Abstract
The resistance of Escherichia coli O157:H7 strains ATCC 43895-, 43895-EPS (an exopolysaccharide [EPS]-overproducing mutant), and ATCC 43895+ (a curli-producing mutant) to chlorine, a sanitizer commonly used in the food industry, was studied. Planktonic cells of strains 43895-EPS and/or ATCC 43895+ grown under conditions supporting EPS and curli production, respectively, showed the highest resistance to chlorine, indicating that EPS and curli afford protection. Planktonic cells (ca. 9 log10 CFU/ml) of all strains, however, were killed within 10 min by treatment with 50 μg of chlorine/ml. Significantly lower numbers of strain 43895-EPS, compared to those of strain ATCC 43895-, attached to stainless steel coupons, but the growth rate of strain 43895-EPS on coupons was not significantly different from that of strain ATCC 43895-, indicating that EPS production did not affect cell growth during biofilm formation. Curli production did not affect the initial attachment of cells to coupons but did enhance biofilm production. The resistance of E. coli O157:H7 to chlorine increased significantly as cells formed biofilm on coupons; strain ATCC 43895+ was the most resistant. Population sizes of strains ATCC 43895+ and ATCC 43895- in biofilm formed at 12°C were not significantly different, but cells of strain ATCC 43895+ showed significantly higher resistance than did cells of strain ATCC 43895-. These observations support the hypothesis that the production of EPS and curli increase the resistance of E. coli O157:H7 to chlorine.
Escherichia coli O157:H7 was first recognized as an enteric pathogen in 1982 (41). It has since been characterized in several laboratories as causing self-limiting diarrhea, hemorrhagic colitis, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura in children and other susceptible groups of individuals (16, 33). Outbreaks of E. coli O157:H7 infections have been primarily associated with eating undercooked ground beef, but a variety of other foods have also been implicated as vehicles. Cross-contamination of foods can occur in food-processing plants and during subsequent handling and preparation, resulting in a wide range of foods being implicated in outbreaks of E. coli O157:H7 infections (4, 26, 32).
A biofilm can be defined as a sessile bacterial community of cells that live attached to each other and to surfaces (10). Attachment and biofilm formation by food-borne pathogens and spoilage microorganisms on food contact surfaces in processing plants are a public health and cross-contamination concern. Biofilms can also form on the surfaces of containers used for harvesting, transporting, and displaying foods at the retail level (4, 6, 11, 21) and can develop on food surfaces (8, 9, 17). E. coli O157:H7 can form biofilms on stainless steel (14, 44, 45), and sloughing of cells may result in cross-contamination of foods during processing (20, 28, 29, 34). The resistance of bacterial cells embedded in biofilm to environmental stresses, such as sanitizers routinely used in the food industry, can be dramatically increased (20, 28, 35, 46, 50).
E. coli O157:H7 is known to produce exopolysaccharides (EPS) (23, 27, 31), which can provide a physical barrier to protect cells against environmental stresses. EPS is also involved in cell adhesion and biofilm formation (18, 49). EPS can serve as a conditioning film on inert surfaces (1), affect cell attachment by functioning as an adhesive or antiadhesive (36), and influence the formation of three-dimensional biofilm structures (12). We have observed that EPS produced by E. coli O157:H7 acts as an antiadhesive, affecting the attachment of cells on the surface of stainless steel (44). Cells in biofilm produced by an EPS-overproducing mutant had enhanced resistance against environmental stresses imposed by nutrient limitation in lettuce juice compared to that of cells in biofilm formed by a strain not overproducing EPS. Resistance of cells embedded in biofilm to sanitizers was not investigated in that study (44).
E. coli O157:H7 has also been shown to produce curli, a thin, coiled fimbriae-like extracellular structure. Unlike nonpathogenic E. coli, curli production by E. coli O157:H7 is uncommon but can occur in association with csgD promoter point mutations (47). Curli produced by nonpathogenic E. coli enhanced the attachment of cells on the surface of polystyrene (40, 48). We studied attachment and biofilm formation of three curli-deficient strains of E. coli O157:H7, as well as curli-producing mutants of the same strains, and concluded that curli production does not affect attachment of cells to stainless steel but does promote biofilm production (45). These discrepant observations may be due in part to differences in hydrophobic characteristics of polystyrene and stainless steel. Stainless steel is moderately hydrophilic, with a negative surface charge (18), whereas polystyrene is hydrophobic. Attachment and detachment of E. coli O157:H7 on lettuce are known to be affected by hydrophobicity, surface charge, and capsule production (24).
Bacterial attachment is influenced by the surface of cells and attachment media as well as by other environmental factors (11, 18, 28). Biofilm formation and subsequent resistance of embedded cells to biocide activity are affected by extracellular structures, such as fimbriae, flagella, and EPS, that often surround cells in protective matrices. Understanding the role of EPS and curli produced by E. coli O157:H7 on attachment, biofilm formation on foods and food contact surfaces, and protection of cells against sanitizers commonly used in processing plants and foodservice settings would provide fundamental information of practical significance for developing intervention strategies to eliminate or control the pathogen.
The objectives of this study were to determine if the production of EPS and curli by E. coli O157:H7 affects attachment and biofilm formation on stainless steel and to determine the influence of EPS and curli production on resistance of cells to chlorine.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli O157:H7 strains ATCC 43895- (curli-deficient strain), ATCC 43895+ (curli-overproducing mutant), and 43895-EPS (spontaneous mutant of ATCC 43895- capable of producing extensive amounts of EPS) were used. Stock cultures kept at −25°C in 15% (wt/vol) glycerol were inoculated into 10 ml of tryptic soy broth (TSB; pH 7.0; BBL/Difco, Sparks, Md.) and incubated at 37°C. Three consecutive 24-h transfers were made before cells were used in experiments. Curli production by each strain was determined by spread plating a cell suspension (0.1 ml) on tryptic soy agar (TSA; pH 7.0; BBL/Difco) supplemented with Congo red (20 μg/ml) and Coomassie brilliant blue (10 μg/ml). Plates were incubated at 22°C for 48 h before examining for evidence of curli production.
Media used.
Cells cultured on modified TSA (mTSA) and heated lettuce juice agar (HLJA) (pH 6.5) were used. Modified TSA (pH 6.5) was prepared by adding HCl (1 N) to TSA (pH 7.0). HLJA was prepared as described by Ryu and Beuchat (42). To test the resistance of E. coli O157:H7 in biofilms formed on stainless steel coupons to chlorine, cells cultured on HLJA were used. Phosphate-buffered saline (PBS) (pH 7.4), which contains (per liter of distilled water) NaCl (8 g), KCl (0.2 g), Na2HPO4 (1.44 g), and KH2PO4 (0.24 g), was used to prepare suspensions of cells produced on mTSA or HLJA. Bacto M9 minimal salt broth (MSB) (pH 7.0) (15) was prepared to grow biofilms on coupons. Approximately 2.25 ml of sodium hypochlorite (NaOCl) (Sigma, St. Louis, Mo.) was added to 500 ml of potassium phosphate buffer (0.05 M, pH 6.8) to give a free chlorine concentration of 200 μg/ml. This solution was diluted in phosphate buffer to give free chlorine concentrations of 20, 50, and 100 μg/ml. Buffer without added NaOCl served as a control solution (0 μg of free chlorine/ml). The concentration of free chlorine was quantitated using a free chlorine colorimeter (model Dr/820; Hach, Loveland, Colo.). Dey-Engley (DE) neutralizing broth (pH 7.4) (BBL/Difco) was used to neutralize chlorine at the end of treatment times. TSA was used to enumerate E. coli O157:H7 in suspensions and in biofilms after treatment with chlorine.
Preparation of stainless steel coupons.
Stainless steel type 304 with number 4 finish was used to prepare coupons (2 by 5 cm). Stainless steel coupons were sonicated in a hot (80°C) alkali detergent solution (FS Pro-Chlor; Zep, Atlanta, Ga.) for 20 min in an ultrasonic water bath (model 250D; VWR, West Chester, Pa.), rinsed in distilled water, sonicated in a 15% phosphoric acid solution at 80°C for 20 min, and rinsed in distilled water with agitation for 20 min. Cleaned coupons were dry sterilized by dry heat treatment prior to use.
Quantification of ECC.
The amount of extracellular carbohydrate complexes (ECC) produced by E. coli O157:H7 cultured under various growth conditions was quantified using the procedure described by Ryu and Beuchat (42). ECC is defined as a composite of carbohydrates secreted by cells or loosely attached to the cell surface which can be detached by heat treatment, and it represents the EPS produced. ECC consists largely of slime and capsule as well as carbohydrates originating from other cellular components, e.g., lipopolysaccharide, cell walls, and degraded EPS. Cryopreserved cell suspensions of strains 43895-EPS, ATCC 43895-, and ATCC 43895+ were thawed and loop transferred (10 μl) to 10 ml of TSB and were incubated at 37°C for 3 days, with transfers at 24-h intervals. Approximately 104 CFU of each strain were inoculated onto the surface of mTSA and HLJA and were incubated at 22°C for 3 days or at 12°C for 5 days. Colonies were collected using a bent glass rod, and the amount of ECC was determined.
Biofilm formation on stainless steel coupons.
E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ grown in TSB at 37°C were loop transferred (10 μl) at 24-h intervals for 3 days. Cultures were serially diluted in 0.1% peptone water, and ca. 104 CFU were surface plated on HLJA, followed by incubating at 22°C for 3 days. Cells were collected using a bent glass rod, suspended in PBS, and diluted to a population of ca. 108 CFU/ml. Cell suspensions (30 ml) were deposited in 50-ml centrifuge tubes, each containing a sterile coupon, and were incubated at 4°C for 24 h to facilitate attachment of cells. Each coupon was then rinsed in 400 ml of PBS by gently moving in a circular motion for 15 s, transferred to a 50-ml centrifuge tube containing 30 ml of MSB, and incubated at 22°C for 2 days or at 12°C for 6 days. Each coupon was rinsed with PBS as described above, transferred to a centrifuge tube containing 30 ml of MSB, and again incubated at 22°C for 2 days or at 12°C for 6 days. The resistance of E. coli O157:H7 in biofilms to chlorine was determined as described below.
Sensitivity of planktonic cells to chlorine.
Suspensions (0.1 ml of 24-h cultures grown at 37°C) of E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ diluted in 0.1% peptone water to give 104 CFU/ml were surface plated on mTSA and HLJA and were incubated at 22°C for 3 days or at 12°C for 5 days. Cells were collected using a sterile bent glass rod, suspended in PBS, and diluted to a population of 108 to 109 CFU/ml. These suspensions contained various amounts of ECC, depending on conditions used to culture cells. Cell suspensions (5 ml) were transferred to sterile 25- by 150-mm screw-cap test tubes. Solutions (5 ml) containing free chlorine at concentrations of 0, 20, 50, and 100 μg/ml were added to test tubes containing cell suspensions and were thoroughly mixed, resulting in chlorine concentrations of 0, 10, 25, and 50 μg/ml. At 1, 3, 5, and 10 min, 2 ml of the chlorinated cell suspension was withdrawn and combined with 2 ml of 2× DE neutralizing broth. Samples of undiluted mixture (0.25 ml in quadruplicate) and mixture serially diluted in 0.1% peptone water (0.1 ml in duplicate) were surface plated on TSA and incubated for 48 h at 37°C before colonies were counted.
Treatment of biofilms with chlorine.
Biofilms of E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ were developed on coupons as described above. Each coupon containing biofilm formed by a single strain was rinsed in PBS and deposited in a 25- by 150-mm screw-cap test tube containing 30 ml of chlorine solution (0, 50, and 200 μg/ml). After treatment for 1, 3, and 5 min, each coupon was transferred to a 50-ml centrifuge tube containing 30 ml of DE neutralizing broth and 3 g of sterile glass beads (425 to 600 μ; Sigma-Aldrich, St. Louis, Mo.). The mixture of a coupon, glass beads, and DE neutralizing broth was vortexed (model Vortexgenie-2; Scientific Industries, Inc., Bohemia, N.Y.) at maximum speed for 1 min. Suspensions (0.1 ml in duplicate) were surface plated on TSA using a spiral plater (Autoplate 4000; Spiral Biotech, Norwood, Mass.) and incubated at 37°C for 48 h to determine populations of cells surviving treatment.
Statistical analysis.
All experiments were replicated three or more times. In experiments involving chlorine treatment, two coupons were examined in each replicate. Data were analyzed using the general linear model of the Statistical Analysis Systems procedure (SAS; SAS Institute, Cary, N.C.). Duncan's multiple-range test and proc t test were used to determine if amounts of ECC produced by E. coli O157:H7 under different culture conditions were significantly different. Significant differences in mean values are presented at a 95% confidence level (P ≤ 0.05).
RESULTS AND DISCUSSION
Quantification of ECC produced by E. coli O157:H7.
Table 1 shows the amount of ECC, calculated on a cell biomass basis, produced by E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ on mTSA and HLJA at 22°C for 3 days or 12°C for 5 days. Strain 43895-EPS consistently produced significantly (P ≤ 0.05) greater amounts of ECC than strains ATCC 43895- and ATCC 43895+ grown under the same culture conditions. With the exception of cells grown on mTSA at 22°C, there was no significant difference in the amount of ECC produced by strains ATCC 43895- and 43895+ under the same growth conditions. When cells were grown on the same medium, strains 43895-EPS and ATCC 43895+ produced significantly larger amounts of ECC at 12 than at 22°C, but strain ATCC 43895- did not produce significantly different amounts at the two temperatures. This is in good agreement with previous observations (43) demonstrating that low nutrient availability (HLJA versus mTSA) and low incubation temperature (12 versus 22°C) favors the production of ECC by E. coli O157:H7.
TABLE 1.
Production of ECC by E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and 43895+ on a per-cell biomass basis as affected by growth medium and temperature
| Medium | Strain | Amt of ECC (μg/ml)a at:
|
|
|---|---|---|---|
| 22°C | 12°C | ||
| HLJA | 43895-EPS | a 1,402 b | a 2,676 a |
| ATCC 43895- | b 103 a | b 152 a | |
| ATCC 43895+ | b 83 b | b 136 a | |
| mTSA | 43895-EPS | a 135 b | a 289 a |
| ATCC 43895- | b 66 a | b 55 a | |
| ATCC 43895+ | c 39 b | b 121 a | |
Amount of ECC produced on a per-cell biomass basis. A standard initial population (104 CFU) of E. coli O157:H7 was inoculated onto each plate and was incubated at 22°C for 3 days or 12°C for 5 days. Values represent the amount of ECC present in suspension at an optical density at 750 nm of 1.0. Within a medium and temperature, values in a column that are not preceded by the same letter are significantly different (P ≤ 0.05). Values in the same row that are not followed by the same letter are significantly different (P ≤ 0.05).
Sensitivity of planktonic cells to chlorine.
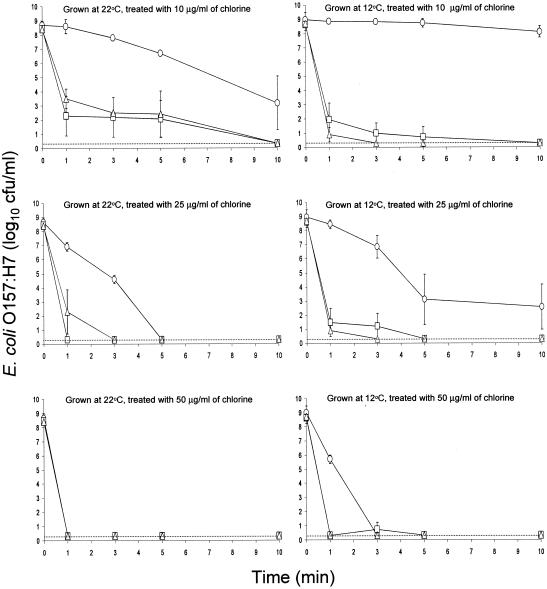
Viability of test strains was not affected by treatment with water. Shown in Fig. 1 are populations of planktonic cells of E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ grown on HLJA at 22 and 12°C and treated with chlorine (10, 25, and 50 μg/ml) for 1, 3, 5, and 10 min. Cell suspensions contained various amounts of ECC (Table 1), depending on strain and growth conditions. Strain 43895-EPS was more resistant than strains ATCC 43895- and ATCC 43895+ to chlorine, indicating that protection was afforded by ECC. The population of strain 43895-EPS grown at 22°C decreased by 5.2 log10 CFU/ml when treated for 10 min with 10 μg of chlorine/ml, but cells grown at 12°C were unaffected by the same treatment. Strain 43895-EPS produced a much larger amount of ECC at 12°C (2,676 μg/ml) than at 22°C (1,402 μg/ml), giving additional support to the hypothesis that EPS protects cells against biocidal activity of chlorine, probably by changing the free chlorine to a nonlethal form before it reached the cell wall. When strain 43895-EPS was treated with chlorine at concentrations of 25 and 50 μg/ml, cells grown at 12°C showed greater resistance than cells grown at 22°C. Populations of cells of strains ATCC 43895- and ATCC 43895+ grown at both temperatures were reduced to <0.3 log10 CFU/ml within 1, 5, and 10 min when treated with 50, 25, and 10 μg of chlorine/ml, respectively.
FIG. 1.
Inactivation of planktonic cells of E. coli O157:H7 strains 43895-EPS (○), ATCC 43895- (□), and ATCC 43895+ (▵) grown on heated lettuce juice agar at 22 and 12°C and treated with 10, 25, and 50 μg of chlorine/ml. The detection limit (dotted line) is 0.3 log10 CFU/ml (2 CFU/ml); bars indicate standard deviations.
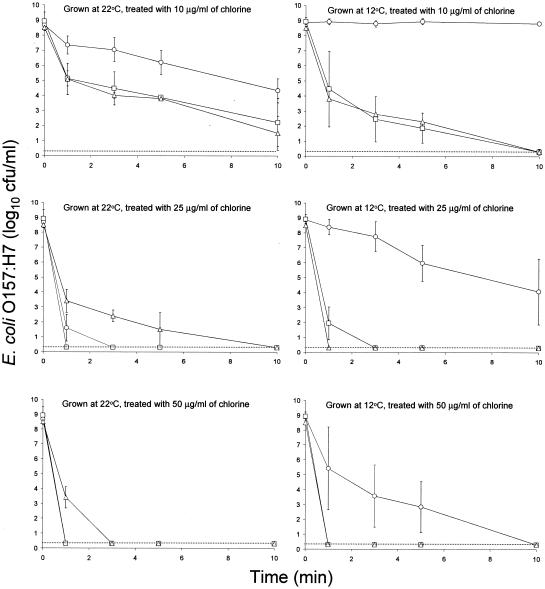
Figure 2 shows the populations of planktonic cells of E. coli O157:H7 strains 43895-EPS, ATCC 43895-, and ATCC 43895+ grown on mTSA at 22°C for 3 days or 12°C for 5 days and treated with chlorine. Treatment of cells with water resulted in an insignificant reduction in population. The amount of ECC produced by strain 43895-EPS on mTSA was significantly less than that produced on HLJA at the same temperature, but cells showed similar resistance to treatment with 10 μg of chlorine/ml. The population of strain 43895-EPS grown at 22°C was reduced from 8.9 log10 CFU/ml to 4.3 log10 CFU/ml by treatment for 10 min with 10 μg of chlorine/ml, but treatment of cells grown at 12°C did not cause a significant reduction. When treated with 25 μg of chlorine/ml, strain 43895-EPS grown at 22°C was inactivated within 3 min, but cells grown at 12°C survived for 10 min. Treatment of strain 43895-EPS grown at 22°C with 50 μg of chlorine/ml inactivated cells within 1 min, but some of the cells grown at 12°C survived for 5 min. Strain 43895- grown at 22 and 12°C survived for 10 min upon treatment with 10 μg of chlorine/ml. Treatment of cells grown at 22 and 12°C with 25 μg of chlorine/ml resulted in inactivation within 1 and 3 min, respectively. Treatment with 50 μg of chlorine/ml inactivated cells within 1 min, regardless of growth temperature.
FIG. 2.
Inactivation of planktonic cells of E. coli O157:H7 strains 43895-EPS (○), ATCC 43895- (□), and ATCC 43895+ (▵) grown on modified tryptic soy agar at 22 and 12°C and treated with 10, 25, and 50 μg of chlorine/ml. The detection limit (dotted line) is 0.3 log10 CFU/ml (2 CFU/ml); bars indicate standard deviations.
Cells of strain ATCC 43895+ grown on mTSA (Fig. 2) generally showed higher resistance to chlorine than did cells grown on HLJA (Fig. 1). Interestingly, resistance was influenced significantly by growth temperature. Cells grown on mTSA at 22°C were reduced from 8.5 log10 CFU/ml to 1.5 log10 CFU/ml after treatment for 10 min with 10 μg of chlorine/ml, whereas cells grown at 12°C were reduced to <0.3 log10 CFU/ml (detection limit). Cells grown at 22°C were detected after treatment for 5 min (1.5 log10 CFU/ml) and 1 min (3.4 log10 CFU/ml) when treated with either 25 or 50 μg of chlorine/ml, respectively, but cells grown at 12°C were inactivated (<0.3 log10 CFU/ml) within 1 min when treated with 25 and 50 μg of chlorine/ml. Because the amount of ECC produced by strain ATCC 43895+ on mTSA at 22°C was significantly lower than that at 12°C, other factors, e.g., curli production, must contribute to the level of chlorine resistance. Olsen et al. (37, 38, 39) demonstrated that curli are typically produced by nonpathogenic E. coli under stress conditions, such as suboptimal growth temperature, low osmolarity, and nutrient deprivation. Environmental factors affecting curli production by E. coli O157:H7 have not been characterized. Although the amount of curli produced by strain ATCC 43895+ was not determined, cells grown on mTSA at 22°C agglutinated in PBS more than those grown on HLJA at 22°C or mTSA at 12°C, as evidenced by subjective observations. Cells of strain ATCC 43895+ grown in TSB at 22°C agglutinated more readily than cells grown at 12°C. This suggests that mTSA may provide a more favorable condition than HLJA for the production of curli, and cells may produce more curli at 22 than at 12°C. Direct measurements of curli production would serve to verify this hypothesis. The observation that strain ATCC 43895+ grown on mTSA at 22°C was more resistant to chlorine than strain ATCC 43895+ grown on HLJA at 22°C or mTSA at 12°C supports the hypothesis that curli production enhances the chlorine resistance.
Results show that EPS produced by E. coli O157:H7 can protect planktonic cells against bactericidal activity of chlorinated water. Curli production may also increase the resistance of planktonic cells to chlorine. Enhanced resistance may not, however, have exceptional practical significance, because the pathogen was rapidly inactivated by treatment with 50 μg of chlorine/ml, a concentration not uncommonly used to sanitize food-processing equipment, regardless of its ability to produce EPS or curli. The role of EPS and curli in protecting cells against chlorine would become significant in situations where lower concentrations of chlorine are applied.
Resistance of cells in biofilm to chlorine.
Biofilms developed by E. coli O157:H7 strains on stainless steel coupons immersed in MSB at 22 and 12°C were treated with chlorine. To simulate biofilm formation on stainless steel in a fresh-cut-lettuce processing plant or in a foodservice facility, cells were grown on HLJA at 22°C for 3 days before suspending in PBS and attaching to stainless steel coupons. Coupons were transferred to fresh MSB after incubation for 2 and 6 days at 22 and 12°C, respectively, for a second immersion period to more easily assess growth and biofilm formation.
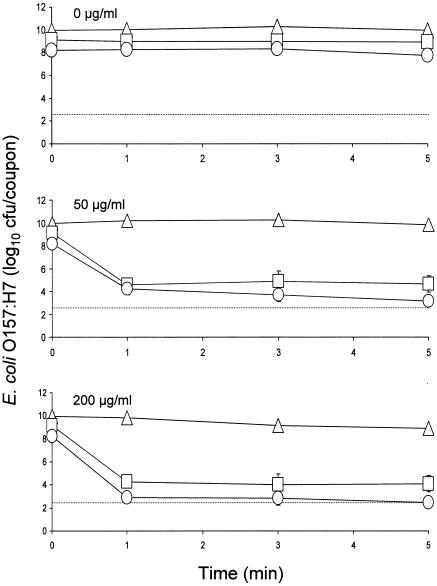
We previously observed that, compared to strain ATCC 43895-, significantly smaller numbers of cells of strain 43895-EPS attached to coupons in PBS, indicating that EPS production was an antiadhesive factor (44). In another study, we observed no significant differences in populations of strains ATCC 43895- and ATCC 43895+ recovered from coupons in PBS (45). In the present study, as biofilms matured in MSB at 22°C, populations of strains 43895-EPS, ATCC 43895-, and ATCC 43895+ increased to 8.2, 9.1, and 10.0 log10 CFU/coupon, respectively (Fig. 3). This shows that populations of strains 43895-EPS and ATCC 43895- increased to similar levels on coupons, indicating that EPS production did not inhibit biofilm formation of strain 43895-EPS. There was no significant difference between populations of strains ATCC 43895- and ATCC 43895+ that attached to coupons in PBS, but the population of strain ATCC 43895+ at the end of the 4-day incubation period during which biofilm was formed was significantly greater than that of strain ATCC 43895-. This suggests that curli production by E. coli O157:H7 did not enhance the attachment of cells on coupons but did enhance biofilm formation. Results also show that mechanisms of attachment of cells on coupons and maturation of biofilm may be different.
FIG. 3.
Inactivation of E. coli O157:H7 strains 43895-EPS (○), ATCC 43895- (□), and ATCC 43895+ (▵) in biofilms formed in minimal salt broth at 22°C upon treatment with 0, 50, and 200 μg of chlorine/ml. The detection limit per coupon (dotted line) is 2.5 log10 CFU. Bars indicate standard deviations.
Inactivation curves for E. coli O157:H7 in biofilms developed on stainless steel coupons at 22°C and treated with 0, 50, and 200 μg of chlorine/ml are shown in Fig. 3. There was no significant reduction in counts when biofilms were treated with water for 5 min. As expected, the resistance of E. coli O157:H7 to chlorine was significantly higher when cells were embedded in biofilms than when they were in planktonic suspensions. The population of strain 43895-EPS decreased from 8.2 log10 CFU/coupon to 4.3 log10 CFU/coupon within 1 min and 3.2 log10 CFU/coupon within 5 min when treated with 50 μg of chlorine/ml. The population of strain ATCC 43895- decreased from 9.1 log10 CFU/coupon to 4.6 log10 CFU/coupon within 1 min and remained constant for 5 min. In contrast, the population of strain ATCC 43895+ did not decrease significantly upon treatment with 50 μg/ml for up to 5 min. Differences in resistance in cells of the three test strains in biofilm treated with 200 μg of chlorine/ml were more clearly evident. The population of strain 43895-EPS decreased to 2.9 log10 CFU/coupon within 1 min and subsequently decreased to an undetectable level at 5 min. The population of strain 43895- decreased to 4.3 log10 CFU/coupon within 1 min and remained constant for an additional 4 min. The number of cells recovered from biofilm formed by strain 43895+ decreased by only 1.1 log10 CFU/coupon after treatment for 5 min with 200 μg of chlorine/ml.
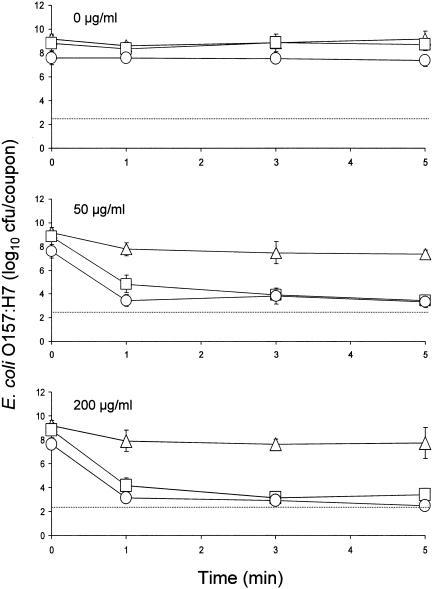
Inactivation curves for cells of E. coli O157:H7 in biofilms produced at 12°C and treated with 0, 50, and 200 μg of chlorine/ml are shown in Fig. 4. There was no significant reduction in counts when biofilms were treated with water for 5 min. Treatment with chlorine at a concentration of 50 μg/ml caused decreases in populations of strains 43895-EPS, ATCC 43895-, and ATCC 43895+ from initial levels of 7.6, 8.8, and 9.2 log10 CFU/coupon to 3.4, 4.8, and 7.8 log10 CFU/coupon, respectively, within 1 min, but counts did not decrease significantly during treatment for an additional 4 min. At a treatment concentration of 200 μg of chlorine/ml, populations of strains 43895-EPS, ATCC 43895-, and ATCC 43895+ were decreased to 3.1, 4.2, and 7.9 log10 CFU/coupon, respectively, within 1 min; however, with the exception of strain 43895-EPS, further reductions were not achieved by treatment for an additional 4 min. The population of strain 43895-EPS in biofilm was reduced to an undetectable level after 5 min. Caution must be taken in comparing the resistance of cells in biofilms formed by strains 43895-EPS and ATCC 43895- to chlorine, because initial populations were significantly different. The concentration of free chlorine is rapidly neutralized by organic materials, rendering cell population a factor in assessing the resistance of cells to chlorine treatment.
FIG. 4.
Inactivation of E. coli O157:H7 strains 43895-EPS (○), ATCC 43895- (□), and ATCC 43895+ (▵) in biofilms formed in minimal salt broth at 12°C upon treatment with 0, 50, and 200 μg of chlorine/ml. The detection limit per coupon (dotted line) is 2.5 log10 CFU. Bars indicate standard deviations.
The production of curli by strain ATCC 43895+ may be more extensive at 22 than at 12°C, based on observations of agglutination characteristics of cells in PBS and TSB. Similar agglutination characteristics were observed when biofilm was developed on coupons immersed in MSB. There was extensive agglutination of cells in MSB when coupons on which strain ATCC 43895+ had attached was immersed in the broth at 22°C. However, when coupons containing strain ATCC 43895+ were kept in MSB at 12°C, cells freely dispersed into the broth. Compared to the number of cells in biofilm formed by strain ATCC 43895+ at 22°C (10.0 log10 CFU/coupon), the number in biofilm formed at 12°C (9.2 log10 CFU/coupon) was significantly lower and was not significantly different than the number formed by strain ATCC 43895-. This is attributed in part to a decrease in production of curli by strain ATCC 43895+ at 12°C. Reduced production of curli may have resulted in entrapment of fewer cells in the biofilm and an increase in the detachment of cells.
Even though cells in biofilm formed by strain ATCC 43895+ showed a greater apparent resistance to chlorine than cells in biofilm formed by strain ATCC 43895- at 22°C (Fig. 3), it cannot be concluded that cells of strain ATCC 43895+ actually had greater resistance. The initial population of strain ATCC 43895+ was about 1 log higher than that of strain ATCC 43895-, thus presenting an approximate 10-fold increase in the amount of organic matter that would more readily neutralize chlorine at the biofilm surface and, in effect, prevent contact of free chlorine with cells embedded in the biofilm. Cells in subsurface areas of biofilm that survive chlorine treatment could subsequently slough off stainless steel and contaminate foods during processing. Populations of strains ATCC 43895- and ATCC 43895+ in biofilms formed at 12°C, on the other hand, were not significantly different. Cells in biofilm formed by strain ATCC 43895+ at 12°C showed markedly higher resistance to treatment with 50 and 200 μg of chlorine/ml than did cells in biofilm formed by strain ATCC 43895- at 12°C (Fig. 4). This indicates that a major factor contributing to increased resistance of cells in biofilm formed by strain ATCC 43895+ was curli production, even though production was reduced at 12°C.
Chlorine is used as a sanitizer to kill spoilage and pathogenic microorganisms on foods as well as to control biofilm formation in food-processing environments (3, 5, 46). However, our observation that treatment with chlorine at concentrations as high at 200 μg/ml does not eliminate E. coli O157:H7 in biofilms was not unexpected. Pathogenic bacteria in biofilms on stainless steel have been shown to have remarkable resistance to sanitizers compared to that of planktonic cells (19, 22, 46). The mechanisms affording protection of cells in biofilms against antimicrobial agents have not been fully elucidated. Accessibility of sanitizers to cells embedded in biofilms, reaction-diffusion kinetics associated with specific antimicrobial agents, slower rates of cell growth, and the expression of antimicrobial-resistant phenotypes in biofilms are among the factors that may affect the efficacy of sanitizers. Frank and Koffi (19) reported that the attachment of Listeria monocytogenes on glass slides, compared to resistance of planktonic cells, enhanced the resistance of cells against sanitizers. Removing adherent cells from the surface resulted in increased sensitivity to a level similar to that of planktonic cells. These observations show that attachment itself can increase the resistance of cells aside from the production of EPS or changes in physiological state. A decrease in the surface area of cells exposed to sanitizers as a result of attachment to glass, stainless steel, or other materials may increase the resistance of cells. It has been reported that subsequent formation of microcolonies on glass slides after attachment of L. monocytogenes provided additional resistance against sanitizers, implying that other factors are also involved in conferring resistance to cells in biofilm (19).
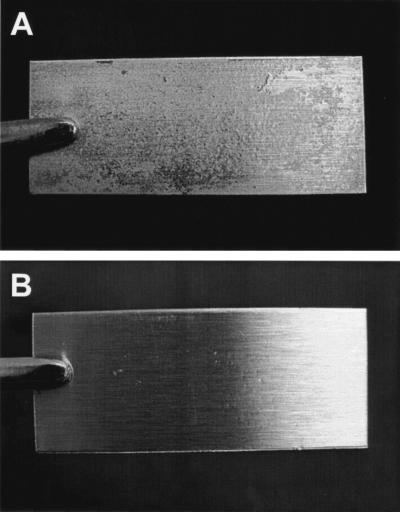
The role of EPS as a diffusion matrix or reaction barrier appears to be a major factor influencing the efficacy of chlorine in killing E. coli O157:H7, particularly in the biofilm habitat. Huang et al. (25) treated Klebsiella pneumoniae and Pseudomonas aeruginosa biofilms on stainless steel surfaces with 2 μg of monochloramine/ml. They observed gradients of respiratory activity within the biofilm, indicating that penetration of monochloramine was retarded. Stewart et al. (46) concluded that penetration of alkaline hypochlorite and chlorosulfamate into biofilms was also retarded. DeBeer et al. (13) measured transient chlorine concentration in biofilms and observed that concentrations were typically 20% or less than that in the treatment solution. In our study, populations of E. coli O157:H7 in biofilms treated with chlorine decreased rapidly within 1 min but subsequently remained fairly constant for the duration of treatment time. This may indicate that cells located on or near the surface of biofilms were immediately killed as a result of contact with free chlorine, but cells in subsurface areas survived because the diffusion of free chlorine was retarded or prevented. Other researchers are in agreement that EPS matrices are major factors responsible for neutralizing or inhibiting diffusion of antimicrobial agents, resulting in limited delivery of active forms to planktonic cells and to cells embedded in biofilm (2, 7, 30). Our study suggests that not only EPS but also curli should be considered as a major barrier to penetration and lethality of antimicrobial agents. Figure 5 shows coupons on which biofilms have been formed by E. coli O157:H7 strains ATCC 43895+ and ATCC 43895- at 22°C. Populations of 10.0 and 9.1 log10 CFU/coupon, respectively, were recovered from these coupons. These photographs provide vivid evidence that curli production greatly enhances the number of cells within biofilms and also increases the volume of biofilm produced.
FIG. 5.
Stainless steel coupons on which biofilms were produced by E. coli O157:H7 strain ATCC 43895+ (A) and strain ATCC 43895- (B) at 22°C. Coupons (2 by 5 cm) are held by forceps.
It is concluded that EPS production by E. coli O157:H7 inhibits the initial attachment of E. coli O157:H7 cells on coupons but does not inhibit biofilm development and maturation. Curli production does not affect the initial attachment of cells to coupons but enhances biofilm development. Biofilm formation and the production of EPS or curli confer resistance to chlorine. Environmental factors affecting curli production by E. coli O157:H7 should be further characterized, with particular attention given to the influence of curli produced under various conditions in protecting E. coli O157:H7 against lethality to chlorine and other sanitizers. The synergistic or additive effects of curli-producing or curli-deficient E. coli O157:H7 and other microorganisms that produce curli or EPS in biofilms, in terms of changes in resistance to sanitizers, should be investigated.
REFERENCES
- 1.Allison, D. G., and I. W. Sutherland. 1987. The role of exopolysaccharides in adhesion of freshwater bacteria. J. Gen. Microbiol. 133:1319-1327. [Google Scholar]
- 2.Ben-Ari, E. T. 1999. Not just slime. Bioscience 49:689-695. [Google Scholar]
- 3.Beuchat, L. R. 1998. Surface decontamination of fruits and vegetables eaten raw: a review. WHO/FSF/FOS/98.2. World Health Organization, Geneva, Switzerland.
- 4.Beuchat, L. R. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 4:413-423. [DOI] [PubMed] [Google Scholar]
- 5.Beuchat, L. R., and J.-H. Ryu. 1997. Produce handling and processing practices. Emerg. Infect. Dis. 3:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackman, I. C., and H. F. Frank. 1996. Growth of Listeria monocytogenes as a biofilm on various food processing surfaces. J. Food Prot. 59:827-831. [DOI] [PubMed] [Google Scholar]
- 7.Boyd, A., and A. M. Chakrabarty. 1995. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J. Ind. Microbiol. 15:162-168. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael, I., I. S. Harper, M. J. Coventry, P. W. J. Taylor, J. Wan, and M. W. Hickey. 1999. Bacterial colonization and biofilm development on minimally processed vegetables. J. Appl. Microbiol. 85:45S-51S. [DOI] [PubMed] [Google Scholar]
- 9.Cooley, M. B., W. G. Miller, and R. E. Mandrell. 2003. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl. Environ. Microbiol. 69:4915-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costerton, J. W. 1995. Overview of microbial biofilms. J. Ind. Microbiol. 15:137-140. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 12.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Beer, D., R. Srinivasan, and P. S. Stewart. 1994. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 60:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewanti, R., and A. C. L. Wong. 1995. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int. J. Food Microbiol. 26:147-164. [DOI] [PubMed] [Google Scholar]
- 15.Difco Laboratories. 1998. Difco manual, 11th ed., p. 277. Becton Dickinson and Company, Sparks, Md.
- 16.Doyle, M. P. 1991. Escherichia coli O157:H7 and its significance in foods. Int. J. Food Microbiol. 12:289-302. [DOI] [PubMed] [Google Scholar]
- 17.Fett, W. F. 2000. Naturally occurring biofilms on alfalfa and other types of sprouts. J. Food Prot. 63:625-632. [DOI] [PubMed] [Google Scholar]
- 18.Frank, J. F. 2000. Microbial attachment to food and food contact surfaces. Adv. Food Nutr. Res. 43:319-370. [DOI] [PubMed] [Google Scholar]
- 19.Frank, J. F., and R. A. Koffi. 1990. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J. Food Prot. 53:550-554. [DOI] [PubMed] [Google Scholar]
- 20.Frank, J. F., J. Ehlers, and L. Wicker. 2003. Removal of Listeria monocytogenes and poultry soil-containing biofilms using chemical cleaning and sanitizing agents under static conditions. Food Prot. Trends 23:654-663. [Google Scholar]
- 21.Gabis, D., and R. E. Faust. 1988. Controlling microbial growth in food processing environments. Food Technol. 4:81-82, 89. [Google Scholar]
- 22.Gilbert, P., D. G. Allison, and A. J. McBain. 2002. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92(Symp. Suppl.):98S-110S. [PubMed] [Google Scholar]
- 23.Grant, W. D., I. W. Sutherland, and J. F. Wilkinson. 1969. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J. Bacteriol. 100:1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan, A. N., and J. F. Frank. 2003. Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol. 87:145-152. [DOI] [PubMed] [Google Scholar]
- 25.Huang, C.-T., F. P. Yu, G. A. McFeters, and P. S. Stewart. 1995. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 61:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute of Food Technologists. 2001. Analysis and evaluation of preventive control measures for the control and reduction/elimination of microbial hazards on fresh and fresh-cut produce. Food and Drug Administration, Washington, D.C. [Online.] http://www.cfsan.fda.gov/∼comm/ift3-toc.html.
- 27.Junkins, A. D., and M. P. Doyle. 1992. Demonstration of exopolysaccharide production by enterohemorrhagic Escherichia coli. Curr. Microbiol. 65:3048-3055. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, C. G., and S. K. Anand. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 42:9-27. [DOI] [PubMed] [Google Scholar]
- 29.Kusumaningrum, H. D., G. Riboldi, W. C. Hazeleger, and R. R. Beumer. 2003. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 85:227-236. [DOI] [PubMed] [Google Scholar]
- 30.Mah, T.-F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 31.Mao, Y., M. P. Doyle, and J. Chen. 2001. Insertion mutagenesis of wca reduces acid and heat tolerance of enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:3811-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng, J., M. P. Doyle, T. Zhao, and S. Zhao. 2001. Enterohemorrhagic Escherichia coli O157:H7, p. 193-213. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, D.C.
- 34.Moore, C. M., B. W. Sheldon, and L.-A. Jaykus. 2003. Transfer of Salmonella and Campylobacter from stainless steel to romaine lettuce. J. Food Prot. 66:2231-2236. [DOI] [PubMed] [Google Scholar]
- 35.Norwood, D. E., and A. Gilmour. 2000. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 88:512-520. [DOI] [PubMed] [Google Scholar]
- 36.Ofek, I., and R. J. Doyle. 1994. Bacterial adhesion to cells and tissues. Chapman and Hall, New York, N.Y.
- 37.Olsen, A., A. Arnqvist, M. Hammar, and S. Normark. 1993. Environmental regulation of curli production in Escherichia coli. Infect. Agents Dis. 2:272-274. [PubMed] [Google Scholar]
- 38.Olsen, A., A. Arnqvist, M. Hammar, S. Sukupolvi, and S. Normark. 1993. The Rpos sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol. Microbiol. 7:523-536. [DOI] [PubMed] [Google Scholar]
- 39.Olsen, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338:652-655. [DOI] [PubMed] [Google Scholar]
- 40.Prigent-Combaret, C., G. Prensier, T. T. L. Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 41.Riley, L. W., R. S. Remis, S. D. Heigerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Harrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 42.Ryu, J.-H., and L. R. Beuchat. 2003. Development of method to quantify extracellular carbohydrate complexes produced by Escherichia coli O157:H7. J. Appl. Microbiol. 95:1304-1314. [DOI] [PubMed] [Google Scholar]
- 43.Ryu, J.-H., and L. R. Beuchat. 2004. Factors affecting production of extracellular carbohydrate complexes by Escherichia coli O157:H7. Int. J. Food Microbiol. 95:189-204. [DOI] [PubMed] [Google Scholar]
- 44.Ryu, J.-H., H. Kim, and L. R. Beuchat. 2004. Attachment and biofilm formation by Escherichia coli O157:H7 on stainless steel as influenced by exopolysaccharide production, nutrient availability, and temperature. J. Food Prot. 67:2123-2131. [DOI] [PubMed] [Google Scholar]
- 45.Ryu, J.-H., H. Kim, J. F. Frank, and L. R. Beuchat. 2004. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett. Appl. Microbiol. 39:359-362. [DOI] [PubMed] [Google Scholar]
- 46.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 47.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2001. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:2367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiner, R., S. Langille, and E. Quintero. 1995. Structure, function and immunochemistry of bacterial exopolysaccharides. J. Ind. Microbiol. 15:339-346. [DOI] [PubMed] [Google Scholar]
- 50.Williams, M. M., and E. B. Braun-Howland. 2003. Growth of Escherichia coli in model distribution system biofilms exposed to hypochlorous acid or monochloramine. Appl. Environ. Microbiol. 69:5463-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]