumbria and Lancashire; 19, Northeast; 20, Wales. (B) Map of annual campylobacter incidence (annual cases per 100,000) by district, shown with district numbers and, in parentheses, corresponding campylobacter rates.
Meteorological data.
Daily meteorological data were obtained from the British Atmospheric Data Centre, which provided access to the Met Office Land Surface Observation Stations data (http://badc.nerc.ac.uk/data/surface/). Daily values for minimum and maximum temperature (degrees Celsius), rainfall (in millimeters), and hours of sunshine were retrieved from 66 meteorological stations throughout England and Wales. Meteorological stations were selected for data completeness over the study period and for an even geographical distribution over the study area. Climatic data were averaged per week and per study region (or district). The number of meteorological stations per study region was as follows: Wales, 9; Northwest, 10; Southwest, 11; North, 13; Central, 12; and Southeast, 11.
Other data.
For England, agricultural data were obtained from the Department for Environment, Food and Rural Affairs (11), and rural information was from the Countryside Agency (http://www.countryside.gov.uk/). For Wales, the Statistical Directorate (56) provided a limited agricultural data set. Information about water companies in the United Kingdom was obtained from the Drinking Water Inspectorate (12).
Analysis.
The statistical software package SAS (version 8.2; SAS Institute Inc., Cary, N.C.) was used to carry out statistical analysis. PROC AUTOREG was used for regression analysis with time series. The rates were transformed with the Freeman-Tukey square root to improve the model assumptions (9), as follows: FTi = (100,000)1/2 {[Ci/Ni]1/2 + [(Ci + 1)/Ni]1/2}, where FTi is the Freeman-Tukey transformed rate in region i, Ci is the corresponding number of campylobacter cases, and Ni is the corresponding population. A spline-fitting procedure (PROC GPLOT) was used to determine peak maxima graphically. This smoothing procedure, suitable for noisy data, corresponds to a cubic spline interpolation in which plotted points do not necessarily fall on the line (46). Throughout the study, a significant statistical difference was declared when P was <0.05.
RESULTS
Epidemiology.
The overall annual campylobacter incidence rate in England and Wales between 1990 and 1999 was 78.4 ± 15.0 (mean ± standard deviation) cases per 100,000, ranging from 29,633 to 52,840 reported cases per year with an average of 40,127 cases per year (Table 1). There were significant statistical differences between regions, with the highest incidence rates observed in the West and the North and the lowest in the Southeast of England and Wales (Table 1; Fig. 1B). An upward trend was observed throughout the decade (Table 1; Fig. 2), with relative increase averaging 53.2% and ranging from 16.9% in the Southeast to 82.1% in the Southwest. Most regions experienced their lowest annual rate of the decade in 1991 and their highest in 1998. The Southeast region, which included London, experienced significantly lower rates and lower relative increases compared to other regions.
TABLE 1.
Mean annual campylobacter incidence (cases per 100,000) by region, overall, by gender, and by age (1990 to 1999)
| Region | Mean ± SD incidenceb | Δ%a 1990-1999 | Incidenceb by gender
|
Incidenceb by age (yrs)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | <1 | 1-4 | 5-19 | 20-34 | 35-49 | 50-69 | ≥70 | |||
| Central | 84.4 ± 20.6 | 80.8 | 89.1 | 70.9 | 144.6 | 130.1 | 46.5 | 110.2 | 80.2 | 66.6 | 42.2 |
| North | 86.6 ± 12.5 | 36.5 | 93.4 | 76.9 | 210.4 | 156.1 | 49.4 | 109.4 | 91.9 | 72.4 | 46.6 |
| Northwest | 77.1 ± 19.7 | 76.4 | 83.4 | 68.5 | 199.5 | 138.6 | 43.0 | 96.3 | 75.3 | 62.7 | 44.5 |
| Southeast | 56.7 ± 6.6 | 16.9 | 61.8 | 50.2 | 112.4 | 97.9 | 33.5 | 70.4 | 54.3 | 48.2 | 35.8 |
| Southwest | 100.2 ± 27.5 | 82.1 | 109.5 | 90.4 | 203.9 | 208.8 | 64.7 | 130.7 | 100.1 | 86.0 | 60.2 |
| Wales | 97.9 ± 19.0 | 50.1 | 106.9 | 86.8 | 226.3 | 195.8 | 62.8 | 129.8 | 99.3 | 76.5 | 48.6 |
| All | 78.4 ± 15.0 | 53.2 | 84.7 | 69.0 | 172.4 | 139.4 | 45.6 | 98.7 | 77.8 | 65.0 | 44.3 |
Percentage increase in campylobacter incidence between 1990 and 1999.
Cases per 100,000.
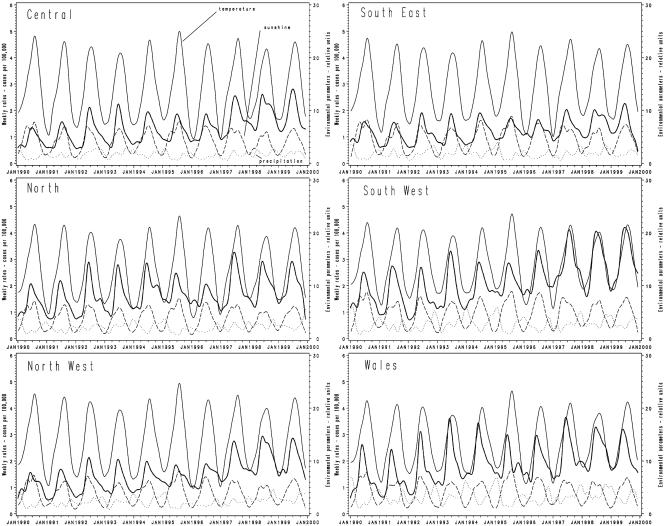
FIG. 2.
Time series of weekly campylobacter incidence (weekly cases per 100,000) and environmental factors, by region. Thick solid line, campylobacter rates per 100,000; thin solid line, temperature; dashed line, precipitation; dotted line, sunshine. Color figures are available at http://www.panix.com/∼vlouis/campy/.
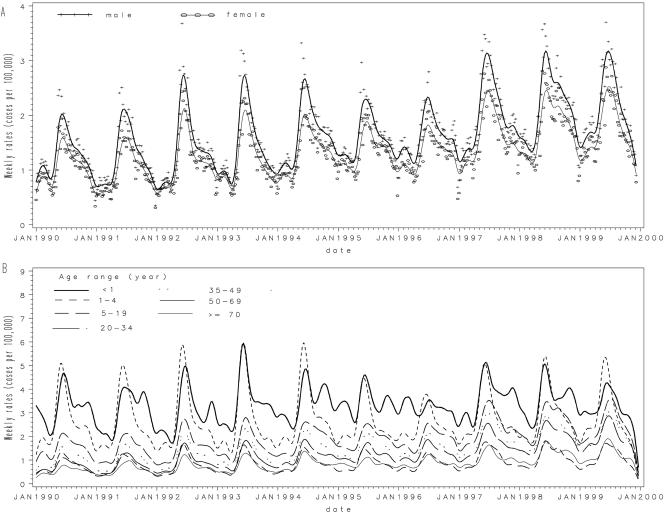
Males represented 53.7% of campylobacter cases in the study and consistently experienced higher rates (Table 1; Fig. 3A). This difference was observed in all regions throughout the time period and in all age groups. Campylobacter incidence rates were highest in children under 5 years of age, and especially in those under 1 year of age (Table 1; Fig. 3B). A second peak was observed in the 20-to-34-year age group, and incidence slowly tapered off with older groups. Overall, the time series showed that patterns by age and gender were observed consistently throughout annual variations.
FIG. 3.
Time series of campylobacter incidence (weekly cases per 100,000) by gender (A) or by age category (in years) (B). The smooth lines represent spline interpolations. For clarity, original data points (+, ○) are shown only in panel A.
Age group and gender pattern.
When age-specific analyses were undertaken, the most marked seasonal variations in campylobacter rates were observed for children under 5 years of age (Fig. 3B). Campylobacter incidence in infants (those less than 1 year old) exhibited multiple peaks, with the highest in spring, while children aged 1 to 4 years experienced a single sharp peak in the spring. The annual peak in the children under 5 preceded the peak in adult groups by an average of 2 weeks (P < 0.01) (Fig. 3B). The annual peak in the male population usually preceded the peak in the female population by 1 or 2 weeks, but the difference between the two groups was not significant (Fig. 3A).
Seasonality.
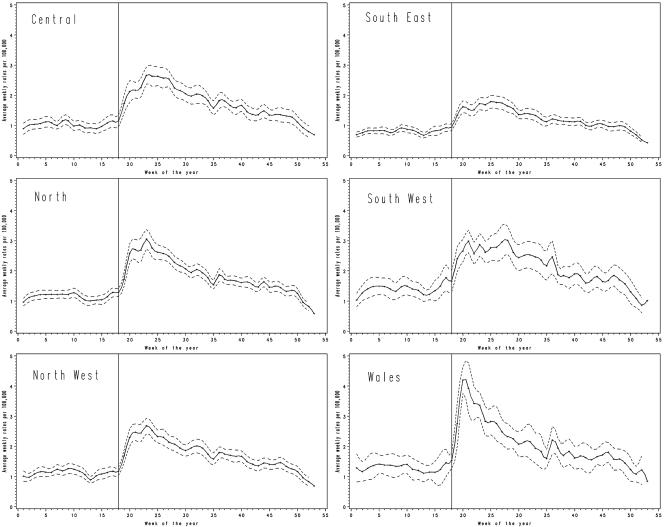
A marked seasonal pattern was observed (Fig. 2), with the seasonal rise starting at the beginning of May and the peak typically occurring between mid-June and mid-July (Fig. 4). The date and shape of the peak varied with geographical location. In Wales a uniquely consistent pattern was observed, with a sharp well-defined spring peak occurring earlier (in early June) than in other areas. By contrast, in the southeast of England the seasonal increase was less pronounced, and the peak incidence was in late June.
FIG. 4.
Campylobacter incidence annual pattern by area. Solid line, average campylobacter rate (weekly cases per 100,000); dotted line, 95% confidence interval around the 10-year mean; vertical line, onset of the annual peak around 1 May.
Environmental factors.
Average weekly temperature, precipitation, and hours of sunshine were included in the analysis. Time series by region showed the relationship of campylobacter incidence to the environmental parameters (Fig. 2).
Multiple regression analysis showed that daily average temperature, precipitation, and sunshine were significantly associated with campylobacter rates (R2 = 0.2342; P < 0.001). Similar results were obtained when a correction for the long-term trend of campylobacter increase was included by adding time into the model (R2 = 0.3329; P < 0.001). Because time series were used, correction for autocorrelated errors was investigated, as it was expected that a value for a given week would be correlated to that of the preceding week. When such correction was included, temperature was found to be the single variable that contributed most to the model and was significant. Nonetheless, the model with all three variables (temperature, precipitation, and hours of sunshine), remained the best-performing model. Even though temperature and sunshine data were expectedly correlated (R2 = 0.5264; P < 0.001), the variable of temperature (whether minimum or maximum daily temperature) performed better in regression models than using the variable of hours of sunshine alone. The effect of different lag periods (i.e., 1, 2, or 3 weeks) was examined, but this had no effect on the findings. Data were also analyzed by region, and similar results were obtained.
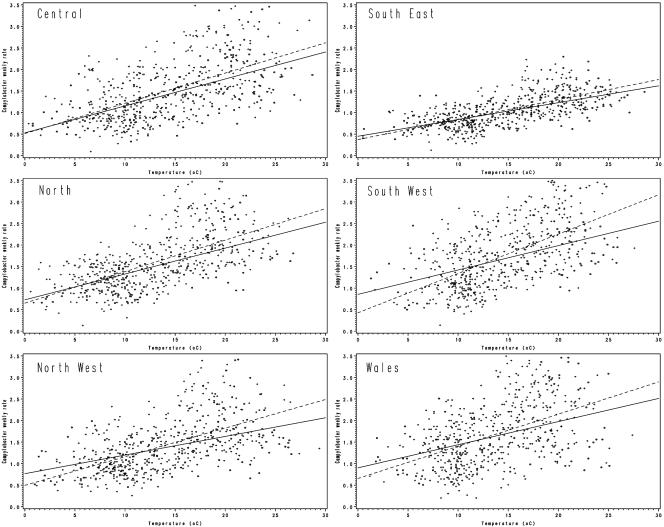
To further investigate the role of rainfall, we examined regression analyses for each region under conditions defined as no rain, when rainfall for the week was <1 mm, or rain, when rainfall was ≥1 mm (Fig. 5). We considered two models, one with temperature alone and one with temperature, rain (as a dichotomous variable), and a term of the interaction between temperature and rain. In all six regions temperature was a highly significant term (P < 0.0001). In four regions, terms involving rain failed to be significant (P > 0.05), and in two regions they were significant but only improved the adjusted R2 by 1%. The R2 values ranged from 0.1919 to 0.4171 when only temperature was in the model.
FIG. 5.
Linear regression of weekly campylobacter rates versus average temperature by geographical area. Circle, no rain (<1 mm in the week); +, rain (≥1 mm in the week). The linear regression line is shown for each case (solid and dotted lines, respectively).
Agriculture and surface water.
At the district level, campylobacter rates significantly correlated with the agricultural data analyzed, namely, total agricultural area, agricultural labor force, cattle, pigs, sheep, and poultry (Table 2). Campylobacter rates were positively correlated with the percentage of rural wards in a district and inversely correlated with population density. When looking at water company supply, it was noted that districts with high campylobacter incidence in the west and north of England and Wales were supplied with over 80% surface water, whereas districts in the southeast with low incidence were supplied with less than 30% surface water. However, a quantitative analysis did not yield a significant correlation (Table 2).
TABLE 2.
Correlation between agricultural data and Freeman-Tukey-transformed campylobacter rates at the district levela
| Variable | Correlationb | P | n | Yr(s) of data availability |
|---|---|---|---|---|
| Total labor employed in agriculture | 0.396 | 0.003 | 55 | 1990,c 1995,c 1999 |
| Total agricultural land | 0.390 | 0.004 | 55 | 1990,c 1995,c 1999 |
| Total cattle | 0.464 | <0.001 | 55 | 1990,c 1995,c 1999 |
| Total pigs | 0.454 | 0.006 | 55 | 1990,c 1995,c 1999 |
| Total sheep | 0.322 | 0.018 | 55 | 1990,c 1995,c 1999 |
| Total poultry | 0.595 | 0.007 | 19 | 1999 |
| Population density | −0.570 | 0.011 | 19 | 1991 |
| % Rural wards in district | 0.544 | 0.020 | 18 | 1991c |
| % of surface water supplied by water co. | 0.348 | 0.325 | 10 | 2001 |
With a 19-district partition that merges North and South London, as required for agricultural data reporting. Statistically significant values are in bold.
Spearman's r value (nonparametric measure of association [45]), ranging from −1 to +1.
Data are missing for Wales.
DISCUSSION
Epidemiology.
The epidemiological results of this study are in general agreement with current knowledge. That is, incidence rates, trends, and patterns for age and gender distribution match observations of campylobacter infections in developed countries (21). The higher male incidence, though typical, has not been fully explained (2, 21). It was observed in all age groups, suggesting a higher susceptibility in males rather than distinctive at-risk behaviors. Higher incidences in children under 5 and, to a lesser extent, young adults, have been reported previously (21). Here we further show the 1-to-4-years age group especially exhibits the seasonal peak of campylobacteriosis. Children under 1 year also showed high campylobacter incidence during the spring peak, but other peaks were also present throughout the year. Protective maternal immunity transferred to infants (42) and weaning at different ages may contribute to the less-clear picture in infants.
Since 1999, a decline in incidence has been observed in the United Kingdom (18). In the United States, the Centers for Disease Control and Prevention observed a slow decrease since 1996 (19, 20). Neither the increase in the 1990s in Europe (21) nor the decline since the end of the decade has been fully explained (19). Advances in food safety and stricter food regulations have probably contributed to the slight decrease in campylobacter incidence over the last few years (20).
Source of bias.
In seeking to explain our observations, it was important to identify potential sources of bias which might have affected our findings. For any emerging pathogen, such as campylobacter, early changes in trend may simply reflect changing laboratory practice rather than real changes in disease incidence. By 1990, however, laboratory methods for campylobacter isolation and reporting of positive results to national surveillance were well established. Clinical practice might also have affected our results. Infants and young children are more likely to be presented to their primary care physician, compared with older age groups, and stool specimens are more likely to be obtained from them, so that age-related estimates of risk might be affected (52). However, there is no evidence to suggest that these effects would vary with season; therefore, the observed seasonal pattern is judged to be real.
Temperature effects will precede measured disease incidence since there are in-built delays, not only those in reporting to national surveillance but also those related to ecological response. The former comprise incubation period, time from disease onset to presentation to a primary care physician, time from stool sample submission to diagnosis, and time taken to report a positive result to national surveillance and, for the latter, ecological factors. We have tried to overcome, at least in part, this lag period by using the date when the stool specimen was received at the primary laboratory in the time series analyses. It should be noted that although the ideal dates would have been case onset dates, these are not consistently recorded in the national surveillance data set.
Regional partitioning produced spatial smoothing and permitted identification of trends and effective presentation of results. We took care to confirm that we were capturing trends and not overlooking important local phenomena. Analysis with data aggregated over both districts and regions yielded congruent results.
Seasonality and environmental factors.
Seasonality was observed throughout England and Wales. The peak observed in the spring was consistent with results of other studies (38), in which different seasonal patterns were observed among countries. However, we show here that the increase in campylobacter incidence is associated with increased temperature. The results also indicate that campylobacter incidence is correlated to agricultural activities. This suggests that environmental factors play a role, at least in part, in the epidemiology of campylobacteriosis. The fact that rural and less populated areas were correlated with higher campylobacter rates is in logical agreement with the links to agricultural activities. In the examination of the link with surface water, the spatial areas of the water company did not overlap well with the epidemiological data. This represented a severe limitation in that the data available allowed only for a crude and limited analysis. A proper analysis would require water data at a finer scale. At this point, a possible link with surface water is intriguing but unproven.
Campylobacter spp. can be readily isolated from surface waters and can survive for weeks in the aquatic environment (28). In surface waters, Campylobacter spp. can be isolated more often at lower temperatures and during the winter (28). This may be explained by the fact that UV light reduces campylobacter survival (39), and warmer temperatures may favor other bacteria out-competing Campylobacter spp. Despite the commonly held belief that Campylobacter spp. cannot grow at temperatures below 30°C (33), it is well established that this organism survives in the environment (4, 33), with the ability to overwinter. Campylobacter spp. are transmitted via a wide array of ecological pathways, such as farm animals, wild birds, and surface water (28). Because of the multiplicity of pathways, prevention of campylobacter infections must be undertaken with considerations other than food safety alone.
Public health implications.
Public health interventions aimed at controlling campylobacter incidence have focused primarily on reducing food-borne infections, in particular those caused by contaminated poultry (16-19). Such interventions are useful and may have contributed to the reduction of campylobacter incidence observed in recent years (20). However, the food-borne route of infection does not fully explain the seasonal peak of campylobacteriosis, as evidenced by the reduced seasonality we observed in the Southeast. Studies have shown that contamination of retail chicken can vary from 0 to 100% (59). It is not unusual to observe a high level of contamination (>40%) year round (8, 17, 62), and with a large proportion of contaminated chicken seasonal variation may be observed but is not always significant (17, 60, 62). Even when campylobacter seasonality is clearly marked in chicken, as studies have shown in the United States (59), Scandinavia (3, 31, 32, 58), The Netherlands (27), and Wales (36), it is difficult to establish a direct correlation between human and chicken campylobacter isolates. Genotypic analyses generally point to a common pool of isolates and multiple reservoirs (7, 32). These results suggest that a common route of infection may be driving both chicken colonization and human campylobacter infections (3, 32, 60). Friedman et al. (21) suggested that water may be that route. Larger farm animals such as cattle and sheep have been shown to shed campylobacter intermittently in their feces (49, 50). The timing of such events usually coincides with stress for the animal, such as change of pasture or birthing, often occurs in the spring, and may contribute to environmental contamination (48). Though campylobacter detection in surface water does not correlate with human cases of campylobacteriosis (28), water plays a complex role in the ecology of campylobacter and its transmission between the environment, wild birds, cattle, sheep, poultry, and humans (28, 33, 48, 54). Campylobacter strains isolated from natural waters may also have different ecological or pathological characteristics than those isolated from clinical sources (51). The interplay between water, which may serve as the reservoir for campylobacter, and the seasonal peak in human campylobacteriosis needs to be refined.
Based on our results, we conclude that the incidence of campylobacter infection is temperature related and that children under 5 years of age are at greatest risk during the seasonal increase, compared with other age groups. Thus, the epidemiology of the seasonal peak in human campylobacter infections may best be understood through studies in infants and young children. Most importantly, however, investigating the role of the waterborne route will shed light on the larger ecological picture involved in seasonal campylobacter transmission.
Acknowledgments
We thank the British Atmospheric Data Centre, which provided access to the Met Office Land Surface Observation Stations data.
REFERENCES
- 1.Adak, G. K., J. M. Cowden, S. Nicholas, and H. S. Evans. 1995. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of Campylobacter infection. Epidemiol. Infect. 115:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 3.Bang, D. D., E. M. Nielsen, K. Knudsen, and M. Madsen. 2003. A one-year study of campylobacter carriage by individual Danish broiler chickens as the basis for selection of Campylobacter spp. strains for a chicken infection model. Epidemiol. Infect. 130:323-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzby, J. C., and T. Roberts. 1997. Economic costs and trade impacts of microbial foodborne illness. World Health Stat. Q. 50:57-66. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2002. Campylobacter infections. [Online.] http://www.cdc.gov/ncidod/dbmd/diseaseinfo/campylobacter_g.htm.
- 7.Colles, F., K. Jones, R. Harding, and M. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 69:7409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corry, J. E., and H. I. Atabay. 2001. Poultry as a source of Campylobacter and related organisms. J. Appl. Microbiol. 90(Suppl.):96S-114S. [DOI] [PubMed] [Google Scholar]
- 9.Cressie, N. A. C. 1993. Statistics for spatial data. Wiley-Interscience, New York, N.Y.
- 10.Denneberg, T., M. Friedberg, L. Holmberg, C. Mathiasen, K. O. Nilsson, R. Takolander, and M. Walder. 1982. Combined plasmapheresis and hemodialysis treatment for severe hemolytic-uremic syndrome following Campylobacter colitis. Acta Paediatr. Scand. 71:243-245. [DOI] [PubMed] [Google Scholar]
- 11.Department for Environment Food and Rural Affairs. 002. Defra: economics and statistics. [Online.] http://statistics.defra.gov.uk/esg/.
- 12.Drinking Water Inspectorate. 2003. A report by the chief inspector. [Online.] http://www.dwi.gov.uk/pubs/annrep01/index.htm.
- 13.Eberhart-Phillips, J., N. Walker, N. Garrett, D. Bell, D. Sinclair, W. Rainger, and M. Bates. 1997. Campylobacteriosis in New Zealand: results of a case-control study. J. Epidemiol. Community Health 51:686-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Effler, P., M. C. Leong, A. Kimura, M. Nakata, R. Burr, E. Cremer, and L. Slutsker. 2001. Sporadic Campylobacter jejuni infections in Hawaii: associations with prior antibiotic use and commercially prepared chicken. J. Infect. Dis. 183:1152-1155. [DOI] [PubMed] [Google Scholar]
- 15.Evans, M. R., C. D. Ribeiro, and R. L. Salmon. 2003. Hazards of healthy living: bottled water and salad vegetables as risk factors for campylobacter infection. Emerg. Infect. Dis. 9:1219-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food Standards Agency. 2001. Report of the study of infectious intestinal disease in England. [Online.] http://www.foodstandards.gov.uk/science/research/research_archive/scientific/intestinal.
- 17.Food Standards Agency. 2001. Survey of Salmonella and Campylobacter contamination of fresh and frozen chicken on retail sale. [Online.] http://www.foodstandards.gov.uk/multimedia/pdfs/campsalmsurvey.pdf.
- 18.Foodborne Disease Strategy Consultative Group. 2002. Incidence and trends in foodborne disease in the UK in the years 2001-2002. [Online.] www.foodstandards.gov.uk/multimedia/pdfs/foodbornediseasetrends.pdf.
- 19.FoodNet. 2002. FoodNet annual report 2001. [Online.] http://www.cdc.gov/foodnet/annual/2001/summary/2001annualreport_pdf.pdf.
- 20.FoodNet. 2003. Preliminary FoodNet data on the incidence of foodborne illnesses—selected sites, United States, 2002. Morb. Mortal. Wkly. Rep. 52:340-343. [PubMed] [Google Scholar]
- 21.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 22.Frost, J., I. Gillespie, and S. O'Brien. 2002. Public health implications of campylobacter outbreaks in England and Wales, 1995-9: epidemiological and microbiological investigations. Epidemiol. Infect. 128:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frost, J., J. Kramer, and S. Gillanders. 1999. Phage typing of Campylobacter jejuni and Campylobacter coli and its use as an adjunct to serotyping. Epidemiol. Infect. 123:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost, J. A. 2001. Current epidemiological issues in human campylobacteriosis. J. Appl. Microbiol. 30(Suppl.):85S-95S. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie, I., S. O'Brien, J. Frost, G. Adak, P. Horby, A. Swan, M. Painter, K. Neal, et al. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannu, T., L. Mattila, H. Rautelin, P. Pelkonen, P. Lahdenne, A. Siitonen, and M. Leirisalo-Repo. 2002. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 41:312-318. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs-Reitsma, W. F., N. M. Bolder, and R. W. Mulder. 1994. Cecal carriage of Campylobacter and Salmonella in Dutch broiler flocks at slaughter: a one-year study. Poult. Sci. 73:1260-1266. [DOI] [PubMed] [Google Scholar]
- 28.Jones, K. 2001. Campylobacters in water, sewage and the environment. J. Appl. Microbiol. 90(Suppl.):68S-79S. [DOI] [PubMed] [Google Scholar]
- 29.Kapperud, G., and S. Aasen. 1992. Descriptive epidemiology of infections due to thermotolerant Campylobacter spp. in Norway, 1979-1988. APMIS 100:883-890. [PubMed] [Google Scholar]
- 30.Kapperud, G., E. Skjerve, N. H. Bean, S. M. Ostroff, and J. Lassen. 1992. Risk factors for sporadic Campylobacter infections: results of a case-control study in southeastern Norway. J. Clin. Microbiol. 30:3117-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapperud, G., E. Skjerve, L. Vik, K. Hauge, A. Lysaker, I. Aalmen, S. M. Ostroff, and M. Potter. 1993. Epidemiologic investigation of risk-factors for campylobacter colonization in Norwegian broiler flocks. Epidemiol. Infect. 111:245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karenlampi, R., H. Rautelin, M. Hakkinen, and M.-L. Hanninen. 2003. Temporal and geographical distribution and overlap of Penner heat-stable serotypes and pulsed-field gel electrophoresis genotypes of Campylobacter jejuni isolates collected from humans and chickens in Finland during a seasonal peak. J. Clin. Microbiol. 41:4870-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenraad, P. M., F. M. Rombouts, and S. H. Notermans. 1997. Epidemiological aspect of thermophilic Campylobacter in water-related environments: a review. Water Environ. Res. 69:52-63. [Google Scholar]
- 34.Locht, H., and K. A. Krogfelt. 2002. Comparison of rheumatological and gastrointestinal symptoms after infection with Campylobacter jejuni/coli and enterotoxigenic Escherichia coli. Ann. Rheum. Dis. 61:448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy, N., and J. Giesecke. 2001. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. Am. J. Epidemiol. 153:610-614. [DOI] [PubMed] [Google Scholar]
- 36.Meldrum, R. J., D. Tucker, and C. Edwards. 2004. Baseline rates of Campylobacter and Salmonella in raw chicken in Wales, United Kingdom, in 2002. J. Food Prot. 67:1226-1228. [DOI] [PubMed] [Google Scholar]
- 37.Neal, K. R., and R. C. Slack. 1997. Diabetes mellitus, anti-secretory drugs and other risk factors for Campylobacter gastro-enteritis in adults: a case-control study. Epidemiol. Infect. 119:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nylen, G., F. Dunstan, S. Palmer, Y. Andersson, F. Bager, J. Cowden, G. Feierl, Y. Galloway, G. Kapperud, F. Megraud, K. Molbak, L. Petersen, and P. Ruutu. 2002. The seasonal distribution of Campylobacter infection in nine European countries and New Zealand. Epidemiol. Infect. 128:383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obiri-Danso, K., N. Paul, and K. Jones. 2001. The effects of UVB and temperature on the survival of natural populations and pure cultures of Campylobacter jejuni, Camp. coli, Camp. lari and urease-positive thermophilic campylobacters (UPTC) in surface waters. J. Appl. Microbiol. 90:256-267. [DOI] [PubMed] [Google Scholar]
- 40.Office for National Statistics. 2003. Population estimates for England and Wales. [Online.] http://www.statistics.gov.uk/popest.
- 41.Pearson, A. D., and T. D. Healing. 1992. The surveillance and control of Campylobacter infection. Commun. Dis. Rep. CDR Rev. 2:R133-R139. [PubMed] [Google Scholar]
- 42.Renom, G., M. Kirimat, A. Georges, J. C. Philippe, and P. M. V. Martin. 1992. High-levels of anti-campylobacter-flagellin IgA antibodies in breast-milk. Res. Microbiol. 143:93-98. [DOI] [PubMed] [Google Scholar]
- 43.Roberts, J., P. Cumberland, P. Sockett, J. Wheeler, L. Rodrigues, D. Sethi, and P. Roderick. 2003. The study of infectious intestinal disease in England: socio-economic impact. Epidemiol. Infect. 130:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues, L., J. Cowden, J. Wheeler, D. Sethi, P. Wall, P. Cumberland, D. Tompkins, M. Hudson, J. Roberts, and P. Roderick. 2001. The study of infectious intestinal disease in England: risk factors for cases of infectious intestinal disease with Campylobacter jejuni infection. Epidemiol. Infect. 127:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SAS. 1990. The CORR procedure, version 6, p. 209-235. SAS procedure guide, 3rd ed. SAS Institute, Inc., Cary, N.C.
- 46.SAS. 1987. SAS/GRAPH guide for personal computers, version 6, p. 62-63. SAS Institute, Inc., Cary, N.C.
- 47.Skirrow, M. 1977. Campylobacter enteritis: a “new” disease. Br. Med. J. 2:9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanley, K., and K. Jones. 2003. Cattle and sheep farms as reservoirs of Campylobacter. J. Appl. Microbiol. 94(Suppl.):104S-113S. [DOI] [PubMed] [Google Scholar]
- 49.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480. [DOI] [PubMed] [Google Scholar]
- 50.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. Seasonal variation of thermophilic campylobacters in lambs at slaughter. J. Appl. Microbiol. 84:1111-1116. [DOI] [PubMed] [Google Scholar]
- 51.Stelzer, W., J. Jacob, and E. Schulze. 1991. Environmental aspects of campylobacter infections. Zentbl. Mikrobiol. 146:3-15. [PubMed] [Google Scholar]
- 52.Tam, C. C., L. C. Rodrigues, and S. J. O'Brien. 2003. The study of infectious intestinal disease in England: what risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. Int. J. Epidemiol. 32:99-105. [DOI] [PubMed] [Google Scholar]
- 53.Tam, C. C., L. R. Rodrigues, and S. J. O'Brien. 2003. Guillain-Barré syndrome associated with Campylobacter jejuni infection in England, 2000-2001. Clin. Infect. Dis. 37:307-310. [DOI] [PubMed] [Google Scholar]
- 54.Thomas, C., H. Gibson, D. J. Hill, and M. Mabey. 1999. Campylobacter epidemiology: an aquatic perspective. J. Appl. Microbiol. 85(Suppl.):168S-177S. [DOI] [PubMed] [Google Scholar]
- 55.U.S. Food and Drug Administration. 2003. Foodborne pathogenic microorganisms and natural toxins handbook. [Online.] http://vm.cfsan.fda.gov/∼mow/chap4.html.
- 56.Wales Statistical Directorate. 2003. Wales in figures. [Online.] http://www.wales.gov.uk/keypubstatisticsforwalesfigures/index.htm.
- 57.Wassenaar, T., and D. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wedderkopp, A., K. O. Gradel, J. C. Jorgensen, and M. Madsen. 2001. Pre-harvest surveillance of Campylobacter and Salmonella in Danish broiler flocks: a 2-year study. Int. J. Food Microbiol. 68:53-59. [DOI] [PubMed] [Google Scholar]
- 59.Willis, W. L., and C. Murray. 1997. Campylobacter jejuni seasonal recovery observations of retail market broilers. Poultry Sci. 76:314-317. [DOI] [PubMed] [Google Scholar]
- 60.Wilson, I. G. 2002. Salmonella and Campylobacter contamination of raw retail chickens from different producers: a six year survey. Epidemiol. Infect. 129:635-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. 2002. The increasing incidence of human campylobacteriosis. Report and proceedings of a W.H.O. consultation of experts. [Online.] http://www.who.int/emc-documents/zoonoses/docs/whocdscsraph20017.pdf.
- 62.Zhao, C., B. Ge, J. De Villena, R. Sudler, E. Yeh, S. Zhao, D. G. White, D. Wagner, and J. Meng. 2001. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., area. Appl. Environ. Microbiol. 67:5431-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]