Abstract
Terminal restriction fragment length polymorphism (T-RFLP) was investigated as a tool for monitoring the human intestinal microflora during antibiotic treatment and during ingestion of a probiotic product. Fecal samples from eight healthy volunteers were taken before, during, and after administration of clindamycin. During treatment, four subjects were given a probiotic, and four subjects were given a placebo. Changes in the microbial intestinal community composition and relative abundance of specific microbial populations in each subject were monitored by using viable counts and T-RFLP fingerprints. T-RFLP was also used to monitor specific bacterial populations that were either positively or negatively affected by clindamycin. Some dominant bacterial groups, such as Eubacterium spp., were easily monitored by T-RFLP, while they were hard to recover by cultivation. Furthermore, the two probiotic Lactobacillus strains were easily tracked by T-RFLP and were shown to be the dominant Lactobacillus community members in the intestinal microflora of subjects who received the probiotic.
The human intestinal microflora is a highly diverse community comprised mainly of anaerobic bacteria. Under normal conditions the composition of this microbial community is relatively stable over long periods of time. However, the human microflora can be negatively affected by external factors, such as antibiotic treatment (25). Disruption of the ecological balance in the intestinal microbial community often results in reduced protection against endogenous and exogenous opportunistic pathogens. Probiotics have been proposed as a supplementary treatment to minimize the disturbances in the intestinal microflora caused by antibiotics. By increasing the number of beneficial microorganisms such as lactobacilli and bifidobacteria in the gastrointestinal tract, probiotics function, at least in part, to buffer the negative impact of antibiotics on the intestinal microflora (12, 20).
A large fraction of the intestinal community is difficult to culture due to their unknown growth requirements, physiological status, or interactions with other members of the community. Previous studies have indicated that 60 to 80% of the organisms in the intestinal tract have not yet been cultivated (9, 23). Molecular tools, based on analyses of 16S rRNA genes, can circumvent the necessity for cultivation and are currently being used to monitor microbial communities in different environmental samples. Recently, molecular approaches, such as fluorescent in situ hybridization (32) and denaturing gradient gel electrophoresis (DGGE) (2), have been used to study antibiotic- or probiotic-induced alterations in the human intestinal flora. For example, a combination of fluorescent in situ hybridization and DGGE was used to study the effect of probiotics (28) and DGGE was used to study the effect of prebiotics (27) on the human fecal microflora. Terminal restriction fragment length polymorphism (T-RFLP) is another molecular fingerprinting technique that has been used for characterization of microbial communities in various environments (1, 7, 14, 18, 22). By using a fluorescently labeled primer when the 16S rRNA genes are amplified, followed by restriction enzyme digestion, the fluorescent terminal restriction fragments (TRFs) can be visualized and registered by an automated sequencing apparatus. In principle, each TRF corresponds to an individual population in the community, and the peak area corresponds to the abundance of that population. Due to the addition of a size standard to every sample, T-RFLP enables comparisons of samples that have not been run on the same gel. Also, T-RFLP is useful as a quantitative technique to assess changes in microbial communities since the relative abundance of a specific population in a community can be easily compared for different treatments or for different sampling periods (7, 13, 30). Recently, T-RFLP has been used to analyze the composition of the human fecal microflora of a strict vegetarian (5) and to compare the microfloras of three different subjects, with specific emphasis on the Bifidobacterium community (21). In the present study, T-RFLP was investigated as a tool for monitoring changes in the human fecal microflora, and the resulting data were compared to data obtained by viable counting. Our hypothesis was that clindamycin-induced disturbances and probiotic strains could be monitored more easily by T-RFLP than by plate counting. The results of this study have broader implications in that T-RFLP was shown to be a useful tool for fingerprinting of individual human fecal microfloras and for monitoring disturbances in the microflora (for example, disturbances due to antibiotic use).
MATERIALS AND METHODS
Experimental setup and sampling.
Eight healthy subjects were included in this study, and they were divided into two groups. Four of the subjects ingested a probiotic yogurt (Arla Foods, Stockholm, Sweden) (250 ml) twice daily for 14 days; this probiotic contained Lactobacillus acidophilus NCFB 1748, Lactobacillus paracasei F19, and Bifidobacterium lactis Bb12 (108 CFU of each strain per ml). The four subjects in the placebo group received standard unflavored yoghurt (Arla Foods) that did not contain any of the probiotic strains added to the active yoghurt, as confirmed by plate counting. All subjects received 150-mg clindamycin capsules (Dalacin; Pharmacia, Stockholm, Sweden) perorally four times a day for 7 days. Administration of clindamycin and administration of yoghurt were initiated on the same day. Stool samples were taken before administration (day 0), on the last day of clindamycin administration (day 7), and after administration on day 21. The samples were stored at −70°C until analysis. None of the subjects had taken any antibiotics 3 months prior to the start of the study. The study was approved by the Local Ethics Committee at Karolinska University Hospital Huddinge, Karolinska Institute, Stockholm, Sweden.
Cultivation.
Fecal specimens were diluted, and all the samples were inoculated onto nonselective and selective agar media as previously described (3). The plates were incubated aerobically for 18 h or anaerobically for 48 h at 37°C, and the culturable microflora was analyzed qualitatively and quantitatively (16). Anaerobic microorganisms were further identified to the genus level by gas-liquid chromatography of metabolites from glucose (16). The lower limit of detection for plate counting was 102 CFU per g of feces.
DNA isolation and PCR conditions.
DNA was extracted from 150 mg of feces by using a MoBio soil DNA isolation kit (MoBio, Solana Beach, Calif.) according to the manufacturer's instructions. The bead-beating step was performed with a flat-bed vortex mixer for 10 min. The 16S rRNA genes in the extracted DNA were amplified by using the general eubacterial primers fD1-FAM (AGAGTTTGATCCTGGCTCAG) (31) labeled at the 5′ end with 6-carboxyfluorescein and 926r (CCGTCAATTCCTTTRAGTTT) (17). For amplification of Lactobacillus spp. and related lactic acid bacteria, the reverse primer Lab-0677r (CACCGCTACACATGGAG) (6) was used together with fD1-FAM. Each PCR was performed with a 50-μl reaction mixture containing 2.5 U of Taq polymerase (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), 1× PCR buffer, 10 nmol of each deoxynucleoside triphosphate (Amersham Pharmacia Biotech), 2.5 μl of dimethyl sulfoxide, 1 μl of template DNA, and 35 pmol of each primer. The cycling program was performed by using a Perkin-Elmer GeneAmp PCR system 2400 thermocycler with the following program: an initial hot start at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 40 s, annealing at 55°C for 40 s, and elongation at 72°C for 1 min. The last cycle ended with an elongation step at 72°C for 7 min. Three replicate PCRs were performed for each sample with the eubacterial primer set. For amplifications in which the Lactobacillus-specific reverse primer was used, one PCR was performed per sample. When bacterial isolates were used as templates for PCRs, 1 μl of a suspension of one colony resuspended in sterile distilled H2O was used, and the reaction conditions were the same as those described above. Extracted DNA and PCR products were analyzed on 1% agarose gels stained with ethidium bromide.
T-RFLP.
For each sample, aliquots (10 μl) of the PCR products were digested with HhaI, HaeIII, or MspI (Promega, Madison, Wis.) separately for 3 h at 37°C in 50-μl (total volume) mixtures. The restricted PCR product was precipitated, and the dried pellet was suspended in 1.25 μl of formamide-dextran-EDTA and 0.25 μl of TAMRA 500 XL size standard (Applied Biosystems Instruments [ABI]). Portions (approximately 0.3 μg) of the DNA fragments were separated according to size by electrophoresis by using an ABI PRISM 377 DNA sequencer (ABI), and the resulting gel image was analyzed by using GeneScan software (ABI). Peaks on the resulting electropherogram represented terminal restriction fragments (TRFs).
Analysis of T-RFLP profiles.
The relative peak area of each TRF (referred to as relative abundance) was calculated by dividing the area of the specific peak by the total area of all the peaks on the electropherogram. Relative abundance values were calculated for all TRFs that were between 50 and 500 bp long and had peak heights of more than 50 fluorescence units. Three replicate PCRs were analyzed for each sample by using all three restriction enzymes mentioned above, which resulted in nine TRF relative abundance profiles for each stool sample. The mean relative peak abundance of each TRF was calculated for subsequent statistical analyses.
The data sets consisted of the mean relative peak abundance values obtained with all three restriction enzymes. The values were transformed by arcsin √p before analysis. The transformed data were analyzed by principal component analysis (PCA) with the multivariate analysis software ADE-4 (29; http://pbil.univ-lyon1.fr/ADE-4). To create dendrograms, Euclidean pairwise distances between day 0, day 7, and day 21 values for each subject were first calculated. The unweighted pair group method with arithmetic averages (UPGMA) was used to calculate the distances between the values for the time points. These calculations were done by using the PHYLIP package (4; http://evolution.genetics.washington.edu/phylip.html). Putative species identification for specific TRFs was performed using the T-RFLP analysis program TAP in the Ribosomal Database Project (RDP) database (11; http://www.cme.msu.edu/RDP).
RESULTS
Results of cultivation.
Marked ecological alterations due to clindamycin administration were found in all subjects. In particular, the numbers of dominant anaerobic microorganisms, such as Bifidobacterium, Eubacterium, Clostridium, and Bacteroides spp., were decreased at day 7, the last day of antibiotic treatment (Table 1). All four subjects in the placebo group were newly colonized by intrinsically clindamycin-resistant non-Escherichia coli Enterobacteriaceae on day 21 (2 weeks after antibiotic administration); two of these subjects were also colonized at day 7. In the group that received the probiotic yoghurt, similar trends were also observed at day 7 for one subject and at day 21 for the remaining three subjects. None of the subjects in either group developed symptoms of diarrhea, although one subject in each group was colonized with low numbers of Clostridium difficile at day 21. On the last sampling day (day 21), the fecal flora of the subjects in the placebo group was still suppressed, while the total viable counts in subjects who received probiotics were mainly restored to pretreatment levels (Table 1).
TABLE 1.
Viable counts of intestinal microorganisms isolated from fecal samples from healthy volunteers collected before, during, and after administration of clindamycin plus the active probiotic (n = 4) or clindamycin plus the placebo (n = 4)
| Microorganism | Median (range) viable count (CFU/g)
|
Median (range) viable count (CFU/g)
|
||||
|---|---|---|---|---|---|---|
| Clindamycin plus probiotic
|
Clindamycin plus placebo
|
|||||
| Day 0 | Day 7 | Day 21 | Day 0 | Day 7 | Day 21 | |
| Enterococcus spp. | 3.2 × 103 (3.0 × 102-5.2 × 105) | 1.5 × 102 (1.0 × 102-5.0 × 106) | 1.2 × 106 (3.3 × 105-2.7 × 107) | 1.2 × 104 (8.0 × 102-6.0 × 105) | <102 (<102-3.0 × 102) | 6.0 × 106 (8.0 × 105-7.0 × 107) |
| E. coli | 2.5 × 105 (<102-1.9 × 107) | 3.0 × 106 (<102-3.0 × 106) | 1.2 × 104 (3.0 × 103-9.1 × 106) | 8.1 × 105 (<102-4.0 × 107) | 2.6 × 107 (<102-1.2 × 108) | 8.5 × 105 (7.0 × 104-3.0 × 107) |
| Klebsiella spp., Citrobacter spp., and Enterobacter spp. | <102 (<102-7.0 × 102) | <102 (<102-3.0 × 106) | 1.2 × 104 (<102-1.2 × 106) | <102 (<102-1.2 × 105) | 1.0 × 106 (<102-6.0 × 107) | 1.1 × 105 (2.0 × 104-6.0 × 107) |
| Lactobacillus spp. | 5.5 × 104 (1.2 × 104-2.7 × 106) | 7.0 × 105 (6.0 × 104-6.0 × 106) | 7.0 × 105 (9.0 × 104-1.4 × 106) | 3.0 × 103 (1.0 × 102-5.0 × 104) | 6.0 × 102 (<102-1.6 × 104) | 5.2 × 103 (4.0 × 102-1.6 × 105) |
| Bifidobacterium spp. | 8.0 × 107 (5.4 × 105-2.4 × 108) | <102 (<102) | 1.5 × 108 (<102-2.6 × 108) | 5.5 × 105 (1.0 × 105-1.2 × 106) | <102 (<102-2.2 × 108) | <102 (<102-3.0 × 105) |
| Clostridium spp. | 1.5 × 105 (<102-3.5 × 105) | <102 (<102-1.3 × 105) | 5.0 × 105 (4.0 × 102-1.5 × 106) | 3.5 × 104 (2.0 × 103-3.0 × 105) | 2.5 × 103 (<102-3.0 × 105) | 2.1 × 105 (1.0 × 102-2.0 × 106) |
| Eubacterium spp. | 1.0 × 104 (<102-1.2 × 109) | <102 (<102) | ≤102 (<102-1.4 × 108) | <102 (<102-4.0 × 107) | <102 (<102) | <102 (<102) |
| Bacteroides spp. | 1.7 × 108 (3.7 × 106-5.3 × 108) | 5.1 × 106 (<102-1.6 × 108) | 7.0 × 108 (<102-1.5 × 109) | 1.9 × 108 (6.0 × 106-6.0 × 108) | 5.0 × 104 (<102-6.0 × 108) | 2.1 × 107 (<102-2.6 × 108) |
| Candida spp. | 4.0 × 102 (<102-2.5 × 103) | 2.0 × 102 (<102-9.8 × 103) | 1.2 × 102 (<102-2.8 × 103) | 1.0 × 102 (<102-3.0 × 102) | 1.1 × 103 (<102-2.3 × 103) | 2.0 × 102 (<102-3.0 × 105) |
Statistical analyses of T-RFLP community profiles.
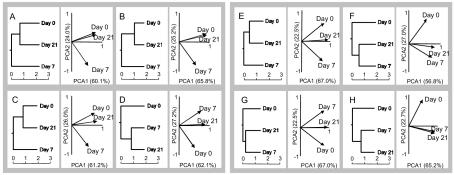
The fecal microflora of each subject was individually characterized and statistically analyzed due to the unique microbial community fingerprints for each individual. Nevertheless, some trends according to treatment and sampling date were observed. The patterns of similarity between days, showing the way that the three sampling periods were related to each other, were the same whether the analysis was done by PCA or UPGMA (Fig. 1). The two statistical descriptive approaches indicated that three of the four subjects in the group that ingested the probiotic yoghurt (subjects A, B, and C) had microbial communities that were more similar on days 0 and 21 than on day 7 (Fig. 1). In particular, this trend was apparent for subjects A and B; for these subjects days 0 and 21 were very near each other on the PCA plots. However, for subject D the original composition of the microbial community had not been reestablished by day 21. By contrast, the intestinal microfloras of the subjects in the placebo group did not return to their original compositions by day 21, as shown by comparisons of days 0 and 21 in the PCA plots for subjects E, F, G, and H (Fig. 1). For three of these four subjects (subjects F, G, and H) the intestinal microfloras on days 7 and 21 showed a higher degree of similarity than the microfloras on day 0 (Fig. 1). This trend was particularly obvious for subject H, in which no tendency for the microflora to return to its original composition by the end of the sampling period was observed.
FIG. 1.
UPGMA dendrograms constructed from Euclidean pairwise distances between time points and corresponding PCA analyses of T-RFLP data from days 0, 7, and 21 for three combined restriction fragment profiles. Subjects A, B, C, and D received a probiotic during antibiotic administration, and subjects E, F, G, and H received ordinary yogurt during antibiotic administration. PCA1, principal component 1; PCA2, principal component 2.
Negative impact of clindamycin on the relative abundance of specific TRFs.
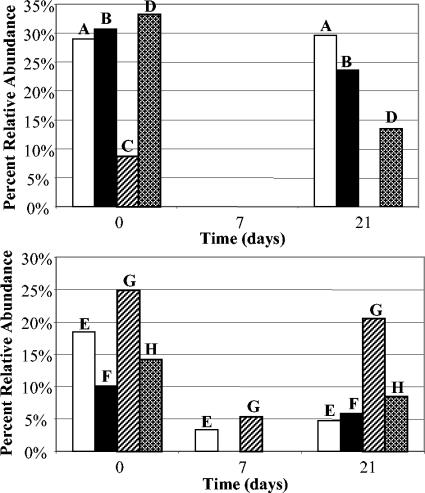
In all eight subjects, specific groups of TRFs (as seen with all three restriction enzymes in separate T-RFLP analyses) were highly negatively affected by clindamycin treatment. A representative example is shown in Fig. 2, in which the results for pooled relative abundance values for TRFs ranging from 222 to 227 bp in the MspI restriction digest profiles for all eight subjects are shown. The results of the two additional restriction fragment profiles showed the same pattern (data not shown). A comparison to entries in the RDP database suggested that the affected TRFs most likely belonged to members of the Clostridium coccoides subgroup (Table 2). In this subcluster the most common genus suggested was Eubacterium. The TRFs assigned to the genus Eubacterium were very dominant in all the community profiles and represented up to 77% of the microbial community of a specific subject at day 0. However, by the last day of clindamycin treatment either most of the putative Eubacterium had disappeared completely or Eubacterium was found only at a low relative abundance. By day 21, in both the placebo group and the active probiotic group, the putative Eubacterium had become reestablished at the original levels or showed a strong tendency toward normalization (Fig. 2).
FIG. 2.
TRFs from the MspI restriction digest representing members of the C. coccoides subgroup that were negatively affected by clindamycin. Each bar represents pooled relative abundance values for a group of TRFs whose sizes range from 222 to 227 bp (typical for this subgroup). Subjects A, B, C, and D (upper panel) received the probiotic, and subjects E, F, G, and H (lower panel) received ordinary yogurt.
TABLE 2.
Highly negatively or positively affected TRF peaks observed and corresponding TRF lengths ascribed to organisms by the RDP database
| Observed TRFs and matching organism | Length of TRF (bp)
|
||
|---|---|---|---|
| HaeIII | HhaI | MspI | |
| Observed TRFs | 238-239 | 188-193 | 222-227 |
| Eubacterium hallii ATCC 27751 | 237 | 188 | 220 |
| Eubacterium cellulosolvens ATCC 43171 | 239 | 190 | 222 |
| Observed TRFs | 272-276 | 188-193 | 222-227 |
| Clostridium clostridiiforme ATCC 25537 | 273 | 190 | 222 |
| Eubacterium formicigenerans ATCC 27755 | 273 | 1,090 | 222 |
| Eubacterium ramulus ATCC 29099 | 275 | 192 | 224 |
| Eubacterium rectale ATCC 33656 | 275 | 192 | 224 |
| Eubacterium ventriosum ATCC 27560 | 275 | 192 | 224 |
| Roseburia cecicola ATCC 33874 | 275 | 192 | 224 |
| Observed TRFs | 317-319 | 188-193 | 222-227 |
| Clostridium symbiosum ATCC 14940 | 317 | 190 | 222 |
| Eubacterium contortum ATCC 25540319 | 319 | 1,092 | 224 |
| Eubacterium eligens ATCC 27750 | 321 | 194 | 226 |
| Observed TRFs | 262-267 | 94-100 | |
| Bacteroides distasonis ATCC 8503 | 263 | 68 | 95 |
| Bacteroides ovatus NCTC 11153 | 265 | 104 | 99 |
| Bacteroides thetaiotaomicron ATCC 29148 | 383 | 104 | 99 |
| Bacteroides uniformis ATCC 8492 | 263 | 102 | 97 |
| Bacteroides vulgatus ATCC 8482 | 263 | 101 | 96 |
| Prevotella melaninogenica ATCC 25845 | 266 | 104 | 99 |
| Observed TRF | 374 | 497 | |
| Escherichia coli K-12 | 39 | 373 | 496 |
| Citrobacter freundii CDC 621-64 | 39 | 373 | 496 |
| Klebsiella sp. strain zlmy | 39 | 371 | 494 |
Positive impact of clindamycin on the relative abundance of specific TRFs.
Several members of the intestinal microflora were positively affected by clindamycin treatment, as shown by increases in the relative abundance values for their TRFs on day 7. These increases in relative abundance could have been due to increases in the sizes of particular populations or more likely were due to decreases in the sizes of other populations that were negatively affected by antibiotic treatment. Some populations present at day 0 showed large increases in relative abundance on day 7 and then decreased again on day 21. For example, in four subjects, irrespective of group, specific TRF clusters dominated the community at day 7. By comparing different isolates obtained from the subjects, we found that the TRFs in these specific TRF clusters were the same length as those typically found in Bacteroides spp. when they are digested with MspI and HaeIII (Table 2). This was also in agreement with an RDP database search that suggested that these TRFs belonged to the genera Bacteroides and Prevotella. Furthermore, in five of the subjects, irrespective of group, a specific TRF (497 bp with MspI and 374 bp with HhaI) (Table 2) appeared only at day 7, and in the profiles the TRF was either the most dominant TRF or one of the dominant TRFs. According to the RDP database, these TRFs corresponded to species belonging to the group containing enterobacters and their relatives in the gamma subdivision of the Proteobacteria. The lengths of these TRFs also matched those of TRFs from E. coli, Klebsiella, and Citrobacter isolates obtained from the subjects.
Tracking probiotic strains.
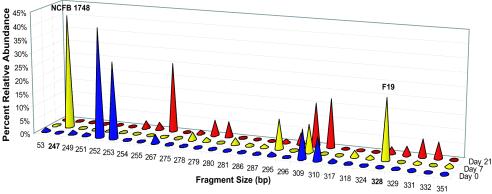
The probiotic strains of lactobacilli used in the active treatment (strains F19 and NCFB 1748) were analyzed in fecal samples by T-RFLP by using the restriction enzyme HaeIII. This enzyme was chosen since both strains could be tracked in the same T-RFLP profiles. The TRF lengths were determined from T-RFLP analyses of pure isolates of the probiotic strains (247 bp for NCFB 1748 and 328 bp for F19) in order to verify the identities in the community patterns of the fecal samples. By using a Lactobacillus-specific reverse primer, the two probiotic strains could easily be detected and distinguished by T-RFLP (Fig. 3). In fact, the two probiotic strains dominated this more defined community in the subjects who received the probiotic; on day 7 NCFB 1748 accounted for 42 to 54% of the community and F19 accounted for 22 to 30% of the community. A representative example is shown in Fig. 3, which shows that NCFB 1748 and F19 accounted for 42 and 22% of the community, respectively, on day 7. By day 21, the strains either were not detected or were present only at very low relative abundance values. Two of the subjects in the placebo group had a 247-bp TRF, which was the same size as the NCFB 1748 TRF; the relative abundance values for these TRFs were low (2.5 and 5%).
FIG. 3.
Relative abundance values for TRFs obtained by using a Lactobacillus-specific reverse primer and HaeIII restriction digestion. Blue cones, day 0; yellow cones, day 7; red cones, day 21. The 247-bp TRF represents L. acidophilus NCFB 1748, and the 328-bp TRF represents L. paracasei F19. The data are for one representative subject who ingested the probiotic (subject A).
DISCUSSION
The results of the present study demonstrate that T-RFLP is a useful tool for investigating the human intestinal microflora. T-RFLP fingerprints of the intestinal microfloras of eight healthy clindamycin-exposed subjects that were treated concomitantly with either a probiotic or a placebo were obtained. Different descriptive statistical approaches, PCA and UPGMA analysis based on the Euclidean pairwise distance, revealed the same patterns for all the subjects. The disturbance of the microfloras seemed to be more prolonged in the placebo group than in the group that received the probiotic. However, due to the unique microbial community fingerprint for each subject and due to the small number of subjects used, it was not possible to use statistics to calculate significant differences between the study groups. Nevertheless, the results demonstrate that T-RFLP is very useful for monitoring changes in the microbial floras of individuals, such as those observed during antibiotic treatment and probiotic ingestion.
When the T-RFLP profiles of different subjects were compared, some TRFs were found in all subjects (e.g., Eubacterium spp.), while other TRFs were unique to individual subjects. This is in agreement with previous studies of DGGE and temperature gradient gel electrophoresis (TGGE) profiles of the human fecal microflora (2, 28, 33). Zoetendal et al. (33) showed by using TGGE that the banding patterns of the fecal microfloras of healthy individuals were constant over time, but they also showed that the banding patterns for individuals were very different. However, all individuals had some bands in common, and the bands corresponded to Eubacterium hallii, among other organisms. The genus Eubacterium is one of the most abundant genera in the gastrointestinal tract, but it is far from the most studied. Cultivation of Eubacterium spp. is considered to be unpredictable, and these organisms are mainly distinguished from other genera based on their lack of easily identifiable metabolic characteristics (15). In the present study, TRFs that were highly negatively affected by clindamycin administration belonged to different species in the C. coccoides subgroup (predominantly Eubacterium spp.), as suggested by the RDP database. This group was easily monitored by T-RFLP but was not successfully recovered by cultivation, probably due to the fastidious nature of the organisms and overgrowth by other bacteria.
Previous studies have shown (10, 19, 24) that clindamycin has a great effect on the anaerobic community. In one of these studies (24), alterations of the fecal microflora in 24 healthy subjects who received clindamycin for 1 week, with or without a probiotic containing two Lactobacillus strains and one Bifidobacterium strain, were monitored by cultivation. The numbers of Lactobacillus spp. and Bacteroides spp. decreased significantly in the subjects who did not receive the probiotic treatment, while the numbers were not significantly affected in the group that also received the probiotic, suggesting that the probiotic had a stabilizing effect on the populations (24). Although relative increases in populations proposed to be Bacteroides and Prevotella spp. were detected by T-RFLP in four subjects in the present study, no increases in the numbers of Bacteroides spp. were observed in the subjects as determined by cultivation. These findings illustrate the fact that different aspects are measured by the two methods. Cultivation-based results, although probably representing only a minor fraction of the total bacterial population, are absolute numbers, while relative abundance values are obtained by T-RFLP.
The counts for members of the Enterobacteriaceae group increased during the antibiotic treatment for almost all of the subjects according to the viable count data. In five of the subjects, this was also apparent in the T-RFLP analysis. However, it was not possible to use T-RFLP to separate species within this group by using the three restriction enzymes. Nevertheless, the results imply that clindamycin positively affects the relative abundance of enterobacterial populations, which is further supported by the fact that enterobacteria are resistant to clindamycin (25).
While ecological disturbances in the intestinal microflora caused by antibiotics often are profound, the quantitative effect of probiotics is minor for the most part (28). Kaplan et al. (8) used T-RFLP to study the intestinal microbial community in rats fed L. acidophilus and showed by PCA that although the most important factor (principal component 1) distinguishing the communities was age, the samples could be separated based on the amount of Lactobacillus administered (principal component 2) (8). In the present investigation, lactobacilli and bifidobacteria were generally isolated in higher numbers on days 7 and 21 for subjects who received the probiotic compound. However, specific strains could not be easily identified by using the cultivation method. When a specific reverse primer was used, the two probiotic Lactobacillus strains could be easily detected in the T-RFLP profiles of the active group at day 7, and both strains were dominant in the Lactobacillus population (Fig. 3). In three of four subjects, these specific strains could no longer be detected by T-RFLP at day 21. This is consistent with a previous report which showed that probiotic bacteria only transiently colonize the gastrointestinal tract and often cannot be detected a few days after the last day of administration (26).
In conclusion, the present results support use of T-RFLP as a tool for obtaining fingerprints of individual human fecal microfloras and for monitoring changes in the fingerprints according to time or treatment. Alterations in the communities in different subjects that were not revealed by the cultivation data could be identified when the fecal samples were analyzed by T-RFLP. Furthermore, when data from three different restriction fragment profiles were used, plausible identities of specific populations in the RDP database could be determined, and the findings were further supported by fingerprinting analyses of specific isolates. In the future, T-RFLP might be useful as a routine method for monitoring disturbances in the human intestinal microflora due to, e.g., changes in diet, illness, or treatment strategies.
Acknowledgments
We acknowledge financial support for this work from Södertörn University College and the Swedish Foundation for Strategic Research Microbes and Man Program (ESPAR project).
REFERENCES
- 1.Chin, K., T. Lukow, S. Stubner, and R. Conrad. 1999. Structure and function of the methanogenic archaeal community in stable cellulose-degrading enrichment cultures at two different temperatures (15 and 30 degrees C). FEMS Microbiol. Ecol. 30:313-326. [DOI] [PubMed] [Google Scholar]
- 2.Donskey, C. J., A. M. Hujer, S. M. Das, N. J. Pultz, R. A. Bonomo, and L. B. Rice. 2003. Use of denaturing gradient gel electrophoresis for analysis of the stool microbiota of hospitalized patients. J. Microbiol. Methods 54:249-256. [DOI] [PubMed] [Google Scholar]
- 3.Edlund, C., G. Beyer, M. Hiemer-Bau, S. Ziege, H. Lode, and C. E. Nord. 2000. Comparative effects of moxifloxacin and clarithromycin on the normal intestinal microflora. Scand. J. Infect. Dis. 32:81-85. [DOI] [PubMed] [Google Scholar]
- 4.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package), 3.5c ed. University of Washington, Seattle.
- 5.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol. Immunol. 46:819-831. [DOI] [PubMed] [Google Scholar]
- 6.Heilig, H. G., E. G. Zoetendal, E. E. Vaughan, P. Marteau, A. D. Akkermans, and W. M. de Vos. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 68:114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jernberg, C., and J. K. Jansson. 2002. Impact of 4-chlorophenol contamination and/or inoculation with the 4-chlorophenol-degrading strain, Arthrobacter chlorophenolicus A6L, on soil bacterial community structure. FEMS Microbiol. Ecol. 42:387-397. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan, C. W., J. C. Astaire, M. E. Sanders, B. S. Reddy, and C. L. Kitts. 2001. 16S ribosomal DNA terminal restriction fragment pattern analysis of bacterial communities in feces of rats fed Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 67:1935-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lidbeck, A., C. Edlund, J. A. Gustafsson, L. Kager, and C. E. Nord. 1988. Impact of Lactobacillus acidophilus on the normal intestinal microflora after administration of two antimicrobial agents. Infection 16:329-336. [DOI] [PubMed] [Google Scholar]
- 11.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercenier, A., S. Pavan, and B. Pot. 2002. Probiotics as biotherapeutic agents: present knowledge and future prospectives. Curr. Pharm. Des. 8:99-110. [DOI] [PubMed] [Google Scholar]
- 13.Mills, D. K., K. Fitzgerald, C. D. Litchfield, and P. M. Gillevet. 2003. A comparison of DNA profiling techniques for monitoring nutrient impact on microbial community composition during bioremediation of petroleum-contaminated soils. J. Microbiol. Methods 54:57-74. [DOI] [PubMed] [Google Scholar]
- 14.Moeseneder, M. M., J. M. Arrieta, G. Muyzer, C. Winter, and G. J. Herndl. 1999. Optimization of terminal-restriction fragment length polymorphism analysis for complex marine bacterioplankton communities and comparison with denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 65:3518-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, W. E. C., and L. V. Holdeman Moore. 1986. Genus Eubacterium Prévot 1938, p. 1353-1373. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 16.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 17.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolcheva, L. G., A. M. Cockshutt, and F. Barlocher. 2003. Determining diversity of freshwater fungi on decaying leaves: comparison of traditional and molecular approaches. Appl. Environ. Microbiol. 69:2548-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orrhage, K., B. Brismar, and C. E. Nord. 1994. Effect of supplements with Bifidobacterium longum and Lactobacillus acidophilus on intestinal microbiota during administration of clindamycin. Microb. Ecol. Health Dis. 7:17-25. [Google Scholar]
- 20.Rolfe, R. D. 2000. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 130:396S-402S. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto, M., H. Hayashi, and Y. Benno. 2003. Terminal restriction fragment length polymorphism analysis for human fecal microbiota and its application for analysis of complex bifidobacterial communities. Microbiol. Immunol. 47:133-142. [DOI] [PubMed] [Google Scholar]
- 22.Scala, D. J., and L. J. Kerkhof. 2000. Horizontal heterogeneity of denitrifying bacterial communities in marine sediments by terminal restriction fragment length polymorphism analysis. Appl. Environ. Microbiol. 66:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan, A., L. Barkholt, and C. E. Nord. 2003. Lactobacillus acidophilus, Bifidobacterium lactis and Lactobacillus F19 prevent antibiotic-associated ecological disturbances of Bacteroides fragilis in the intestine. J. Antimicrob. Chemother. 52:308-311. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 26.Tannock, G. W. 1999. Analysis of the intestinal microflora: a renaissance. Antonie Leeuwenhoek 76:265-278. [PubMed] [Google Scholar]
- 27.Tannock, G. W., K. Munro, R. Bibiloni, M. A. Simon, P. Hargreaves, P. Gopal, H. Harmsen, and G. Welling. 2004. Impact of consumption of oligosaccharide-containing biscuits on the fecal microbiota of humans. Appl. Environ. Microbiol. 70:2129-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannock, G. W., K. Munro, H. J. Harmsen, G. W. Welling, J. Smart, and P. K. Gopal. 2000. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl. Environ. Microbiol. 66:2578-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thioulouse, J., D. Chessel, S. Dolédec, and J.-M. Olivier. 1997. ADE-4: a multivariate analysis and graphical display software. Statistics Comput. 7:75-83. [Google Scholar]
- 30.Tom-Petersen, A., T. D. Leser, T. L. Marsh, and O. Nybroe. 2003. Effects of copper amendment on the bacterial community in agricultural soil analyzed by the T-RFLP. FEMS Microbiol. Ecol. 46:53-62. [DOI] [PubMed] [Google Scholar]
- 31.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welling, G. W., A. C. M. Wildeboer-Veloo, N. W. M. Lemmers, R. Tian, Q. Gao, G. C. Raangs, R. H. J. Tonk, G. J. Jansen, J. E. Degener, and J. M. Harmsen. 2002. Fluorescent in situ hybridization (FISH) as a tool in intestinal bacteriology. Biosci. Microflora 20:115-120. [Google Scholar]
- 33.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]