Abstract
The vigorous proliferation of Ralstonia solanacearum OE1-1 in host intercellular spaces after the invasion of host plants is necessary for the virulence of this bacterium. A folate auxotroph, RM, in which a mini-Tn5 transposon was inserted into pabB encoding para-aminobenzoate synthase component I, lost its ability to vigorously proliferate in intercellular spaces along with its systemic infectivity and virulence after inoculation into roots and infiltration into leaves of tobacco plants. Complementation of RM with the pabB gene allowed the mutant to multiply in intercellular spaces and to cause disease. In tobacco plants that were pretreated with folate, RM was able to vigorously proliferate in the intercellular spaces and cause disease. Interestingly, when it was inoculated through cut stems, the mutant multiplied in the plants and was virulent. Moreover, the mutant multiplied well in stem fluids but not in intercellular fluids, suggesting that the folate concentration within intercellular spaces may be a limiting factor for bacterial proliferation. Therefore, folate biosynthesis contributes to the vigorous proliferation of bacteria in intercellular spaces and leads to systemic infectivity resulting in virulence.
Bacterial wilt caused by Ralstonia solanacearum (29) is one of the most devastating bacterial plant diseases and causes severe losses in many important crops in the tropics, subtropics, and warm-temperature regions worldwide (16). R. solanacearum is a soilborne vascular bacterium. This bacterium generally invades plant roots via wounds or the natural openings from which secondary roots subsequently emerge (16, 20). After invasion, bacteria first colonize and proliferate in intercellular spaces and then invade xylem vessels. Once bacteria have invaded the vessels, they multiply and travel rapidly throughout the entire plant. The plants then wilt when sap flow is reduced by the presence of large numbers of bacteria and the exopolysaccharide (EPS) slime that they produce (26).
R. solanacearum OE1-1 is pathogenic for tobacco plants and induces necrotic lesions in tobacco leaves 60 h after infiltration (17, 18). Although a compatible R. solanacearum strain, GMI1000, is pathogenic for tomato plants, similar to OE1-1, it is nonpathogenic to tobacco plants and elicits a hypersensitive response 24 h after infiltration of tobacco leaves, in contrast to OE1-1 (2, 5, 6, 18, 19, 27). OE1-1 possesses hrp genes similar to those of R. solanacearum GMI1000 (2, 18, 19, 27). These genes encode proteins which are parts of the type III secretion machinery, and their expression is regulated by HrpB (11, 27). Type III secretion machinery-deficient mutants of OE1-1 lose the ability to colonize and proliferate in intercellular spaces, resulting in a loss of the ability to induce necrotic lesions and a lack of virulence (18). Therefore, type III effectors secreted through the type III secretion machinery of OE1-1 are involved in not only its virulence but also its induction of necrotic lesions in infiltrated tobacco leaves (18). Moreover, a harpin-like protein, PopA, is secreted through the type III secretion machinery (3) for 3 h after invasion to allow the bacteria to prevent the induction of host resistance, resulting in a vigorous proliferation of bacteria in intercellular spaces and causing disease (19). Proliferation of the bacteria in intercellular spaces may thus be required for their virulence, but there is little information on this aspect of pathogenicity other than that found with the tryptophan auxotroph of R. solanacearum K60, which is avirulent in tobacco and tomato plants as a result of severe growth limitations caused by the small amounts of free tryptophan in xylem sap (7).
For this study, we isolated the avirulent mutant RM after transposon mutagenesis of OE1-1. This mutant lost the ability to vigorously proliferate in intercellular spaces but not in xylem vessels. The results of genetic and biological studies with the RM mutant suggest that folate biosynthesis by this bacterium is essential for its vigorous growth in intercellular spaces and for subsequent disease in the host.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used for this study are listed in Table 1. R. solanacearum strains were grown at 30°C in PY medium (28) and Boucher's medium (5) as a nutrient-rich and a minimal medium, respectively. Escherichia coli strains were grown in LM medium (13) at 37°C. The following antibiotics and chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) and were used in selective media in the indicated amounts: ampicillin, 50 μg/ml; kanamycin, 50 μg/ml; spectinomycin, 50 μg/ml; folate, 30 μM; and para-aminobenzoate (PABA), 2 μM.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−endA1 relA φ80 lacZΔM15 hsdR17 supE44 thi-1 recA1 gyrA96 | 13 |
| R. solanacearum | ||
| OE1-1 | Wild-type, race 1, biovar 1 | 18 |
| RM | OE1-1 derivative, carries mini-Tn5, Spr | This study |
| RM/pabB | Transformant of RM with pUCD3101-pabB, Kmr/Spr | This study |
| Plasmids | ||
| pBluescript SK | Apr | Stratagene |
| pUCD3101 | pLAFR3 derivative, Kmr | 17 |
| pTn-pabB | 6.8-kb fragment inserted next to mini-Tn5 in pBluescript SK, Apr Spr | This study |
| pUCD3101-pabB | 1.8-kb BamHI and XbaI fragment containing pabB in pUCD3101, Kmr | This study |
Populations of OE1-1 and RM in vitro in five independent experiments were assayed by the use of Hara-Ono plates (15) and Hara-Ono plates containing spectinomycin, respectively.
Virulence assays.
Tobacco plants (Nicotiana tabacum cv. Bright Yellow) were grown in 7.5-cm-diameter pots (Nippori, Gifu, Japan) containing a mixture of vermiculite and peat moss (3:1) in a growth room at 25°C with 10,000 lx of light for 16 h per day and were watered with fivefold-diluted Hoagland's solution (17). The tobacco plants were inoculated with bacteria by either root dip inoculation, stem inoculation, or leaf infiltration, as follows. For root dip inoculation (17), the roots of tobacco plants were soaked in a bacterial suspension of 1.0 × 108 CFU/ml for 30 min and then washed in running water. The plants were then grown in water culture pots with fivefold-diluted Hoagland's solution. For stem inoculation (23), plants were inoculated by stabbing a 200-μl disposable pipette tip containing 100 μl of 1.0 × 108 CFU/ml into the third leaf axil down from each plant's apex, and then inoculated plants were placed in a 25°C growth room and the pipette tips were removed after the entire bacterial suspension entered the stem. For leaf infiltration (18), a bacterial suspension of 1.0 × 108 CFU/ml in a 50-μl volume was inoculated into tobacco leaves by use of a disposable 1-ml syringe. For all assays, inoculum concentrations were determined spectrophotometrically and confirmed by dilution plating (17). All inoculated plants were grown in a growth room at 25°C with 10,000 lx of light for 16 h per day.
For folate applications, 5-week-old test plants were grown in water culture pots (water culture pot no. 1; Yamato Plastic, Tokyo, Japan) with fivefold-diluted Hoagland's solution containing 500 μM folate for 2 days in a 25°C growth room and then were inoculated by root dip inoculation.
The plants were coded and inspected daily for wilting symptoms for 14 days. The disease was rated as follows: 0, no wilting; 1, 1 to 25% wilting; 2, 26 to 50% wilting; 3, 51 to 75% wilting; and 4, 76 to 100% wilting or dead (23). Each assay was repeated in five successive trials. Within each trial, each strain was used to inoculate 12 plants.
Bacterial populations in tobacco plants.
Roots were excised 1, 2, 3, 4, and 5 days after inoculation (DAI) from five of each set of tobacco plants that were inoculated with bacteria through the roots. Stems between the third and fourth leaf axils down from the apex of the plants inoculated in the petioles were detached 1, 2, 3, 4, and 5 DAI. The bacterium-infiltrated area (2 cm2) of the tobacco leaves was excised from five of each set of plants 1, 2, 3, 4, and 5 DAI. Each sample was ground in 1 ml of distilled water by use of a mortar and pestle. A 0.1-ml sample of the original suspension or a 10-fold serial dilution was spread onto three plates of Hara-Ono medium. The medium contained spectinomycin at 50 μg/ml or kanamycin at 50 μg/ml for RM and RM/pabB, respectively. The colonies were counted after 2 days of incubation at 30°C.
A plate-printing assay was performed as described by Kanda et al. (19). Ten days after infiltration with OE1-1 and RM, bacterium-infiltrated leaves were detached and cut into four pieces with razor blades. The stems of the tobacco plants were also cut into three pieces. The cut site of each piece was placed on Hara-Ono medium for a few seconds, and the plates were then incubated at 30°C for 3 days.
General DNA manipulations.
The isolation of genomic DNA, plasmid DNA manipulations, PCR, and Southern hybridization analysis were performed according to standard techniques (25). Restriction enzymes were obtained from Takara (Ohtsu, Japan). R. solanacearum was transformed by electroporation as described by Allen et al. (1). Double-stranded DNA sequencing templates were prepared with GenElute plasmid miniprep kits (Sigma). Sequences were determined by use of an automated DNA sequencer (ABI Prism 3100-Avant genetic analyzer; Applied Biosystems, Tokyo, Japan). DNA sequence data were analyzed with DNASIS Mac software (Hitachi Software Engineering, Yokohama, Japan).
Transposon mutagenesis.
A mini-Tn5 transposon derivative, mini-Tn5 Sm/Sp, carrying the spectinomycin resistance gene (8) was electroporated into strain OE1-1. Spectinomycin-resistant transformants were isolated after incubation on Hara-Ono medium containing spectinomycin and were subsequently screened for alterations in their plant-associated phenotypes.
Mini-Tn5 insertion site in genomic DNA of RM.
PstI-digested genomic DNA from strain RM was ligated into PstI-digested pBluescript SK (Stratagene, La Jolla, Calif.), and E. coli DH5α was transformed with the resulting plasmid. A spectinomycin-resistant transformant carrying pTn-pabB, which contained 6.8 kb of the DNA fragment, was obtained, and the nucleotide sequence of the DNA region adjacent to the mini-Tn5 was determined by use of the primer 5′-CCCCGGGAATTCGGCCTAGG-3′ (named I-end), based on the nucleotide sequence of mini-Tn5 Sm/Sp (8).
Complementation of RM with pUCD3101-pabB.
A 1.8-kb DNA fragment containing pabB was PCR amplified from the genomic DNA of OE1-1 by the use of Ex Taq DNA polymerase (Takara) and the primers 5′-CGGGATCCTACGACCAGTACGGCCATGC-3′ (named pabB-BamHI [the additional BamHI site is underlined]) and 5′-GCTCTAGACTAAGCGACGGGCGGCTC-3′ (named pabB-XbaI [the additional XbaI site is underlined]), which were designed based on data from a genomic analysis of GMI1000 (24). The BamHI- and XbaI-digested PCR product was ligated into the BamHI and XbaI sites of pUCD3101 (17) to create pUCD3101-pabB, which was electroporated into RM to create RM/pabB.
Bacterial proliferation in intercellular fluids and stem fluids.
The third leaves down from the plant apex of 5-week-old tobacco plants were infiltrated with 50 μl of distilled water by use of a disposable 1-ml syringe. The intercellular fluid from the leaves and the stem fluid were extruded by the method of de Wit and Spikman (9) and sterilized by membrane filtration (0.2-μm pore size) (Minisart CE; Sartorius, Gottingen, Germany). Triplicate test tubes containing 1 ml of sterilized intercellular fluids or stem fluids were prepared, and each tube was inoculated with 10 μl of cell suspension to give an initial optical density at 600 nm of 0.01. The cultures were incubated for 24 h at 30°C with shaking and then diluted fivefold with fresh medium, and the absorbance was measured at 600 nm.
Nucleotide sequence accession number.
The nucleotide sequence of pabB of strain OE1-1 was deposited in DDBJ/GenBank/EMBL under accession number AB164392.
RESULTS
Selection of a transposon mutant, RM, deficient in the ability to proliferate in intercellular spaces.
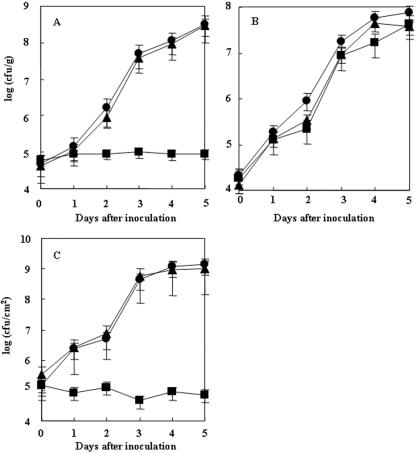
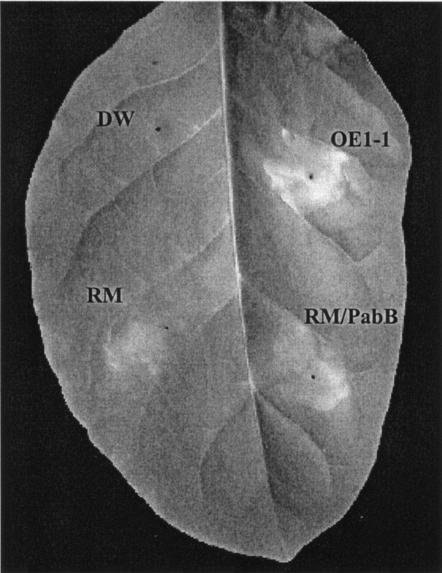
When strain OE1-1 was applied to plants by root dip inoculation, the bacterial population in the roots increased to 4.9 × 107 CFU/g by 3 DAI (Fig. 1A), and the plants completely wilted by 8 DAI (Fig. 2A). When plants were inoculated with OE1-1 by stem inoculation, the bacterial population in stems increased to 7.6 × 108 CFU/g by 5 DAI (Fig. 1B), and the plants completely wilted by 9 DAI (Fig. 2B). When tobacco leaves were infiltrated with OE1-1, necrotic lesions were induced 72 h after infiltration (Fig. 3), and the bacterial population in the OE1-1-infiltrated area reached 1.4 × 109 CFU/cm2 by 5 DAI (Fig. 1C). The plants wilted completely by 12 DAI (Fig. 2C).
FIG. 1.
In planta growth of R. solanacearum for tobacco plants. Eight-week-old tobacco plants were inoculated by either dipping the roots of the plants in a bacterial suspension of R. solanacearum OE1-1 (circles), RM (squares), or RM complemented with pabB (RM/pabB) (triangles) at 1.0 × 108 CFU/ml for 30 min (A), stabbing a 200-μl disposable pipette tip containing 100 μl of medium with 1.0 × 108 CFU/ml into the third leaf axil down from the plant apex (B), or inoculating a bacterial suspension of 1.0 × 108 CFU/ml in approximately 100 μl of medium into the leaves by use of a disposable syringe (C). Each assay was repeated in five successive trials.
FIG. 2.
Virulence of R. solanacearum. Eight-week-old tobacco plants were inoculated with R. solanacearum OE1-1 (circles), RM (squares), and RM complemented with pabB (RM/pabB) (triangles) by root dip inoculation, stem inoculation, and leaf infiltration, and populations of bacteria were determined for the roots (A), stems (B), and infiltrated areas of the leaves (C), respectively. Each assay was repeated in five successive trials.
FIG. 3.
Necrotic lesions in tobacco leaves 3 days after infiltration with either R. solanacearum OE1-1, RM, RM/pabB (RM complemented with pabB), or distilled water (DW).
Among the spectinomycin-resistant transposon mutants obtained, mutants which were not virulent in tobacco plants but retained the ability to induce necrotic lesions in infiltrated tobacco leaves 72 h after infiltration were identified. One such mutant, RM, was analyzed further. When RM infiltrated tobacco leaves, the bacterial population in the infiltrated tobacco leaves did not change much, remaining at 4.6 × 104 to 1.3 × 105 CFU/cm2 from 2 to 5 DAI (Fig. 1C), suggesting that RM had lost the ability to vigorously proliferate in intercellular spaces. RM also lost its virulence for tobacco plants that were inoculated by root dipping (Fig. 2A). After root dip inoculation, the population of RM in roots did not change from 3 to 5 DAI (Fig. 1A). On the other hand, when RM was inoculated into plant stems, the mutant population in the stems remarkably increased to 4.3 × 107 CFU/g by 5 DAI (Fig. 1B), and the plants wilted by 12 DAI (Fig. 2B).
In a plate-printing assay, although OE1-1 was detected in all areas by 10 DAI, RM was never detected beyond the area of leaf infiltration. These results suggest that RM had lost the ability to vigorously proliferate in intercellular spaces, thereby resulting in a loss of the ability to move into the periphery through intercellular spaces and to a lack of virulence.
pabB-containing RM mutant.
Nucleotide sequence analysis of pTn-pabB, which contained a 6.8-kb PstI fragment including the spectinomycin resistance gene of mini-Tn5 from RM, showed that the mini-Tn5 was inserted into the putative pabB gene, which is predicted to encode PABA synthase component I. PABA synthase component I is a component of 4-amino-4-deoxychorismate (ADC) synthase (4, 12, 21). ADC is synthesized from chorismate by ADC synthase. ADC is a substrate for the synthesis of PABA, which is a substrate for the synthesis of folate. Folate is an essential cofactor for one-carbon transfer reactions.
The open reading frame of OE1-1 pabB consists of 1,881 nucleotides. The deduced amino acid sequence of the PabB protein of OE1-1 showed a high identity (99.8%) to that of a GMI1000 protein (RSc2633). A 1.8-kb DNA fragment containing pabB was ligated into pUCD3101, and pUCD3101-pabB was created. RM was transformed with pUCD3101-pabB, and a kanamycin-resistant transformant, RM/pabB, was obtained. RM/pabB was virulent in tobacco plants inoculated by root dipping (Fig. 2A) and infiltration into leaves (Fig. 2C), similar to OE1-1. Populations of RM/pabB in roots (Fig. 1A) and infiltrated leaf areas (Fig. 1C) rapidly increased in a similar manner to those of OE1-1. These results suggest that the ability of RM to proliferate in intercellular spaces can be complemented by pabB.
Ability of RM to grow in vitro.
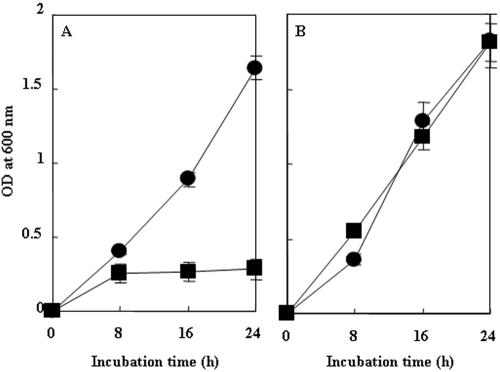
To elucidate whether RM was a folate synthesis-deficient mutant, we analyzed its ability to grow in vitro. OE1-1 grew rapidly both in a rich medium and in a minimal medium, reaching 5.0 × 109 (Fig. 4A) and 4.8 × 108 CFU/ml (Fig. 4B) 24 h after incubation, respectively. Although RM grew in rich medium to 2.0 × 109 CFU/ml 24 h after incubation, similar to OE1-1 (Fig. 4A), the mutant did not grow well in minimal medium, and the population only reached 2.0 × 107 CFU/ml (Fig. 4B). On the other hand, although RM/pabB grew more slowly than OE1-1 in minimal medium until 24 h after incubation, the population of the transformant reached 2.5 × 108 CFU/ml 32 h after incubation, similar to that of OE1-1 (Fig. 4B), suggesting that the ability of RM to grow in minimal medium can be complemented by pabB. Since transformants of OE1-1 and RM with pUCD3101 grew in minimal medium similarly to OE1-1 and RM, respectively (data not shown), multiple copies of pabB may affect bacterial growth in minimal medium.
FIG. 4.
In vitro growth of R. solanacearum. R. solanacearum cells were incubated in PY medium (A) or Boucher's medium (B). The strains used were OE1-1 (closed circles), RM (closed squares), RM complemented with pabB (RM/pabB) (closed triangles), RM with 2 μM PABA (×), and RM with 30 μM folate (open diamonds). Each assay was repeated in five successive trials.
In minimal medium containing 1 and 5 μM folate, RM did not grow much and grew slower than OE1-1, respectively (data not shown). On the other hand, when it was incubated in minimal medium containing 2 μM PABA or 30 μM folate, RM grew as well as OE1-1, and the population of the mutant reached 2.5 × 108 CFU/ml 16 h after incubation (Fig. 4B). These results suggest that the addition of 2 μM PABA or 30 μM folate can rescue the ability of RM to grow in minimal medium.
Virulence of RM for folate-pretreated tobacco plants.
To test whether the virulence of RM could be complemented by a treatment with folate, we incubated tobacco plants in Hoagland's solution containing 500 μM folate for 2 days and then inoculated them by root dipping into RM or OE1-1. Tobacco plants inoculated with RM wilted by 12 DAI, suggesting that the virulence of RM for tobacco plants was restored by the pretreatment with folate (Fig. 5).
FIG. 5.
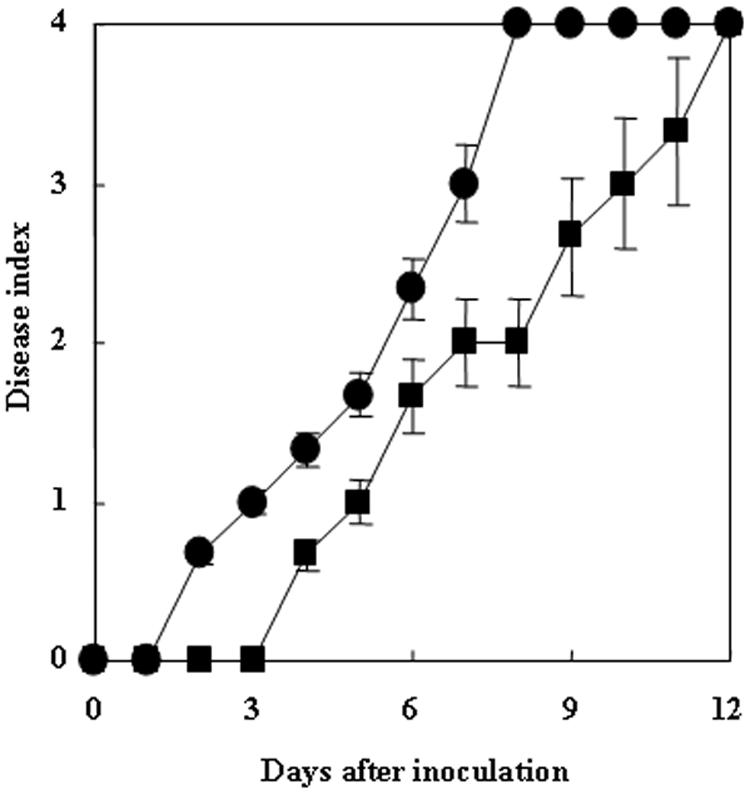
Virulence of R. solanacearum in folate-pretreated tobacco plants. Tobacco plants were grown in water culture pots with fivefold-diluted Hoagland's solution containing 500 μM folate for 2 days in a growth room and then were inoculated by root dipping with R. solanacearum OE1-1 (circles) or RM (squares). Each assay was repeated in five successive trials.
Proliferation of RM in intercellular fluids and stem fluids.
OE1-1 grew vigorously both in intercellular fluids (Fig. 6A) and in stem fluids (Fig. 6B). While RM grew vigorously as well as OE1-1 in stem fluids (Fig. 6B), it did not grow well compared to OE1-1 in intercellular fluids (Fig. 6A).
FIG. 6.
Growth of R. solanacearum OE1-1 (circles) and RM (squares) in intercellular fluids (A) and stem fluids (B). Each assay was repeated in five successive trials.
DISCUSSION
All cells, both prokaryotic and eukaryotic, require reduced folate cofactors for the biosynthesis of a diverse range of cellular components (4). The OE1-1 pabB mutant generated for this study lost the ability to grow vigorously in nutrient-poor medium. PABA, an intermediate in the tetrahydrofolate biosynthetic pathway in a variety of bacteria (4, 12, 21), is synthesized from chorismate in two steps. In the first step, chorismate and glutamine are converted to ADC and glutamate by ADC synthase, which is composed of dissimilar subunits, PabA and PabB. Glutamine aminotransferase encoded by pabA generates ammonia, which is used along with chorismate in the second step, catalyzed by aminodeoxychorismate synthase encoded by pabB. The restoration of the ability of RM to grow vigorously in a nutrient-poor medium containing either folate or PABA suggests that the destruction of pabB results in a loss of the ability to synthesize PABA, leading to a loss of the ability to synthesize folate. Therefore, RM is a folate auxotroph.
Plants are able to synthesize tetrahydrofolate de novo, similar to bacteria (14, 22). As determined by an analysis of the subcellular distribution of folate in pea leaves, folate is distributed in a ratio of 30:3:67 between the mitochondrial fraction, the chloroplast fraction, and a fraction including the cytosol, the nucleus, and the vacuole (10). Plants synthesize PABA in chloroplasts and use it to synthesize folate in mitochondria (22). However, there are no reports on the distribution of PABA and folate in the intercellular spaces and xylem vessels. In this study, although the folate biosynthesis-deficient mutant had the ability to utilize external folate, the mutant lost its ability to grow in intercellular spaces and intercellular fluids. These results suggest that the folate concentration in plants is not sufficient for the growth of RM in intercellular spaces. Therefore, the biosynthesis of folate is essential for the vigorous proliferation of bacteria in intercellular spaces.
Coplin et al. (7) reported that the relative concentrations of methionine and leucine in xylem vessel fluid were sufficiently different to explain the susceptibility of tobacco plants and the resistance of tomato plants to methionine and leucine auxotrophs of R. solanacearum K60. They also reported that a severe limitation of the growth of tryptophan auxotrophs in plants leads to a loss of their virulence, not only when they are directly inoculated into xylem vessels, but also when they are used to infiltrate leaves (7). On the other hand, RM also lost its virulence for tomato plants and eggplants when it was used to infiltrate leaves (data not shown). Moreover, the results of this study suggest that the loss of virulence of the folate auxotroph may be dependent on a loss of the ability to vigorously proliferate due to the low levels of folate in intercellular spaces.
After vigorous proliferation in intercellular spaces, R. solanacearum accumulates around the stele of the plant and then breaks into and fills the xylem vessels by the action of its cellulolytic enzymes on vessel walls or its release from a tylose formed by infected cells adjacent to xylem vessels (26). The bacteria then systemically infect entire plants through xylem vessels in the stems (16, 17). Interestingly, RM retained its ability to grow vigorously in the stems and stem fluid. This evidence suggests that there may be enough folate or PABA in xylem vessels for bacterial proliferation. On the other hand, RM persisted in the infiltrated sites, suggesting that the folate auxotroph lost its systemic infectivity. The loss of the ability to vigorously proliferate in intercellular spaces may result in a loss of systemic infectivity, suggesting that the proliferation of bacteria in intercellular spaces may be required for invasion into xylem vessels. Therefore, the biosynthesis of folate contributes to the vigorous proliferation of bacteria in intercellular spaces and to the subsequent systemic infection leading to virulence.
Acknowledgments
We thank T. Okuno, Kyoto University, for his critical reading of the manuscript.
This work was supported by grants-in-aid for scientific research on priority area B (16380037) and priority area A (15028214) to Y.H. from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Allen, C., Y. Huang, and L. Sequeira. 1991. Cloning of genes affecting polygalacturonase production in Pseudomonas solanacearum. Mol. Plant-Microbe Interact. 4:147-154. [Google Scholar]
- 2.Arlat, M., C. L. Gough, C. Zischek, P. Barberis, A. Trigalet, and C. Boucher. 1992. Transcriptional organization and expression of the large hrp gene cluster of Pseudomonas solanacearum. Mol. Plant-Microbe Interact. 5:187-193. [DOI] [PubMed] [Google Scholar]
- 3.Arlat, M., F. Van Gijsegum, J. C. Huet, J. C. Pernollet, and C. A. Boucher. 1994. PopA1, a protein which induces a hypersensitive-like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanacearum. EMBO J. 13:543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermingham, A., and J. P. Derrick. 2002. The folic acid biosynthesis pathway in bacteria: evaluation of potential for antibacterial drug discovery. BioEssays 24:637-648. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, C. A., P. A. Barberis, P. A. Trigaret, and D. A. Demery. 1985. Transposon mutagenesis of Pseudomonas solanacearum: isolation of Tn5-induced avirulent mutants. J. Gen. Microbiol. 131:2449-2457. [Google Scholar]
- 6.Boucher, C. A., F. Van Gijsegem, P. Barberis, M. Arlat, and C. Zischek. 1987. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J. Bacteriol. 169:5626-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coplin, D. L., L. Sequeira, and R. S. Hanson. 1974. Pseudomonas solanacearum: virulence of biochemical mutants. Can. J. Microbiol. 20:519-529. [DOI] [PubMed] [Google Scholar]
- 8.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wit, P. J. G., and G. Spikman. 1982. Evidence for the occurrence of race- and cultivar-specific elicitors of necrosis in intercellular fluids of compatible interactions of Cladosoprium fulvum and tomato. Physiol. Plant Pathol. 21:1-11. [Google Scholar]
- 10.Gambonnet, B., S. Jabrin, S. Ravanel, M. Karan, R. Douce, and F. Rebeille. 2001. Folate distribution during higher plant development. J. Sci. Food Agric. 81:835-841. [Google Scholar]
- 11.Genin, S., C. L. Gough, C. Zischek, and C. A. Boucher. 1992. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol. Microbiol. 6:3065-3076. [DOI] [PubMed] [Google Scholar]
- 12.Green, J. M., B. P. Nichols, and R. G. Matthews. 1996. Folate biosynthesis, reduction, and polyglutamylation, p. 665-673. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, A. D., and J. F. Gregory III. 2002. Synthesis and turnover of folates in plants. Curr. Opin. Plant Biol. 5:244-249. [DOI] [PubMed] [Google Scholar]
- 15.Hara, H., and K. Ono. 1983. Ecological studies on the bacterial wilt of tobacco, caused by Pseudomonas solanacearum E. F. Smith. I. A selective medium for isolation and detection of P. solanacearum. Bull. Okayama Tob. Exp. Stn. 42:127-138. [Google Scholar]
- 16.Hayward, H. C. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29:65-87. [DOI] [PubMed] [Google Scholar]
- 17.Hikichi, Y., Y. Nakazawa-Nasu, S. Kitanosono, K. Suzuki, and T. Okuno. 1999. The behavior of genetically lux-marked Ralstonia solanacearum in grafted tomato cultivars resistant or susceptible to bacterial wilt. Ann. Phytopathol. Soc. Jpn. 65:597-603. [Google Scholar]
- 18.Kanda, A., S. Ohnishi, H. Tomiyama, H. Hasegawa, M. Yasukohchi, A. Kiba, K. Ohnishi, T. Okuno, and Y. Hikichi. 2003. Type III-secretion machinery deficient mutants of Ralstonia solanacearum lose their ability to colonize, proliferate and induce host responses immediately after invasion, resulting in loss of their virulence. J. Gen. Plant Pathol. 69:250-257. [Google Scholar]
- 19.Kanda, A., M. Yasukohchi, K. Ohnishi, A. Kiba, T. Okuno, and Y. Hikichi. 2003. Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant-Microbe Interact. 16:447-455. [DOI] [PubMed] [Google Scholar]
- 20.Kelman, A., and L. Sequeira. 1965. Root-to-root spread of Pseudomonas solanacearum. Phytopathology 55:304-309. [Google Scholar]
- 21.Nicols, B. P., A. M. Seibold, and S. Z. Doktor. 1989. para-Aminobenzoate synthesis from chorismate occurs in two steps. J. Biol. Chem. 264:8597-8601. [PubMed] [Google Scholar]
- 22.Ravanel, S., H. Cherest, S. Jabrin, D. Grunwald, Y. Surdin-Kerjan, R. Douce, and F. Rebeille. 2001. Tetrahydrofolate biosynthesis in plants: molecular and functional characterization of dihydrofolate synthetase and three isoforms of folylpolyglutamate synthetase in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98:15360-15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, D. P., T. P. Denny, and M. A. Schell. 1988. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J. Bacteriol. 170:1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salanoubat, M., S. Genin, F. Artiguenave, J. Gouzy, S. Mangenot, M. Arlat, A. Billault, P. Brottier, J. C. Camus, L. Cattolico, M. Chandler, N. Choisne, C. Claudel-Renard, S. Cunnac, N. Demange, C. Gaspin, M. Lavie, A. Moisan, C. Robert, W. Saurin, T. Schiex, P. Siguier, P. Thebault, M. Whalen, P. Wincker, M. Levy, J. Weissenbach, and C. A. Boucher. 2002. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 415:497-502. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schell, M. A. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory array. Annu. Rev. Phytopathol. 38:263-292. [DOI] [PubMed] [Google Scholar]
- 27.Van Gijsegem, F., C. Gough, C. Zischek, E. Niqueux, M. Arlat, S. Genin, P. Barberis, S. German, P. Castello, and C. Boucher. 1995. The hrp gene cluster of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol. Microbiol. 15:1095-1114. [DOI] [PubMed] [Google Scholar]
- 28.Wakimoto, S., T. Uematsu, and H. Mukoo. 1968. Bacterial canker disease of tomato in Japan. 1. Isolation and identification of the causal bacteria, and resistance of tomato varieties against the disease. Bull. Natl. Inst. Agric. Sci. Ser. C 22:269-279. [Google Scholar]
- 29.Yabuuchi, E., Y. Kosako, I. Yano, H. Hotta, and Y. Nishiuchi. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff, 1973) comb. nov., Ralstonia eutropha (Davis, 1962) comb. nov. Microbiol. Immunol. 39:897-904. [DOI] [PubMed] [Google Scholar]