Abstract
The microbicidal activity of four different biocides was studied in synthetic metalworking fluid (MWF) against Mycobacterium immunogenum, a suspected causative agent for hypersensitivity pneumonitis, and Pseudomonas fluorescens, a representative for the predominant gram-negative bacterial contaminants of MWF. The results indicated that M. immunogenum is more resistant than P. fluorescens to the tested formaldehyde-releasing biocides (Grotan and Bioban), isothiazolone (Kathon), and phenolic biocide (Preventol). Kathon was effective against mycobacteria at lower concentrations than the other three test biocides in MWF. In general, there was a marked increase in biocidal resistance of both the test organisms when present in MWF matrix compared to saline. Increased resistance of the two test organisms to biocides was observed when they were in a mixed suspension (1:1 ratio). The results indicate the protective effect of the MWF matrix against the action of commonly used biocides on the MWF-colonizing microbial species of occupational health significance, including mycobacteria.
Metalworking fluid (MWF) is used as lubricant, coolant, and/or metal removing agent in machining operations. The modern synthetic metalworking fluids, which contain organic and inorganic salts, hydrocarbons, organic esters, and lubricating fluids, are excellent sources of nutrition for microorganisms (5). Microbial contamination of MWF has been frequently associated with occupational health problems, such as hypersensitivity pneumonitis due to inhalation of aerosolized MWF bacteria (9, 10) and dermatitis due to MWF microflora exposure (1), that occur in metalworkers. The occurrence of mycobacteria in MWF and their potential occupational health significance has been highlighted (8, 10, 18). Mycobacterium immunogenum, a nontuberculous Mycobacterium species, has been reported in MWF and implicated as possible causative agent of hypersensitivity pneumonitis in machine workers who have been exposed to it (21, 23, 24). Among other MWF microbial communities, pseudomonads often constitute the major fraction of gram-negative organisms responsible for endotoxin release and accumulation in the fluids, resulting in occupational health hazards in metalworkers (2). In modern machine industries, the most commonly used method to control microbial contamination is the use of chemical biocides. However, little information is available on evaluation of relative efficacy of commercial biocides against microbial genera or species associated with metalworking fluids. Our aim here was to investigate the potential effectiveness of the commonly used commercial formaldehyde (HCHO) and nonformaldehyde (non-HCHO) biocides in metalworking fluids against two widely recognized bacterial genera of occupational health concern: Mycobacterium and Pseudomonas. To our knowledge, this is the first report to investigate the effect of any biocide against the MWF-associated Mycobacterium species alone and in the presence of common microbial cocontaminant Pseudomonas.
Microorganisms and culture conditions
The MWF isolate M. immunogenum (ATCC 700506) and a representative gram-negative organism in MWF, Pseudomonas fluorescens (ATCC 13525), were used. All experiments were performed with Middlebrook 7H9 (MB7H9) broth and MB7H10 agar (Difco Laboratories, Sparks, Md.) supplemented with 10% oleic acid-albumin-dextrose-catalase enrichment medium (BD Biosciences, Sparks, Md.) and 0.5% glycerol. The bacterial cultures of M. immunogenum and P. fluorescens were grown to exponential phase in 40 ml of broth with continuous shaking (150 rpm) at 37°C for 120 h and at 25°C for 24 h, respectively, to a cell density equivalent to a 120 Klett reading (108 CFU/ml) measured by using a Klett photoelectric colorimeter (Klett, New York, N.Y.).
Biocides and metalworking fluid
Four commercial biocides were evaluated, including two HCHO-releasing biocides, Grotan (Troy Chemical Corp., Newark, N.J.) and Bioban CS 1135 (DOW Chemical Co., Midland, Mich.). The active ingredient in Grotan is hexahydro-1,3,5-tris(2-hydroxyethyl)-s-triazine (78.9%). The active ingredient in Bioban is 4,4-dimethyloxazolidine-3,4,4-trimethyloxazolidine (76%). An isothiazolone group biocide, Kathon 886 MW (Rohm & Haas Co., Philadelphia, Pa.) with 14.1% 5-chloro-2-methyl-4-isothiazolin-3-one as the active ingredient and a phenolic biocide, Preventol CMK 40 (Bayer Chemicals Corp., Pittsburgh, Pa.), with 4-chloro-3-methylphenol sodium salt (46.13%) as the active ingredient were also tested.
A commercial synthetic metalworking fluid at a commonly used working concentration of 5% (vol/vol) was filter sterilized and used as the test matrix. Saline was used as the control matrix for comparison.
Biocide efficacy test
The efficacy of the four biocides in fluid suspension tests was studied based on logarithmic reductions in viable counts of the test organisms at different concentrations of biocides with various contact times. The log reductions were estimated by converting initial and posttreatment bacterial counts to log10 values and then subtracting the mean of the final log10 values from the initial log10 value. The initial log value was obtained from the mean of the values for the control containing no biocide.
For both test organisms, the same batch of culture suspension was used in all treatments with a given test biocide to minimize experimental variation. The test organisms were serially diluted to have 108 CFU/ml in MWF and saline matrices. Aliquots (1 ml) of the organisms suspension was placed into 1.5-ml microcentrifuge tubes and treated with individual biocides. Experiments were also conducted with the two organisms in a mixed suspension (1:1). A single biocide at various final concentrations (100, 1,000, 10,000, and 100,000 ppm) was added to test sample and incubated at room temperature for various contact times (15, 30, 45, and 60 min). Survival of the test organisms was determined by taking 100-μl aliquot from each treatment and spread plating on Middlebrook agar, followed by incubation at 37°C for 120 h (M. immunogenum) and 25°C for 24 h (P. fluorescens). For experiments with mixed suspensions of M. immunogenum and P. fluorescens, selective counts for each test organism were obtained by incubating two different sets of Middlebrook agar plates at two different temperatures of incubation, one set at 37°C for M. immunogenum for 120 h and the other set at 25°C for P. fluorescens for 24 h.
Determination of MIC of biocides
The precise MICs were determined from the biocide concentration range and contact time resulting in 100% kill of test organisms in the two different matrices (MWF and saline). The MIC represented the minimum concentration required to attain complete loss of cultivability of the test organism as determined by spread plating.
Statistical analysis
Log reductions were compared by using analysis of variance by a mixed model with SAS/STAT software (SAS Institute, Inc., Cary, N.C.).
Biocidal activity against M. immunogenum
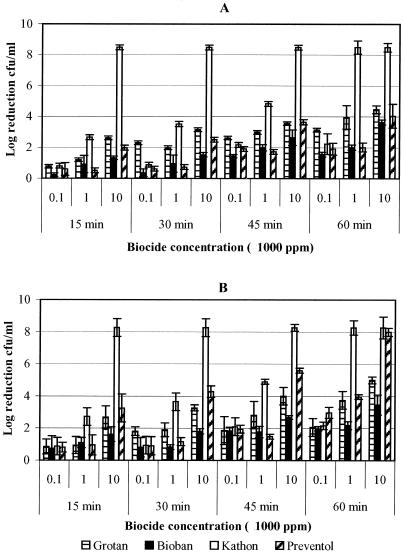
The results represented in Fig. 1 indicate the biocidal activity of four different biocides against pure suspension of M. immunogenum in MWF and saline matrices. Log reductions at the highest concentration (100,000 ppm) of biocides are not included in the figure since these treatments led to 100% kill of the test organisms at all contact times. M. immunogenum showed greater log reductions when treated with the isothiazolone biocide, Kathon, than with the other non-HCHO biocide (Preventol) or with either of the two HCHO biocides (Grotan and Bioban). Kathon was observed to be significantly (P < 0.0001) more effective at lowest exposure time (15 min) with 10,000 ppm biocide concentration, as shown by a log reduction of ≥8 as against ≤2-log reductions observed with other test biocides (Fig. 1A). There was no significant (P = 0.5030) matrix effect (MWF versus saline) on Kathon biocidal activity toward M. immunogenum, although there was a difference in the log reductions in the two matrices. Between the two HCHO biocides, Grotan was observed to be more effective than Bioban in MWF, as evidenced by significant differences (P = 0.0311) in the log reductions (Fig. 1A). However, there were no significant log reductions between these two HCHO biocides in saline except at high concentrations (P = 0.0234; Fig. 1B). In the presence of Preventol, M. immunogenum showed a significantly (P = 0.0395) greater log reduction in saline than in MWF (Fig. 1).
FIG. 1.
Determination of the efficacy of four biocides (Grotan, Bioban, Kathon, and Preventol) presented as the logarithmic reduction in the cell concentration of M. immunogenum in pure suspension. The biocides were tested at various concentrations for different contact times (15, 30, 45, and 60 min). (A) Log10 reduction of M. immunogenum in MWF; (B) log10 reduction in saline.
As in the pure suspension, Kathon biocide was observed to be most effective against M. immunogenum in mixed suspension compared to other biocides (Fig. 2). At lower biocide concentrations (100 and 1,000 ppm) with a 15-min exposure time, there were no significant differences (P = 0.4195) in cell concentrations (≤2-log reductions) for all four biocides in both matrices (MWF and saline). However, Kathon was twice as effective at 10,000 ppm as the other biocides in MWF (Fig. 2A). In a comparison of the HCHO biocides, there was no clear trend in the biocidal activities of Grotan and Bioban in both MWF and saline matrices. In contrast, non-HCHO biocides (Kathon and Preventol) were comparable in saline showing no significant differences (P = 0.3700; Fig. 2B) in cell concentrations. In general, M. immunogenum in mixed suspension showed greater resistance than in pure suspension to all biocides based on the log reduction of cell concentration.
FIG. 2.
Determination of the efficacy of four biocides (Grotan, Bioban, Kathon, and Preventol) presented as logarithmic reduction in the cell concentration of M. immunogenum in mixed suspension. The biocides were tested at various concentrations for different contact times of 15, 30, 45, and 60 min. (A) Log10 reduction patterns of M. immunogenum in MWF fluid; (B) log10 reduction patterns in saline matrix.
Biocidal activity against P. fluorescens
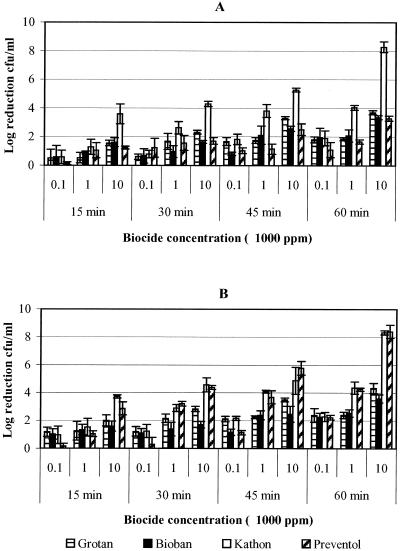
The relative susceptibility of P. fluorescens to the four test biocides is presented in Fig. 3. The data for 10,000 and 100,000 ppm biocide concentrations and 45 and 60 min exposure times are not presented because of 100% kill of the test organism under these conditions. P. fluorescens was found to be relatively more susceptible to all test biocides than M. immunogenum, showing maximum log reduction (≥8) in cell concentration within 15 and/or 30 min contact time (Fig. 3); the corresponding log reduction value for the cell concentration of M. immunogenum being ≤2 (Fig. 1 and 2). In MWF fluid, Kathon was the most effective since it caused maximum log reduction of cell concentration (≥8 logs) at a minimum biocide concentration (10 ppm) in a 30-min exposure compared to the other test biocides, which required 1,000 ppm (Fig. 3A) for this level of inhibition. These differences were significant (P < 0.0001). However, in saline, Kathon was as effective as Bioban and Preventol (≥6-log reductions in cell concentration for each), and this activity was significantly (P < 0.0148) higher than that of Grotan (≥4-log reduction in cell concentration; Fig. 3A).
FIG. 3.
Determination of the efficacy of four biocides (Grotan, Bioban, Kathon, and Preventol) presented as logarithmic reduction in the cell concentration of P. fluorescens in pure and mixed suspensions. The biocides were used at various concentrations for different contact times of 15, 30, 45, and 60 min. (A) Log10 reduction of P. fluorescens in pure suspension in MWF and saline; (B) log10 reduction of P. fluorescens in mixed suspension in MWF and saline matrices.
A greater susceptibility of P. fluorescens to Kathon biocide was also observed in MWF fluid with mixed suspension (Fig. 3B). In contrast, Bioban was observed to be more effective in saline than Kathon and other biocides (Fig. 3B). However, at 1,000 ppm, Preventol was as effective as Bioban, and the differences in log reduction of cell concentration were not significant (P = 0.1008).
MICs of biocides
Based on the MICs, M. immunogenum was more resistant than P. fluorescens (Table 1). Among the four biocides, Kathon showed highest biocidal activity (least MICs) against both test organisms in MWF fluid. The observed Kathon MICs for pure and mixed suspensions were 600 and 3,000 ppm for M. immunogenum and 20 and 100 ppm for P. fluorescens, respectively. However, Bioban was very effective in saline against P. fluorescens with MICs of 30 and 60 ppm for pure and mixed suspensions, respectively. In general, the HCHO biocides (Grotan and Bioban) were least effective (showing highest MICs) against M. immunogenum in both fluid matrices, whereas Grotan and Preventol were least effective (showing highest MICs) against P. fluorescens in MWF (Table 1).
TABLE 1.
MICs of biocide for M. immunogenum and P. fluorescens in pure and mixed suspensions treated with biocides in MWF and saline matrices
| Biocide (type) | MIC of biocide (ppm)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
M. immunogenum (60 min)
|
P. fluorescens (30 min)
|
|||||||
| Pure suspension
|
Mixed suspension with P. fluorescens
|
Pure suspension
|
Mixed suspension with M. immunogenum
|
|||||
| MWF | Saline | MWF | Saline | MWF | Saline | MWF | Saline | |
| Grotan (HCHO) | 25,000 | 13,000 | 32,000 | 23,000 | 1,000 | 80 | 2,000 | 300 |
| Bioban (HCHO) | 42,000 | 24,000 | 25,000 | 23,000 | 400 | 30 | 900 | 60 |
| Kathon (isothiazolone) | 600 | 900 | 3,000 | 4,000 | 20 | 100 | 100 | 200 |
| Preventol (phenolic) | 12,000 | 3,000 | 14,000 | 6,000 | 800 | 100 | 4,000 | 600 |
The MIC value corresponded to the lowest concentration that caused complete growth inhibition (100% kill) of the test organism at a specified contact time (60 min for M. immunogenum and 30 min for P. fluorescens).
Relative resistance of M. immunogenum versus P. fluorescens
Our results show that M. immunogenum possesses significantly higher resistance to all biocides, since it requires higher concentration of biocides or a longer contact time compared to Pseudomonas. Mycobacteria have been known for their resistance against other antimicrobial agents compared to the relatively more sensitive gram-negative and gram-positive bacteria. The hydrophobic, waxy, and impermeable nature of mycobacterial cell wall, which contains mycolic acids, arabinogalactan and high-molecular-weight lipids, have been postulated to be responsible for their antimicrobial resistance (3). The observed resistance of Pseudomonas in presence of the test biocides up to certain concentrations is consistent with the earlier reports on the biocide resistance of this genus. For instance, P. fluorescens has been reported to cause the deterioration of Grotan BK biocide (14) as a result of the transformation of HCHO molecules to formic acid (20). In another report, Kathon resistance of P. fluorescens was ascribed to its ability to degrade this biocide in sewage waters (6). Another species of this genus, P. aeruginosa, had shown resistance to formaldehyde and 1,3,5-tris-(2-hydroxyethyl)-s-triazine, which are the active components of Grotan BK (20).
Relative efficacy of the test biocides in MWF versus saline
Kathon (Isothiazolone) biocide was observed to be effective at lower concentrations compared to the other test biocides, belonging to the HCHO-releasing (Grotan and Bioban) and the phenolic (Preventol) types. The Kathon efficacy was particularly more pronounced in MWF compared to saline. Since the isothiazolone has been reported to be activated in alkaline pH (17), the prevalence of high pH in MWF matrix (pH 8 to 10) might have caused an increased Kathon biocidal activity in MWF compared to saline. The increased biocidal activity in MWF might also be due to the synergistic interaction between the biocide and the corrosion inhibitor component, sodium tolyl triazole, of synthetic metalworking fluid used in this study (15).
The relatively greater resistance of M. immunogenum against HCHO-releasing biocides compared to Kathon might be due to the action of mycothiol, a unique thiol compound produced in mycobacteria and actinomycetes, which detoxifies the active component formaldehyde (13). In addition, the hydrophobic and waxy nature of mycobacterial cell wall, which discourages the interaction of formaldehyde and thus provides protection against HCHO biocides (7). However, further studies may be required to prove these phenomena in M. immunogenum. The resistant nature of mycobacteria against biocides was also indicated in earlier studies on hospital hygiene, wherein M. chelonae was found to be resistant to glutaraldehyde and other aldehydes (22). Between the two HCHO biocides used in the present study, Grotan was observed to be more effective than Bioban against M. immunogenum and P. fluorescens in MWF matrix. This could be explained by the fact that Grotan releases three HCHO molecules per molecule on hydrolysis compared to one HCHO molecule from Bioban (4, 19). Both the HCHO-releasing biocides showed appreciable difference in biocidal activity against M. immunogenum and P. fluorescens between MWF and saline matrices, indicating protective effect of the MWF matrix.
Preventol (phenolic) was intermediate, between Kathon (Isothiazolone) and the HCHO biocides (Grotan and Bioban), in biocidal activity against M. immunogenum. However, with P. fluorescens, Preventol was in general less effective than other biocides particularly in MWF. The Preventol results are consistent with the assumption that phenolic biocides are weak in physical interaction with lipophilic components of the bacterial cell wall, and this could be responsible for the observed resistance of M. immunogenum and particularly P. fluorescens to Preventol (11). Phenolic biocides are more active at acid and neutral pH (16), and this could also be one of the reasons for comparatively increased biocidal activity of Preventol in saline (neutral pH) compared to MWF (alkaline pH). However, further investigation is required to prove these possible mechanisms.
Cocontaminant effect
The cocontaminant effect on biocidal activity toward the individual test organisms was studied with the test organisms in a mixed suspension (1:1 ratio). M. immunogenum was found to be more resistant to both HCHO and non-HCHO biocides in mixed suspension with P. fluorescens than in pure suspension, and this was observed in both the MWF and saline matrices. Three of the four biocides—Grotan (1.3-fold), Kathon (5-fold), and Preventol (3-fold)—showed various decrease in biocidal activity in mixed bacterial suspensions. The relative biocidal resistance of P. fluorescens in mixed suspension with the tested biocides in either matrix was also increased, except for Kathon. An increased biocidal resistance of both M. immunogenum and P. fluorescens in mixed suspension treatment compared to pure suspension treatment suggests a possible mutual protective mechanism among the microbial communities in metalworking fluid environment, leading to their enhanced ability to resist biocide. We used the same number of cells of both test organisms in the 1:1 mixed suspensions to that used in experiments with pure suspension. Therefore, the biocide may have been diluted, distributed, or adsorbed in a differential manner between the two test organisms. As a result, the actual concentration of the biocide contacting either organism in mixed suspension experiments was less than the added concentration, thereby leading to greater survival or protection from killing. Interestingly, Bioban showed the increased biocidal activity against mixed suspension of P. fluorescens in saline matrix, the reason for that is not clear.
In conclusion, both mycobacteria and the pseudomonads have the ability to tolerate the biocides albeit to a varying extent. M. immunogenum is significantly more tolerant to the tested biocides. These observations point to the limited usefulness of the commercial biocides commonly applied for microbial control in MWF particularly against mycobacteria. Moreover, it is evident from the collected data that metalworking fluid matrix enhances survival of mycobacteria in presence of biocides at otherwise inhibitory concentrations in other aqueous media such as saline. These observations emphasize the importance of evaluating each commercial biocide against appropriate problem strains of bacteria commonly prevalent in MWF and study the effect of fluid matrix and cocontaminants for such evaluation.
Acknowledgments
This study was supported by grant 1RO1OH007364 (to J.S.Y.) from the National Institute of Occupational Safety and Health, The Centers for Disease Control and Prevention. The U.S. Council for Automotive Research-Environmental Research Consortium (USCAR-ERC) supported the initial methods optimization part of the study.
We thank Milacron management and the USCAR-ERC for obtaining the commercial biocides from different sources. We further acknowledge Linda Levin, Biostatistics Division, University of Cincinnati, for the help in statistical analysis.
REFERENCES
- 1.Awosika-Olumo, A. I., K. L. Trangle, and L. F. Fallon. 2003. Microorganism-induced skin disease in workers exposed to metal working fluids. Occup. Med. 53:35-40. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein D. I., Z. L. Lummus, G. Santilli, J. Siskosky, and I. L. Bernstein. 1995. Machine operator's lung. A hypersensitivity pneumonitis disorder associated with exposure to metalworking fluid aerosols. Chest 108:636-641. [DOI] [PubMed] [Google Scholar]
- 3.Brennen, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmeir, T., J. Geier, J. P. Lepoittevin, and P. J. Frosch. 2002. Patch test reactions to Biobans in metalworkers are often weak and not reproducible. Contact Derm. 47:27-31. [DOI] [PubMed] [Google Scholar]
- 5.Buers, K. L. M., E. L. Prince, and C. J. Knowles. 1997. The ability of selected bacterial isolates to utilize components of synthetic metal-working fluids as sole sources of carbon and nitrogen for growth. Biotechnol. Lett. 19:791-794. [Google Scholar]
- 6.Chazal, P. M. 1995. Pollution of modern metalworking fluids containing biocides by pathogenic bacteria in France. Reexamination of chemical treatments accuracy. Eur. J. Epidemiol. 11:1-7. [DOI] [PubMed] [Google Scholar]
- 7.Eager, R. G., J. Leder, J., and A. B. Theis. 1986. Glutaraldehyde: factors important for microbicidal efficacy, p. 32-49. Proceedings of the Third Conference on Progress in Chemical Disinfection. SUNY, Binghamton, N.Y.
- 8.Falkinham, J. O. 2003. Mycobacterial aerosols and respiratory disease. Emerg. Infect. Dis. 9:763-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox, J., H. Anderson, T. Moen, G. Gruetzmacher, L. Hanrahan, and J. Fink. 1999. Metal working fluid-associated hypersensitivity pneumonitis: an outbreak investigation and case-control study. Am. J. Ind. Med. 35:58-67. [DOI] [PubMed] [Google Scholar]
- 10.Kreiss, K., and J. Cox-Ganser. 1997. Metal working fluid-associated hypersensitivity pneumonitis: a workshop summary. Am. J. Ind. Med. 32:423-432. [DOI] [PubMed] [Google Scholar]
- 11.Maillard, J. Y. 2002. Bacterial target sites for biocide action. J. Appl. Microbiol. 92:16S-27S. [PubMed] [Google Scholar]
- 12.Moore, J. S., M. Christensen, R. W. Wilson, R. J. Wallace, Jr., Y. Zhang, D. R. Nash, and B. Shelton. 2000. Mycobacterial contamination of metalworking fluids: involvement of a possible new taxon of rapidly growing mycobacteria. AIHAJ 61:205-213. [DOI] [PubMed] [Google Scholar]
- 13.Newton, G. L., and R. C. Fahey. 2002. Mycothiol biochemistry. Arch. Microbiol. 178:388-394. [DOI] [PubMed] [Google Scholar]
- 14.Onyekwelu, I. U., E. O. Bennett, and J. E. Gannon. 1981. The effective life of preservatives in cutting fluids concentrates. Triology 1981:7-9. [Google Scholar]
- 15.Rossmoore, H. W. 1995. Biocides for metalworking lubricants and hydraulic fluids, p. 133-156. In H. W. Rossmoore (ed.), Handbook of biocide and preservative use. Blackie Academic & Professional, New York, N.Y.
- 16.Rossmoore, H. W., and L. A. Rossmoore. 1991. Effect of microbial growth products on biocide activity in metalworking fluids. Int. Biodeterior. 27:145-156. [Google Scholar]
- 17.Rossmoore, L. A., and H. W. Rossmoore. 1994. Metalworking fluid microbiology, p. 247-271. In J. P. Byers (ed.), Metalworking fluids. Marcel Decker, Inc., New York, N.Y.
- 18.Shelton, G. B., W. D. Flanders, and G. K. Morris. 1999. Mycobacterium sp. as a possible cause of hypersensitivity pneumonitis in machine workers. Emerg. Infect. Dis. 5:270-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sondossi, M., H. W. Rossmoore, and J. W. Wireman. 1986. The effect of fifteen biocides on formaldehyde-resistant strains of Pseudomonas aeruginosa. J. Ind. Microbiol. 1:87-96. [Google Scholar]
- 20.Sondossi, M., H. W. Rossmoore, and R. Williams. 1989. Relative formaldehyde resistance among bacterial survivors of biocide-treated metalworking fluid. Int. Biodeterior. 25:423-437. [Google Scholar]
- 21.Trout, D., D. N. Weissman, D. Lewis, R. A. Brundage, A. Franzblau, and D. Remick. 2003. Evaluation of hypersensitivity pneumonitis among workers exposed to metal removal fluids. Appl. Occup. Environ. Hyg. 18:953-960. [DOI] [PubMed] [Google Scholar]
- 22.van Klingeren, B., and W. Pullen. 1993. Glutaraldehyde resistant mycobacteria from endoscope washers. J. Hosp. Infect. 25:147-149. [DOI] [PubMed] [Google Scholar]
- 23.Wallace, R. J., Jr., Y. Zhang, R. W. Wilson, L. Mann, and H. Rossmoore. 2002. Presence of a single genotype of the newly described species Mycobacterium immunogenum in industrial metalworking fluids associated with hypersensitivity pneumonitis. Appl. Environ. Microbiol. 68:5580-5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav, J. S., I. Khan, F. Fakhari, and M. B. Soellner. 2003. DNA-based methodologies for rapid detection, quantification, and species- or strain-level identification of respiratory pathogens (mycobacteria and pseudomonads) in metalworking fluids. Appl. Occup. Environ. Hyg. 18:966-975. [DOI] [PubMed] [Google Scholar]