Abstract
Bacteriochlorophyll a-containing aerobic anoxygenic phototrophs (AAnP) have been proposed to account for up to 11% of the total surface water microbial community and to potentially have great ecological importance in the world's oceans. Recently, environmental and genomic data based on analysis of the pufM gene identified the existence of α-proteobacteria as well as possible γ-like proteobacteria among AAnP in the Pacific Ocean. Here we report on analyses of environmental samples from the Red and Mediterranean Seas by using pufM as well as the bchX and bchL genes as molecular markers. The majority of photosynthesis genes retrieved from these seas were related to Roseobacter-like AAnP sequences. Furthermore, the sequence of a novel photosynthetic operon organization from an uncultured Roseobacter-like bacterial artificial chromosome retrieved from the Red Sea is described. The data show the presence of Roseobacter-like bacteria in Red and Mediterranean Sea AAnP populations in the seasons analyzed.
Recent reports suggest that aerobic anoxygenic phototrophs (AAnP) form a significant fraction of the marine bacterioplankton community (16, 17). Known cultured planktonic AAnP belong to only a few restricted groups within the Alphaproteobacteria (39) and include Roseobacter and Erythrobacter spp. Until recently, Erythrobacter spp. were among the more commonly cultured bacteriochlorophyll a-containing bacteria recovered from the open ocean (15, 16). The functionality of their photosynthetic apparatus was recently proven by kinetic bacteriochlorophyll fluorescence measurements and CO2 fixation assays (16). These bacteria, however, are not capable of strictly photoautotrophic growth, as they require a supply of organic carbon (15).
Most of the genes required for the formation of bacteriochlorophyll-containing photosystems in both anaerobic and aerobic anoxygenic phototrophs are clustered in a contiguous, 45-kb chromosomal region. These include the bch genes coding for enzymes in the bacteriochlorophyll biosynthetic pathway and the puf operon genes coding for the subunits of the light-harvesting complex (pufB and pufA) and the L and M subunits of the reaction center (RC; pufL and pufM). Analyses of photosynthetic superoperons recovered on large genome fragments of naturally occurring bacterioplankton have provided evidence for several different types of AAnP in coastal Pacific waters (7). Interestingly, it was recently reported that in some cultured AAnP species (Roseobacter litoralis and Staleya guttiformis) the photosynthetic superoperon is located on a linear plasmid and not on the chromosome (28).
Recently, pufM genes were used to assess the remarkable diversity of different aerobic (7) and anaerobic (1, 14) anoxygenic photosynthetic assemblages. Proteins in the bacteriochlorophyll biosynthetic pathway were also recently used as phylogenetic markers for different anoxygenic phototrophs (7). It was shown that Roseobacter and Roseobacter-like bacteria constitute a significant proportion of AAnP in the Pacific Ocean, together with bacteria related to cultured anaerobic Betaproteobacteria and Gammaproteobacteria (7). In contrast to results of culture-dependent studies, no photosynthetic genes or cDNA belonging to Erythrobacter spp. (α-4 subclass of Proteobacteria) were retrieved in any of the Pacific Ocean samples analyzed so far (7).
Until recently, only six defined Roseobacter and Roseobacter-like (Rhodobacteraceae) species with the capability for aerobic anoxygenic photosynthesis have been described. These include Roseobacter litoralis and Roseobacter denitrificans, isolated from marine algae and sediments (31); S. guttiformis (19) and Roseovarius tolerans (18), isolated from a hypersaline Antarctic lake; and Roseivivax halotolerans and Roseivivax halodurans, isolated from a hypersaline lake in Australia (34). No seawater isolates from the genera Sagittula, Silicibacter, or Sulfitobacter or from the newly discovered Roseobacter clade-affiliated cluster have yet been found to have the capacity to perform aerobic anoxygenic photosynthesis (24, 30). Many marine Roseobacter-like AAnP isolates now await to be better defined, and include strains isolated by O. Béjà et al. (7), M. Allgaier et al. (3), and M. Koblízek, P. G. Falkowski, and Z. S. Kolber (unpublished data).
To better describe the nature and diversity of planktonic, aerobic anoxygenic photosynthetic bacteria in oligotrophic seas, we screened surface water from the Red and Mediterranean Seas with primers previously designed to amplify both pufL and pufM (a 1.6-kb fragment) (7). These primers match well-conserved sequences in the pufLM operon and will target most pufLM sequences currently available in the GenBank database (7, 25). PufM sequences from different marine AAnP isolates were also retrieved in order to better resolve the tree topology.
Parallel to the use of pufM primers, other primers (originally designed to amplify nifH genes [23]) were used to amplify bch genes (bchL and bchX) from the same water samples. Common reductase was suggested as the possible ancestor for Mg tetrapyrrole biosynthetic proteins as well as nitrogenase (38). NifH and BchL were found to be remarkably similar by both Fujita et al. (11) and Burke et al. (8), and NifH was used as an outgroup for studying phylogenetic relationships among different BchL homologs from both bacteria and plants (9, 38). These degenerate general nifH primers (23) target the same conserved regions on both bchL and bchX (amino acids regions GKGGIGKS and VCGGFAMP; see alignment figure in Burke et al. showing the alignment of nitrogenases and chlorophyll iron proteins [9]). The use of different chlorophyll biosynthetic pathway genes (bchL, for example) enables the comparison of genes found in both AAnP and oxygenic phototrophs. We also identified a new bacterial artificial chromosome (BAC) clone from the AAnP bacteria in these waters and studied the genetic organization of its photosynthetic operon. Using these three independent molecular markers (the pufM and bch genes and a BAC clone with the AAnP photosynthetic operon), we tried to identify the main AAnP populations in Red and Mediterranean oligotrophic waters.
Nucleotide sequence accession numbers. Nucleotide sequences have been deposited in the GenBank database under accession numbers AY671989, AY671990 to AY672016, AY672018 to AY672043, and AY675565 to AY675576. The sequence of eBACred25D05 was deposited under accession number AY671989.
MATERIALS AND METHODS
Sample collection.
Red Sea samples (taken from waters at 29.28°N, 34.55°E and 27.17°N, 34.22°E) were collected in February 1999 (depths, 5 and 50 m; fraction diameter, >0.45 μm) (20), and in November 2002 (29.28°N, 34.55°E; depths of 0, 30,50 and 100 m; fraction diameter, 0.2 to 1.6 μm). Mediterranean samples were collected from the Alborán Sea (36.0°N, 4.25°W) in May 1998 (depths, 5 and 50 m; fraction diameter, 0.2 to 2 μm) and from the Catalano-Balear Sea (42.7°N, 2.8°E) in January 2000 (depths, 5 and 60 m; fraction diameter, 0.2 to 5 μm). Samples were also collected off the Israeli Coast in October 2002 (depth, 66 m; fraction diameter, 0.2 to 1.6 μm) and in January 2003 (33.95°N, 31.09°E; depths, 70 and 100 m; fraction diameter, 0.2 to 1.6 μm). The preservation and extraction of DNA from all samples were done as previously described (21).
Large-insert genomic library construction from environmental DNA.
The BAC library was constructed from plankton samples collected from coastal water (depth, 7 m) pumped from the dock at the Interuniversity Institute for Marine Sciences in Eilat in May 2002 (29).
Strain isolation.
The AAnP strains were isolated as described previously (15) with the following modification: in addition to a standard f/2 vitamin mix, all the media were enriched with 2 μM nicotinic acid (vitamin B3) which is required by the Roseobacter species. In June 2001, strain BS90 was isolated on the northwest shelf of the Black Sea and strain BS110 was isolated from deep (62-m) Mediterranean waters of the Bosporus (Koblízek et al., unpublished data); strain COL2P was collected on the French Mediterranean coast in September 2000; strain SO3 was isolated in the Southern Ocean at 62° 0′ S, 170° 46′ W in February 2002; and strains SY0P1 and SY0P2 were collected on the Sydney beach, Australia, in September 2002. The presence of bacteriochlorophyll a-containing organisms was tested by using an infrared fast repetition rate fluorometer (17). Pure isolates were typically grown in organic rich medium composed of f/2 medium supplemented by 0.5 g of peptone and 0.1 g of yeast extract per liter. Isolates were grown under conditions of an alternating light-dark cycle (12 h of light-12 h of dark), illuminated by a bank of luminescent tubes providing white light of about 100 μmol photon/m2/s at room temperature.
PCR amplification.
PCR amplification was carried out in a total volume of 25 μl containing 10 ng of template DNA, a 200 μM concentration of each deoxynucleoside triphosphate, 1.5 mM MgCl2, 2.5 U of BIO-X-ACT DNA polymerase (Bioline) or Ex-Taq (Takara), and a 0.2 μM concentration of each primer. The following degenerate oligonucleotide primers were used: pufLfwd (5′-CTKTTCGACTTCTGGGTSGG-3′)and pufMrev (5′-CCATSGTCCAGCGCCAGAA-3′) for the pufLM genes (7) and GKGGIGKSfwd (5′-GGHAARGGHGGHATHGGNAARTC-3′) and VCGGFAMPrev (5′-GGCATNGCRAANCCVCCRCANAC-3′) for the bch genes (23). The amplification program consisted of 92°C for 4 min and 40 cycles at 92°C for 1 min, 50°C for 1 min, and 68°C for 1.6 min for the pufLM segment and for 45 s for the amplification of the bch genes. PCR-amplified pufLM and bch genes from each depth of the different sampling sites were ligated into the pDrive cloning vector (QIAGEN). Clones were analyzed by EcoRI and AluI restriction fragment length polymorphism analysis before sequencing of representative restriction fragment length polymorphism groups. Sequencing of the pufLM fragment required an additional internal primer (pufM570fwd, 5′-CAGTTACTTTATTTTTCACAAC-3′).
Phylogenetic inference.
Sequence alignments were performed by using the program Clustal X version 1.81 (36). Neighbor-joining (NJ) and maximum-parsimony (MP) analyses were conducted on amino acid data sets by using the PAUP* program, version 4.0b10 (35). Default parameters were used in all analyses. Bootstrap resampling of NJ (1,000 replicates) and MP (1,000 replicates) trees was performed in all analyses to provide confidence estimates for the inferred topologies.
RESULTS AND DISCUSSION
Detection of pufM sequences.
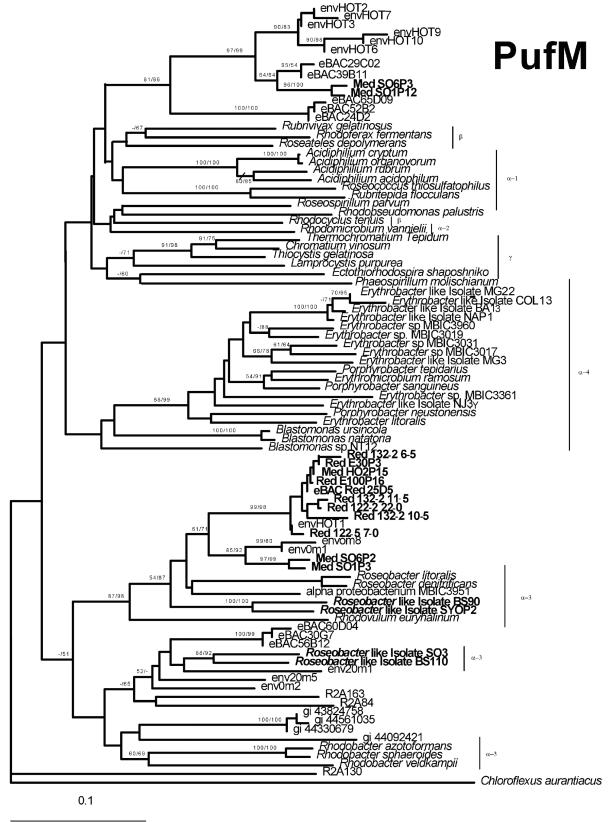
The phylogenetic tree of PufM proteins (Fig. 1) encompassed three major clades, separating between α-3, α-4, and α-1/α-2/β/γ representatives. Most of the environmental pufM clones from the Red and Mediterranean Seas fell into one group based on PufM phylogeny (Fig. 1). These clones were highly similar to PufM sequences previously identified in the Pacific Ocean (Fig. 1, envHOT1, env0m8, and env0m1) and are related to PufM proteins from Roseobacter and Roseobacter-like groups.
FIG. 1.
Phylogenetic relationships of PufM sequences of AAnP bacteria. Evolutionary relationships were determined by NJ analysis (see Materials and Methods). The green nonsulfur bacteria Chloroflexus aurantiacus was used as an outgroup. Sequences that were amplified by PCR in this study are indicated in boldface. Bootstrap values (NJ/MP) greater than 50% are indicated above the branches. The scale bar represents the number of substitutions per site.
Surprisingly, no pufM clones from a previously reported Pacific Ocean environmental α-proteobacterial group (Fig. 1, eBACs 30G07, 60D04, and 56B12) could be detected in Red or Mediterranean Sea waters and only two sequences (Fig. 1, SO6P3 and SO1P12) related to Pacific Ocean γ-like (J. C. Cho and S. Giovannoni, personal communication) proteobacterial environmental groups (Fig. 1, eBACs 29C02, 39B11 and 24D02, 52B02, and 65D09) were found.
These observations could indicate the absence of the pufM clones in Red or Mediterranean Sea waters in the seasons sampled or the inability of the primers used to detect these PufM groups. The second explanation seems less likely, since the same primers were used to detect diverse Pacific α- and β- or γ-proteobacterial PufM types. Nevertheless, the possibility of mismatches in the primer region used remains.
Interestingly, when a Red Sea BAC library was screened with the same pufLM primers, a positive BAC clone was detected (eBACred25D05). This BAC was related to the sequences amplified via direct PCR on environmental samples and fell with PufM proteins from the Roseobacter and Roseobacter-like groups. This alone gives a certain indication for the abundance of this AAnP group because only abundant bacterial groups will be represented in a given BAC or fosmid library.
Detection of bchX and bchL sequences.
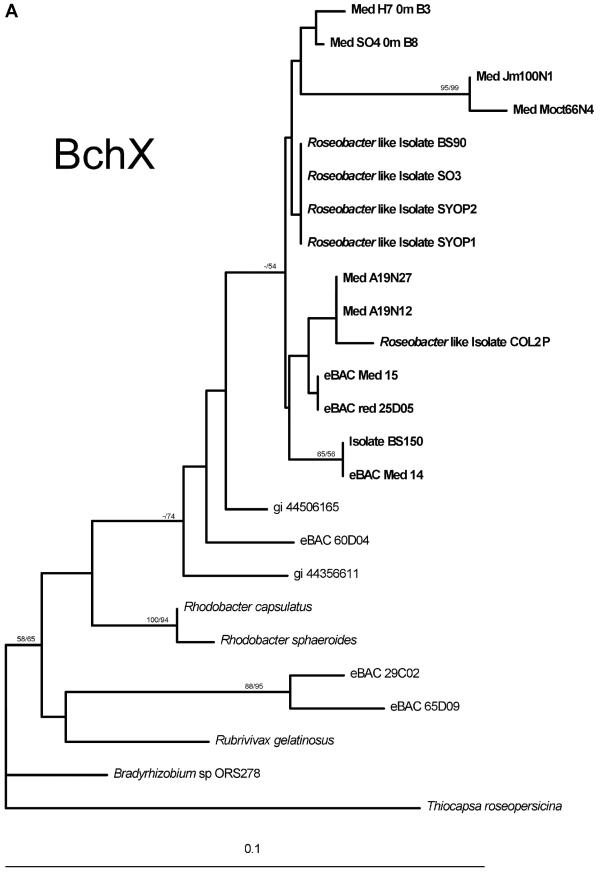
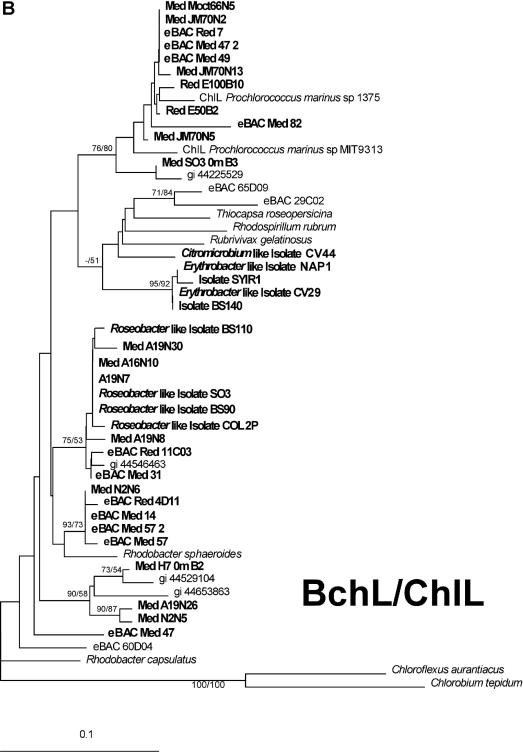
Environmental BchX protein sequences were clearly related to BchX sequences from Roseobacter-like isolates (Fig. 2A), and again, as in the pufM case, no sequences related to BchX sequences from Pacific α- or γ-proteobacterial AAnP groups were detected (represented in the tree by sequences from eBAC 60D04, 29C02 and 65D09). BchX from the Red Sea BAC clone (eBACred25D05) was also positioned within the Roseobacter-like group. The picture generated with the environmental BchL and ChlL sequences was more complex. Eleven of the sequences were related to ChlL proteins from cyanobacteria and probably represent diversity within the Prochlorococcus and Synechococcus groups. Seven of the retrieved BchL environmental sequences were related to Roseobacter-like isolates while four sequences (Fig. 2B, MedN2N6, eBACRed4D11, eBACMed14, and eBACMed57_2) were related to BchL from Rhodobacter sphaeroides; four sequences (MedA19N26, MedN2N5, MedH70mB2, and eBACMed47) fell between sequences represented by Rhodobacter sphaeroides BchL proteins and proteins from a Pacific Alphaproteobacteria AAnP (eBAC 60D04). The BchX and BchL trees include sequences from environmental samples, newly isolated Roseobacter strains (Koblízek et al., unpublished data) as well as from environmental large-insert BACs isolated from the Mediterranean and Red Seas (G. Sabehi and O. Béjà, unpublished data). Future sequencing of these Roseobacter-related BACs should help in assigning them phylogenetic groups and resolve the positions of some BchL sequences (MedA19N26 and MedN2N5, for example).
FIG. 2.
Phylogenetic analyses of the BchX and BchL proteins. (A) Phylogenetic tree of the BchX protein. (B) Phylogenetic tree of the BchL and ChlL proteins. Bootstrap values (NJ/MP) greater than 50% are indicated above the branches. The scale bar represents the number of substitutions per site.
Analyses of a photosynthetic operon from a Roseobacter-like containing BAC.
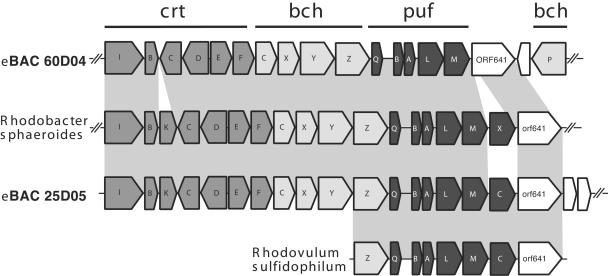
The clone eBACred25D05, which, based on PCR analysis, contained both the pufM and bchX genes and represents the sequences from the Roseobacter-like AAnP group, was fully sequenced, and the photosynthetic operon was compared with operons of cultured proteobacteria and uncultured AAnP BACs (7). This BAC clone, which is related to Roseobacter bacteria (on the basis of pufM sequences) (Fig. 1), most closely resembles the photosynthetic operons from the cultured bacteria Rhodovulum sulfidophilum (22), Rhodobacter capsulatus (2), and Rhodobacter sphaeroides (10, 26) in both organization and gene content. The superoperonal gene arrangement, crtEF-bchCXYZ-puf, found in Rhodobacter sphaeroides, Rhodobacter capsulatus and Rubrivivax gelatinosus is conserved in eBACred25D05 (Fig. 3), suggesting that the photosynthetic apparatus in the bacteria from which this BAC insert originated is functional. Unfortunately, no whole photosynthetic superoperon from any cultured AAnP is yet available to compare to the operonal organization of eBACred25D05 or to other environmental uncultured photosynthetic superoperons containing BACs (7).
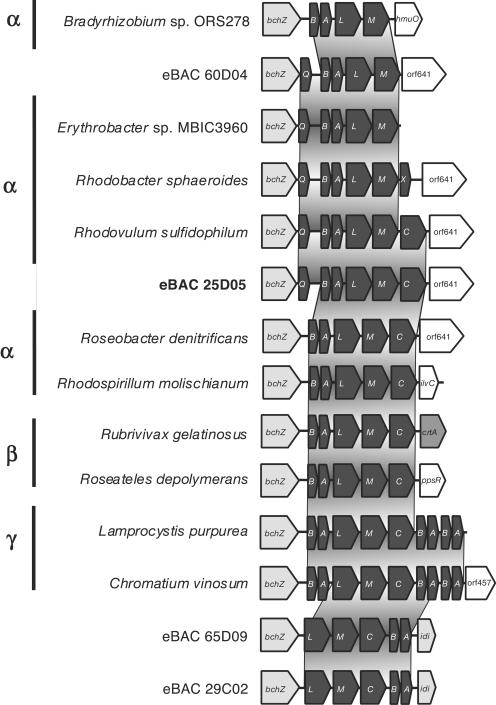
FIG. 3.
Schematic comparison of photosynthetic operons of Rhodobacter sphaeroides, eBAC60D04, eBAC25D05, and Rhodovulum sulfidophilum. Open reading frame (orf) abbreviations use nomenclature defined previously (references 10 and 13). Predicted open reading frames are in shaded according to biological category: bch, bacteriochlorophyll biosynthesis genes; crt, carotenoid biosynthesis genes; puf, light-harvesting and reaction center genes. White boxes indicate nonphotosynthetic and hypothetical proteins with no known function. Homologous regions are connected by gray areas.
A more comprehensive comparison was made with the region surrounding light-harvesting complex I (pufAB) and the RC (pufLM). Two types of RC are known in purple bacteria. One has a tightly bound subunit of a c-type cytochrome (pufC). The other accepts electrons directly from water-soluble electron carriers such as cytochrome c2 (cycA) (27). Considerable diversity exists in this region in different proteobacteria, including those derived from environmental BAC clones (7) (Fig. 4). Clone eBACred25D05 contains the gene pufC and also contains the gene pufQ, implicated in spectral complex assembly (6, 12), but lacks the pufX gene suggested to be involved in electron transfer from the RC to the bc1 complex (4, 5). The organization of this operon, which consisted of six genes, pufQ, pufB, pufA, pufL, pufM, and pufC, is new in photosynthetic bacteria in the sense that pufQ and pufC coexist. Such an organization has been found so far only in Rhodovulum species (37).
FIG. 4.
Schematic comparison of puf operons. Open reading frame (orf) abbreviations and color codes are as described in the legend of Fig. 3. Homologous regions are connected by gray areas.
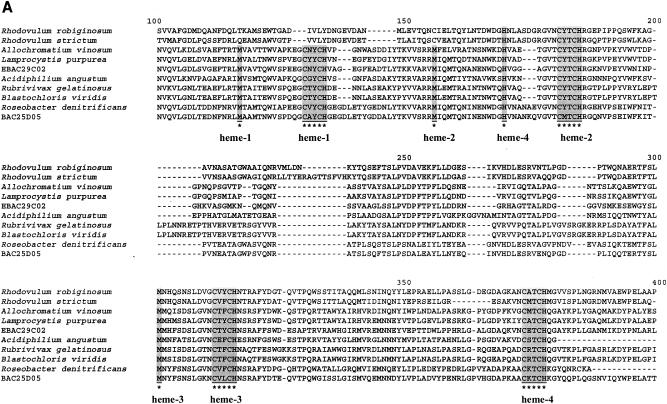
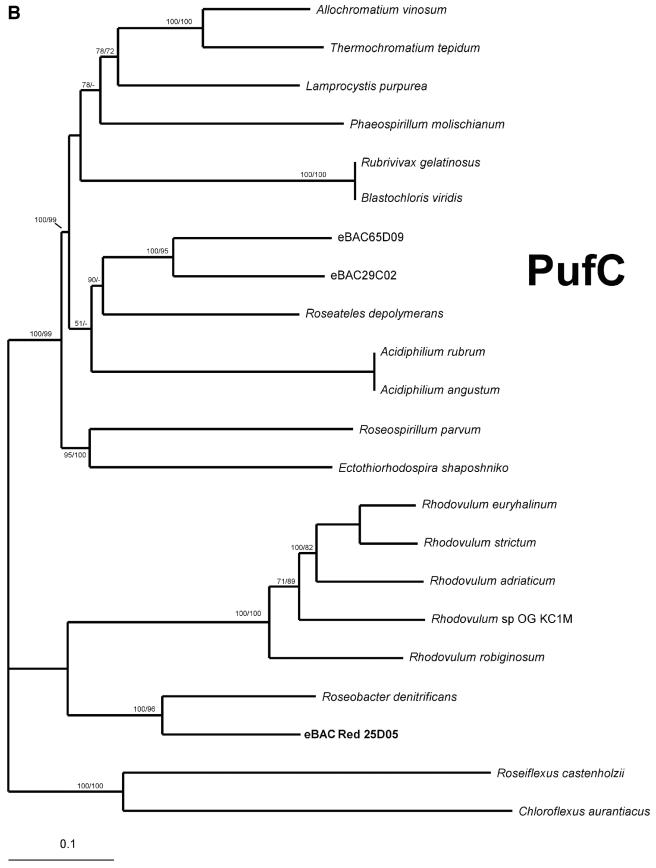
As opposed to the PufC proteins from Rhodovulum species, which contain only three possible heme-binding motifs (37), PufC from clone eBACred25D05 contains all four possible heme-binding motifs, as does Roseobacter denitrificans PufC, and therefore belongs to the classical tetraheme cytochrome family (Fig. 5A). Furthermore, eBACred25D05 PufC shows the highest homology to Roseobacter denitrificans PufC (70% [244 of 347 residues] protein identity) and branches close to Roseobacter denitrificans PufC with high bootstrap values (Fig. 5B).
FIG. 5.
Heme-binding domains and phylogeny in c-type cytochrome proteins. (A) Multiple alignment of PufC amino acid sequences and predicted heme-binding domains are shaded and indicated by asterisks. (B) Phylogenetic analyses of PufC proteins. Bootstrap values (NJ/MP) greater than 50% are indicated above the branches. The scale bar represents the number of substitutions per site. Heme binding domains are underlined.
Concluding remarks.
Our results now show the presence of Roseobacter and Roseobacter-like bacteria in aerobic anoxygenic photosynthetic populations in the oligotrophic Red and Mediterranean Seas. Two recent publications show the dominance of these groups in the eastern Pacific Ocean. By using real time PCR, it was shown that Roseobacter groups account for up to 40% of the bacterial population in certain areas in Monterey Bay (33). Furthermore, these groups account for up to 26% of all rRNA identified in surface BAC libraries constructed from Monterey Bay samples (32). Therefore, the Roseobacter group appears to be a substantial component of marine bacterial assemblages, and our data imply that some can be involved in oceanic aerobic anoxygenic photosynthetic activity.
The role of these groups and their seasonal dominance in different oceanic regions will have to be further monitored by real-time PCR or different hybridization assays to distinguish between the different α groups and between these groups and the β or γ oceanic AAnP groups. Furthermore, additional data from more quantitative methods, such as infrared fast repetitive rate fluorometry, infrared epifluorescence microscopy, and/or pigment analysis are important for obtaining a more complete picture about the distribution and diversity of AAnP in these environments.
Acknowledgments
We thank Anton Post for kindly providing Red Sea samples and the captain and crew of the RV Shikmona for expert assistance at sea.
This work was supported in part by the Israel Science Foundation grant 434/02 and Human Frontiers Science Program P38/2002 (O.B.), EU project PICODIV EVK3-CT1999-00021 (R.M.), and GACR project 206/03/P079, institutional concept grant AV0Z5020903 and Czech MSMT project LN00A141 (M.K.).
REFERENCES
- 1.Achenbach, L. A., J. Carey, and M. T. Madigan. 2001. Photosynthetic and phylogenetic primers for detection of anoxygenic phototrophs in natural environments. Appl. Environ. Microbiol. 67:2922-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti, M., D. H. Burke, and J. E. Hearst. 1995. Structure and sequence of the photosynthesis gene cluster, p. 1083-1106. In R. E. Blankenship, M. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 3.Allgaier, M., H. Uphoff, and I. Wagner-Dobler. 2003. Aerobic anoxygenic photosynthesis in Roseobacter clade bacteria from diverse marine habitats. Appl. Environ. Microbiol. 69:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barz, W. P., F. Francia, G. Venturoli, B. A. Melandri, A. Vermeglio, and D. Oesterhelt. 1995. Role of PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 1. PufX is required for efficient light-driven electron transfer and photophosphorylation under anaerobic conditions. Biochemistry 34:15235-15247. [DOI] [PubMed] [Google Scholar]
- 5.Barz, W. P., A. Vermeglio, F. Francia, G. Venturoli, B. A. Melandri, and D. Oesterhelt. 1995. Role of the PufX protein in photosynthetic growth of Rhodobacter sphaeroides. 2. PufX is required for efficient ubiquinone/ubiquinol exchange between the reaction center QB site and the cytochrome bc1 complex. Biochemistry 34:15248-15258. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, C. E., and B. L. Marrs. 1988. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc. Natl. Acad. Sci. USA 85:7074-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Béjà, O., M. T. Suzuki, J. F. Heidelberg, W. C. Nelson, C. M. Preston, T. Hamada, J. A. Eisen, C. M. Fraser, and E. F. DeLong. 2002. Unsuspected diversity among marine aerobic anoxygenic phototrophs. Nature 415:630-633. [DOI] [PubMed] [Google Scholar]
- 8.Burke, D. H., M. Alberti, and J. E. Hearst. 1993. The Rhodobacter capsulatus chlorin reductase-encoding locus, bchA, consists of three genes, bchX, bchY, and bchZ. J. Bacteriol. 175:2407-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke, D. H., J. E. Hearst, and A. Sidow. 1993. Early evolution of photosynthesis: clues from nitrogenase and chlorophyll iron proteins. Proc. Natl. Acad. Sci. USA 90:7134-7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhary, M., and S. Kaplan. 2000. DNA sequence analysis of the photosynthesis region of Rhodobacter sphaeroides 2.4.1T. Nucleic Acids Res. 28:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita, Y., Y. Takahashi, M. Chuganji, and H. Matsubara. 1992. The nifH-like (frxC) gene is involved in the biosynthesis of chlorophyll in the filamentous cyanobacterium Plectonema boryanum. Plant Cell Physiol. 33:81-92. [PubMed] [Google Scholar]
- 12.Gong, L., J. K. Lee, and S. Kaplan. 1994. The Q gene of Rhodobacter sphaeroides: its role in puf operon expression and spectral complex assembly. J. Bacteriol. 176:2946-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi, N., J. Harada, S. Nagashima, K. Matsuura, K. Shimada, and K. V. P. Nagashima. 2001. Horizontal transfer of the photosynthetic gene cluster and operon re-arrangement in purple bacteria. J. Mol. Evol. 52:333-341. [DOI] [PubMed] [Google Scholar]
- 14.Karr, E. A., W. M. Sattley, D. O. Jung, M. T. Madigan, and L. A. Achenbach. 2003. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic lake. Appl. Environ. Microbiol. 69:4910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koblízek, M., O. Béjà, R. R. Bidigare, S. Christensen, B. Benetiz-Nelson, C. Vetriani, M. K. Kolber, P. G. Falkowski, and Z. S. Kolber. 2003. Isolation and characterization of Erythrobacter sp. strains from the upper ocean. Arch. Microbiol. 180:327-338. [DOI] [PubMed] [Google Scholar]
- 16.Kolber, Z. S., F. G. Plumley, A. S. Lang, J. T. Beatty, R. E. Blankenship, C. L. VanDover, C. Vetriani, M. Koblízek, C. Rathgeber, and P. G. Falkowski. 2001. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science 292:2492-2495. [DOI] [PubMed] [Google Scholar]
- 17.Kolber, Z. S., C. L. Van Dover, R. A. Niderman, and P. G. Falkowski. 2000. Bacterial photosynthesis in surface waters of the open ocean. Nature 407:177-179. [DOI] [PubMed] [Google Scholar]
- 18.Labrenz, M., M. D. Collins, P. A. Lawson, B. J. Tindall, P. Schumann, and P. Hirsch. 1999. Roseovarius tolerans gen. nov., sp. nov., a budding bacterium with variable bacteriochlorophyll a production from hypersaline Ekho Lake. Int. J. Syst. Bacteriol. 49:137-147. [DOI] [PubMed] [Google Scholar]
- 19.Labrenz, M., B. J. Tindall, P. A. Lawson, M. D. Collins, P. Schumann, and P. Hirsch. 2000. Staleya guttiformis gen. nov., sp. nov. and Sulfitobacter brevis sp. nov., alpha-3-Proteobacteria from hypersaline, heliothermal and meromictic antarctic Ekho Lake. Int. J. Syst. Evol. Microbiol. 50:303-313. [DOI] [PubMed] [Google Scholar]
- 20.Lindell, D., and A. F. Post. 2001. Ecological aspects of ntcA gene expression and its use as an indicator of the nitrogen status of marine Synechococcus spp. Appl. Environ. Microbiol. 67:3340-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massana, R., A. E. Murray, C. M. Preston, and E. D. DeLong. 1997. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara channel. Appl. Environ. Microbiol. 63:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda, S., M. Yoshida, K. V. P. Nagashima, K. Shimada, and K. Matsuura. 1999. A new cytochrome subunit bound to the photosynthetic reaction center in the purple bacterium, Rhodovulum sulfidophilum. J. Biol. Chem. 274:10795-10801. [DOI] [PubMed] [Google Scholar]
- 23.Mehta, M. P., D. A. Butterfield, and J. A. Baross. 2003. Phylogenetic diversity of nitrogenase (nifH) genes in deep-sea and hydrothermal vent environments of the Juan de Fuca Ridge. Appl. Environ. Microbiol. 69:960-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran, M. A., J. M. González, and R. P. Kiene. 2003. Linking a bacterial taxon to sulfur cycling in the sea: studies of the marine Roseobacter group. Geomicrobiol. J. 20:375-388. [Google Scholar]
- 25.Nagashima, K. V. P., A. Hiraishi, K. Shimada, and K. Matsuura. 1997. Horizontal transfer of genes coding for the photosynthetic reaction centers of purple bacteria. J. Mol. Evol. 45:131-136. [DOI] [PubMed] [Google Scholar]
- 26.Naylor, G. W., H. A. Addlesee, L. C. D. Gibson, and C. N. H. Hunter. 1999. The photosynthesis gene cluster of Rhodobacter sphaeroides. Photosynth. Res. 62:121-139. [Google Scholar]
- 27.Nitschke, W., and S. M. Dracheva. 1995. Reaction center-associated cytochromes, p. 775-805. In R. E. Blankenship, M. Madigan, and C. E. Bauer (ed.), Anoxygenic photosynthetic bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 28.Pradella, S., M. Allgaier, C. Hoch, O. Pauker, E. Stackebrandt, and I. Wagner-Dobler. 2004. Genome organization and localization of the pufLM genes of the photosynthesis reaction center in phylogenetically diverse marine Alphaproteobacteria. Appl. Environ. Microbiol. 70:3360-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabehi, G., O. Béjà, M. T. Suzuki, C. M. Preston, and E. F. DeLong. 2004. Different SAR86 subgroups harbour divergent proteorhodopsins. Environ. Microbiol. 6:903-910. [DOI] [PubMed] [Google Scholar]
- 30.Selje, N., M. Simon, and T. Brinkhoff. 2004. A newly discovered Roseobacter cluster in temperate and polar oceans. Nature 427:445-448. [DOI] [PubMed] [Google Scholar]
- 31.Shiba, T., Y. Shioi, K. Takamiya, D. C. Sutton, and C. R. Wilkinson. 1991. Distribution and physiology of aerobic bacteria containing bacteriochlorophyll a on the east and west coasts of Australia. Appl. Environ. Microbiol. 57:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki, M. T., C. M. Preston, O. Béjà, R. J. de la Torre, G. F. Steward, and E. F. DeLong. Quantitative phylogenetic screening of ribosomal RNA gene-containing clones in bacterial artificial chromosome (BAC) libraries from different depths in Monterey Bay. Microb. Ecol., in press. (First published 9 November 2004; doi:10.1007/s00248-004-0213-5 [http://springerlink.metapress.com/app/home/contribution.asp?wasp=gmultklqxp3v19vhhl5x&referrer=parent&backto=issue,13,51;journal,1,60;linkingpublicationresults,1:100365,1.) [DOI] [PubMed]
- 33.Suzuki, M. T., C. M. Preston, F. P. Chavez, and E. F. DeLong. 2001. Quantitative mapping of bacterioplankton populations in seawater: field tests across an upwelling plume in Monterey Bay. Aquat. Microbiol. Ecol. 24:117-127. [Google Scholar]
- 34.Suzuki, T., Y. Muroga, M. Takahama, and Y. Nishimura. 1999. Roseivivax halodurans gen. nov., sp. nov. and Roseivivax halotolerans sp. nov., aerobic bacteriochlorophyll-containing bacteria isolated from a saline lake. Int. J. Syst. Bacteriol. 2:629-634. [DOI] [PubMed] [Google Scholar]
- 35.Swofford, D. L. 2002. PAUP* v. 4.0b10: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland, Mass.
- 36.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukatani, Y., K. Matsuura, S. Masuda, K. Shimada, A. Hiraishi, and K. V. P. Nagashima. 2004. Phylogenetic distribution of unusual triheme to tetraheme cytochrome subunit in the reaction center complex of purple photosynthetic bacteria. Photosynth. Res. 79:83-91. [DOI] [PubMed] [Google Scholar]
- 38.Xiong, J., and C. E. Bauer. 2002. Complex evolution of photosynthesis. Annu. Rev. Plant Biol. 53:503-521. [DOI] [PubMed] [Google Scholar]
- 39.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]