Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by dysfunctions in social interactions resulting from a complex interplay between immunogenetic and environmental risk factors. Autoimmunity has been proposed as a major etiological component of ASD. Whether specific autoantibodies directed against brain targets are involved in ASD remains an open question. Here, we identified within a cohort an ASD patient with multiple circulating autoantibodies, including the well-characterized one against glutamate NMDA receptor (NMDAR-Ab). The patient exhibited alexithymia and previously suffered from two major depressive episodes without psychotic symptoms. Using a single molecule-based imaging approach, we demonstrate that neither NMDAR-Ab type G immunoglobulin purified from the ASD patient serum, nor that from a seropositive healthy subject, disorganize membrane NMDAR complexes at synapses. These findings suggest that the autistic patient NMDAR-Abs do not play a direct role in the etiology of ASD and that other autoantibodies directed against neuronal targets should be investigated.
Keywords: autism spectrum disorder, autoimmunity, infection, glutamate, single-molecule tracking
Abstract
El trastorno del espectro autista (TEA) es un trastorno del neurodesarrollo caracterizado por dísfunciones en las interacciones sociales que se traducen en un complejo interjuego entre factores de riesgo ambientales e inmunogenéticos. Se ha propuesto a la autoinmunidad como un componente etiológico importante en el TEA. Sigue pendiente saber si hay autoanticuerpos específicos dirigidos contra blancos cerebrales involucrados en el TEA. En este artículo se identificó, dentro de una cohorte, un paciente con TEA con múltiples autoanticuerpos circulantes, incluyendo uno bien caracterizado contra el receptor de glutamato NMDA (NMDAR-Ab). El paciente presentaba alexitimia y previamente había tenido dos episodios depresivos mayores sin síntomas psicóticos. Mediante técnica de imágenes de molécula única se demostró que ni la γ inmunoglobulina purificada del NMDAR-Ab del suero del paciente con TEA, ni la de un sujeto sano seropositivo desorganizaban los complejos de membrana del NMDAR en las sinapsis. Estos hallazgos sugieren que los auto-anticuerpos del NMDAR de pacientes autistas no juegan un papel directo en la etiología del TEA y que se deben investigar otros autoanticuerpos dirigidos contra blancos neuronales.
Abstract
Les troubles du spectre autistique (TSA) sont des maladies neurodéveloppementales caracterisées par des dysfonctions des interactions sociales provoquées par un jeu complexe entre des facteurs de risque immunogénétiques et environnementaux. L'auto-immunité a été proposée comme composant étiologique majeur des TSA. Il reste à savoir si des auto-anticorps spécifiques dirigés contre des cibles cérébrales sont impliqués dans les TSA. Dans cet article, nous identifions au sein d'une cohorte, un patient TSA ayant de nombreux auto-anticorps circulants dont celui très connu contre le récepteur NMDA du glutamate (NMDAR-Ab). Ce patient présente une alexithymie et a eu antérieurement deux épisodes dépressifs caractérisés sans symptômes psychotiques. Grâce à l'utilisation d'une technique d'imagerie de molécule unique, nous démontrons que ni l'immunoglobuline γ purifiée NMDAR-Ab sérique du patient TSA ni celle d'un patient sain ayant le même anticorps ne désorganisent les complexes membranaires NMDAR au niveau synaptique. Ces résultats semblent indiquer que les auto-anticorps NMDAR-Ab de patients autistes ne jouent pas de rôle direct dans I'étiologie des TSA et que d'autres autoanticorps dirigés contre des cibles neuronales devraient faire l'objet de recherches.
Introduction
Autism spectrum disorder (ASD) is characterized by impairments in social interactions and verbal and nonverbal communication, along with repetitive patterns of behaviors and interests. ASD prevalence has continued to increase over the last decades, affecting approximately 1% of the population worldwide.1 Although its etiology is still unknown, it involves genetic, immunological, and environmental risk factors,2,3 leading to severe alterations in neuronal network formation early in life. Multiple epidemiological studies provide strong evidence for a connection between maternal infection during the first two trimesters of pregnancy and the development of ASD in offspring.4-6 For instance, inflammatory cytokines could reach the fetal brain and alter key physiological and neurodevelopmental processes.7 In addition, mothers with autoimmune disorders show an increased risk of giving birth to children with ASD,8 supporting the etiological role of an autoimmune dysfunction in patients with ASD. Consistently, autoantibodies against fetal brain targets have been identified in the sera of ASD patients' mothers.9 These autoantibodies, for which targets are yet undefined, produce long-term behavioral consequences in newborn rodents if injected into the dams.10
Autoantibodies against neuronal receptors have been increasingly identified in neuropsychiatric disorders and today constitute one of the hottest topics in psychiatry.11 This has generated great hope for a better understanding of the molecular dysfunctions underlying these disorders and identification of patients who could benefit from immunotherapy. The best-characterized disorder in which autoantibodies directed against brain targets directly contribute to psychiatric symptoms is anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis. The NMDAR autoantibodies (NMDAR-Ab) target an extracellular epitope of the GluN1-NMDAR subunit and profoundly alter receptor trafficking and signaling.12,13 NMDAR-Abs were also detected in two adults with ASD who consequently developed anti-NMDA encephalitis.14 Intriguingly, it was reported that a 9-year-old boy initially suffering from an anti-NMDAR encephalitis subsequently exhibited ASD-like symptoms,15 suggesting a potential link between the NMDAR-Ab and the emergence of ASD. Here, we investigated the presence and putative molecular pathogenecity of NMDAR-Ab in a cohort of ASD patients, using the single-particle tracking method in live dissociated hippocampal neurons.
Materials and methods
Patient cohort
ASD patients and controls (healthy) were recruited via the InFoR-Autism program (I'lnserm, la Fondation FondaMental et Roche - Autism), which is a 2-year, multicenter, longitudinal, non-drug study. Children, adolescents, and adult patients presenting with ASD, and age- and gender-matched healthy subjects were recruited in three French specialist centers for ASD under the auspices of the FondaMental Foundation (Créteil, Paris and Bordeaux). Subjects were evaluated with a battery of diagnostic tools, including a French version of the ADI-R (Autism Diagnostic Interview - Revised), and the ADOS (Autism Diagnostic Observation Schedule). All adult subjects had several blood samplings planned over the course of the study for genetic, biochemical, and immunological studies.
Detection of NMDA-receptor autoantibodies (NMDAR-Ab) in sera
We tested serum samples of a subpopulation of the InFor-Autism cohort (24 ASD patients and 18 healthy controls) for the presence of NMDAR-Ab using a cell-based assay previously described in the literature.12 Briefly, human embryonic kidney cells (HEK293) were transfected with the GluN1-NMDAR subunit fused to green fluorescent protein (GFP), along with the GluN2B-NMDAR subunit. After a 48-hour expression period, live HEK cells were incubated 3 hours with the subjects' sera (1/20 in saturation buffer). Then, fixed HEK cells were incubated with anti-human type G immunoglobulin (IgG) coupled to Alexa 555. Viewed by fluorescence microscopy, an overlap of both green and red staining indicated a subject's seropositivity for NMDAR-Ab. Samples were considered seropositive for NMDAR-Ab when a clear overlap was confirmed by three different readers in three independent assays.
Primary cell culture and single quantum dot (QD) tracking
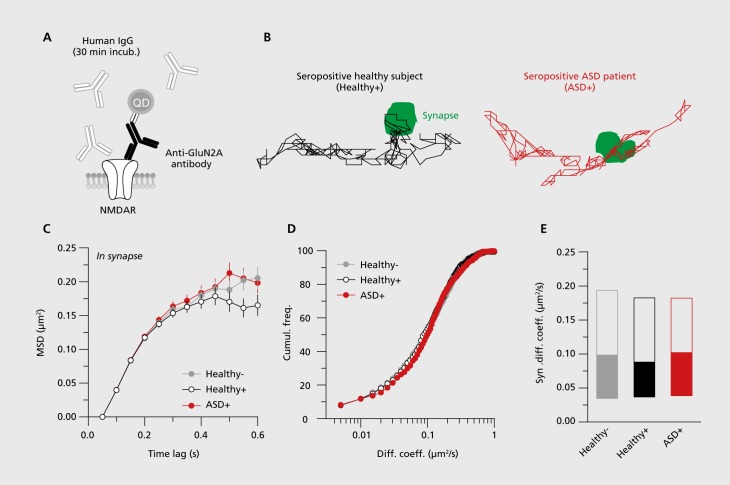
As previously described in the literature,12 dissociated hippocampal neurons were prepared from E18 Sprague-Dawley rats. Quantum dot (QD) tracking of the endogenous GluN2A-NMDAR subunit was performed on live hippocampal neurons at day 13 to day 15 in vitro. Neurons were first incubated (30 min) with purified IgG (5μg/mL) from either the seropositive ASD patient (ASD+), the seropositive healthy subject (healthy+), or seronegative healthy subjects (healthy-) (Figure 1). QD labeling and microscopy were performed as previously described.12 Briefly, hippocampal neurons were incubated (10 min) with anti-GluN2A-NMDAR subunit antibodies (Agro-Bio, 1/200). Neurons were then washed and incubated (10 min) with QDs coupled to an anti-rabbit F(ab) fragment (Life Technologies, 1/20 000). Mitotracker Green (Life Technologies, 1/20000) was used as an endogenous synaptic marker. Images were obtained with an acquisition time of 50 milliseconds with up to 500 consecutive frames. The instantaneous diffusion coefficient, D, was calculated for each trajectory, from linear fits of the first four points of the mean square displacement (MSD) versus time function using MSD(t)= <r2>(t)= 4Dt.
Figure 1. NMDAR autoantibodies from the seropositive healthy subject (Healthy+) and the seropositive ASD patient (ASD+) do not differ in their capacity to alter synaptic NMDAR dynamics. (A) Schematic representation of the experimental design. Before tracking GluN2A-NMDAR-QD complexes, hippocampal cultures (13 to 15 days in vitro) were incubated for 30 minutes with different purified type G immunoglobulin (IgG) samples from a seropositive healthy subject from the cohort (Healthy+), a seropositive ASD patient from the cohort (ASD+), or three pooled healthy seronegative subjects (Healthy-). (B) Representative trajectories of GluN2A-NMDAR-QD complexes (500 frames, 50 ms acquisition) at the plasma membrane in the presence of NMDAR-Ab from Healthy+ and ASD+. Synapses (in green) are identified with Mitotracker. Scale bar: 500 nm. (C) Synaptic mean square displacement (MSD) of GluN2A-NMDAR-QD complexes in the absence (Healthy-) or presence (Healthy+, ASD+) of NMDAR-Ab. (D) Cumulative frequency of the synaptic GluN2A-NMDAR-QD complexes diffusion coefficient in the absence (Healthy-) or presence (Healthy+, ASD+) of NMDAR-Ab. (E) Comparison of GluN2A-NMDAR diffusion coefficient in the absence (Healthy-) or presence (Healthy+, ASD+) of NMDAR-Ab at glutamate synapses (median diffusion coefficient ࢱ 25%-75% IQR, Healthy- = 0.107 μm2/s, IQR= 0.034-0.197 μm2/s, n= 492; Healthy+ = 0.089 μm2m/s, interquartile range (IQR) = 0.036-0.185 μm2/s, n= 490; ASD+ = 0.102 μm2/s, IQR= 0.039-0.186 μm2/s, n= 555; P= 0.81 Kruskal-Wallis test). Anti-GluN2A, anti-N-methyl-D-aspartate receptor 2A; ASD, autism spectrum disorder; ASD+, seropositive autism spectrum disorder patient; Cumui. freq., cumulative frequency; Diff. coeff., diffusion coefficient; Healthy+, seropositive healthy subject; Healthy-, seronegative healthy subject; IgG, type G immunoglobulin; QD, quantum dot; MSD, mean square displacement; NMDAR, N-methyl-D-aspartate receptor; NMDAR-Ab, N-methyl-D-aspartate receptor autoantibody.
Results
Using a classical cell-based assay, as described in the literature,12 we investigated the presence of circulating NMDAR-Ab in healthy subjects and patients with ASDs. Out of the 42 normal subjects and patients tested for NMDAR-Ab, only one healthy subject free of any neurological or psychiatric disorder and one ASD patient were seropositive for NMDAR-Ab. This ASD patient is a 53-year-old man, initially examined at the Expert Center for Autism (Centre Expert Asperger, Créteil, France). On initial evaluation, the patient was well oriented in time and space. However, his speech volume and production was low, with monotonous prosody. The affect was restricted in range, flat and relatively incoherent to his described internal state. Thought process and content were normal. There was no suicidal ideation, delusions, hallucinations, or memory trouble at the time of the interview. The patient presented with a specific phobia and a delayed sleep phase syndrome. A lack of nonverbal and verbal communication was noted (see ADOS score in Table I). The patient exhibited alexithymia, difficulties in apprehending intentions, and a lack of empathy, consistent with an altered theory of mind. Sensory peculiarities (hypersensitivity to noise and hyposensitivity to low temperatures) were also reported. These symptoms met criteria for ASD without intellectual deficiency (Table II). ASD symptoms emerged during childhood, as the patient was isolated, suffered from severe social anxiety, and acquired speech later than average. In adulthood, the patient had recurrent episodes of panic attacks, generalized anxiety, and suffered from two major depressive disorder episodes without psychotic symptoms (at age 37 and 43). The patient had no history of auditory or visual hallucination, no delusional thinking, no suicidal attempts, and the neurological examination was normal.
TABLE I. ADOS (Autism Diagnostic Observation Schedule) 1 module 4 algorithm scores from the autism spectrum disorder (ASD+) patient.
| Domains | Scores |
| Language and communication | |
| A-4 | 0 |
| A-8 | 1 |
| A-9 | 0 |
| A-10 | 1 |
| Total communication | 2 |
| Reciprocal social interaction | |
| B-1 | 0 |
| B-2 | 1 |
| B-6 | 2 |
| B-8 | 0 |
| B-9 | 0 |
| B-10 | 1 |
| B-11 | 1 |
| Total reciprocal social interaction | 5 |
| Total communication + social interaction | 7 |
| Imagination/creativity | |
| C-1 | 1 |
| Stereotyped hebavior and restricted interest | |
| D-1 | 0 |
| D-2 | 0 |
| D-4 | 0 |
| D-5 | 0 |
| Total | 0 |
| Other abnormal behaviors | |
| E-1 | 0 |
| E-2 | 0 |
| E-3 | 0 |
| Total | 0 |
TABLE II. Intellectual quotient (IQ) and Hamilton Anxiety and Depression Scales scores from the seropositive autism spectrum disorder (ASD+) patient. PRI, Perceptual Reasoning Index; PSI, Processing Speed Index; WAIS IV, Wechsler Adult Intelligence Scale IV; WMI, Working Memory Index; VCI, Verbal Comprehension Index .
| VCI=108 | ||
| PRI=122 | ||
| Cognitive level | WAIS IV | WMI=120 |
| PSI=137 | ||
| Hamilton anxiety score | 1 | |
| Hamilton depression score | 0 |
We then explored the possibility that the circulating NMDAR-Ab from this patient, and not from the healthy+ subject, altered NMDAR trafficking and signaling,12 possibly contributing to the symptomatology.15 For this, NMDAR-Ab IgGs were purified from the ASD patient and the seropositive healthy subject, and both IgGs were used to perform single-nanoparticle tracking on surface NMDAR in live hippocampal neurons (Figure 1A). Incubation for at least 30 minutes with purified IgGs from either the ASD patient or the seropositive healthy subject did not alter the surface trafficking of GluN2A-NMDAR when compared with seronegative healthy subjects (Figure 1B-E). This was assessed by measuring the MSD curves and instantaneous diffusion coefficients of surface GluN2A-NMDAR (Figure 1B-E). Together, these data indicate that the NMDAR-Ab purified from the ASD patient or from a seropositive healthy subject do not alter the surface trafficking of NMDAR and thus probably have no effect on NMDAR signaling.
Discussion
Regarding the accumulating evidence that immune imbalance could be one of the factors responsible for neurodevelopmental brain disorders such as ASD, we investigated the autoimmune status of a cohort of ASD patients. We reveal that a fraction of these patients exhibited a high content of circulating autoantibodies, reflecting an overall autoimmune dysregulation. Among the autoantibodies, we specifically explore the presence and molecular pathogenicity of NMDAR-Ab in ASD patients. Indeed, the report that a 9-year-old boy initially suffering from an anti-NMDAR encephalitis subsequently exhibited ASD-like symptoms15 suggests an intriguing link between the NMDAR-Ab and the emergence of ASD. These autoantibodies were best characterized in anti-NMDAR encephalitis patients that exhibit, among others, psychotic symptoms and catatonia, followed by neurologic deterioration.13,16 Using the innovative single-particle tracking approach, we explored with very high sensitivity whether the NMDAR-Ab from the ASD patient altered the trafficking of the NMDAR, as previously demonstrated with NMDAR-Ab purified from anti-NMDAR encephalitis patients.12 Our data demonstrate that NMDAR-Ab from the ASD patient did not alter the basal trafficking of surface NMDAR in hippocampal glutamate synapses. Although this report is based on the molecular exploration from only one seropositive patient, it suggests that NMDAR-Ab isolated in ASD patients (or in healthy subjects) do not alter the NMDAR signaling and probably are not involved in the ASD etiology. Noteworthy, this finding is consistent with the fact that NMDAR-Ab induced major psychotic symptoms that were never diagnosed in this patient. However, other autoantibodies have been detected in ASD patients,17 some with specific brain targets. Based on the pathological autoimmune status of this patient and the therapeutic benefit of immunotherapy in ASD patients,17 it would be of great interest to screen for autoantibodies against other neuronal targets involved in brain cell communication and that have been related to ASD.
Acknowledgments
We thank Delphine Bouchet and Pauline Durand for technical assistance on cell cultures, and members of all laboratories for constructive discussions.
This study is part of clinical trial C07-33 sponsored by Inserm. It was granted approval by the local ethics committee (Comité de Protection des Personnes) on November 14, 2008, authorized by the French authorities (French National Agency for Medicines and Health Products Safety [ANSM] 80738-B70 on August 11, 2008), and registered in a public trials registry (NCT02628808). All study participants gave their informed written consent to participation, in line with French ethical guidelines. It was coordinated by the FondaMental Foundation and carried out thanks to the following organisms and establishments: Public Hospitals of Paris (APHP), Bordeaux University Hospital Center (CHU Bordeaux), Charles Perrens Hospital, Robert Debré and Henri Mondor's Center of Clinical Investigation.
It was financially supported by the Roche Institute, by the Investissements d'Avenir programs managed by the French National Research Agency (ANR) under reference ANR-11-IDEX-0004-02, ANR-10-COHO-10-01, ANR-12-SAMA-0014, by the French National Center for Scientific Research (CNRS), by the Medical Research Foundation (FRM), by Labex Bordeaux BRAIN, and the Ministry of Higher Education and Research (MESR).
Selected abbreviations and acronyms
- ASD
autism spectrum disorder
- NMDAR
N-methyl-D-aspartate receptor
- NMDAR-Ab
N-methyi-D-aspartate receptor autoantibody
- QD
quantum dot
- ASD+
seropositive autism spectrum disorder patient
- Healthy+
seropositive healthy subject
- Healthy-
seronegative healthy subject
- IgG
immunoglobulin type G
- MSD
mean square displacement
Contributor Information
Hélène Gréa, University of Bordeaux, Interdisciplinary Institute for Neuroscience (HNS), Mixed Research Unit (UMR) 5297, Bordeaux, France; National Center for Scientific Research (CNRS), IINS UMR 5297, Bordeaux, France (Equal contribution).
Isabelle Scheid, University Paris Est Créteil, Psychiatry department, University Hospital Henri Mondor, Public Hospitals of Paris (AP-HP), University Hospital Department of Personalized Neurology and Psychiatry (DHU PePSY), France; Translational Psychiatry Laboratory, National Institute of Health and Medical Research (Inserm) U955, France; FondaMental Foundation, France.
Alexandru Gaman, University Paris Est Créteil, Psychiatry department, University Hospital Henri Mondor, Public Hospitals of Paris (AP-HP), University Hospital Department of Personalized Neurology and Psychiatry (DHU PePSY), France; Translational Psychiatry Laboratory, National Institute of Health and Medical Research (Inserm) U955, France; FondaMental Foundation, France.
Véronique Rogemond, Institut NeuroMyoGene Inserm U1217/CNRS UMR 5310, Lyon, France; Lyon Civil Hospitals, Neurological Hospital, Bron, France; University of Lyon - University Claude Bernard Lyon 1, Lyon, France.
Sandy Gillet, Institut NeuroMyoGene Inserm U1217/CNRS UMR 5310, Lyon, France; Lyon Civil Hospitals, Neurological Hospital, Bron, France; University of Lyon - University Claude Bernard Lyon 1, Lyon, France.
Jérôme Honnorat, Institut NeuroMyoGene Inserm U1217/CNRS UMR 5310, Lyon, France; Lyon Civil Hospitals, Neurological Hospital, Bron, France; University of Lyon - University Claude Bernard Lyon 1, Lyon, France.
Federico Bolognani, Roche Institute, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Christian Czech, Roche Institute, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Céline Bouquet, Roche Institute, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Elie Toledano, Roche Institute, Boulogne-Billancourt Cedex, France.
Manuel Bouvard, Aquitane Autism Resource Center, Charles Perrens Hospital, Bordeaux.
Richard Delorme, Robert Debré Hospital, Paris, France.
Laurent Groc, University of Bordeaux, Interdisciplinary Institute for Neuroscience (IINS), Mixed Research Unit (UMR) 5297, Bordeaux, France; National Center for Scientific Research (CNRS), IINS UMR 5297, Bordeaux, France; FondaMental Foundation, France (Equal seniority).
Marion Leboyer, University Paris Est Créteil, Psychiatry department, University Hospital Henri Mondor, Public Hospitals of Paris (AP-HP), University Hospital Department of Personalized Neurology and Psychiatry (DHU PePSY), France; Translational Psychiatry Laboratory, National Institute of Health and Medical Research (Inserm) U955, France; FondaMental Foundation, France.
REFERENCES
- 1.Elsabbagh M., Divan G., Koh YJ., et al Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafeiriou Dl., Ververi A., Dafoulis V., Kalyva E., Vargiami E. Autism spectrum disorders: the quest for genetic syndromes. Am J Med Genet Part B Neuropsychiatr Genet. 2013;162(4):327–366. doi: 10.1002/ajmg.b.32152. [DOI] [PubMed] [Google Scholar]
- 3.Sealey LA., Hughes BW., Sriskanda AN., et al Environmental factors in the development of autism spectrum disorders. Environ Int. 2016;88:288–298. doi: 10.1016/j.envint.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atladottir HÓ., Thorsen P., Østergaard L., et al Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 6.Zerbo O., Qian Y., Yoshida C., Grether JK., Van de Water J., Croen LA. Maternal infection during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2015;45(12):4015–4025. doi: 10.1007/s10803-013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deverman BE., Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Zhong X., Jiang L., et al Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Behav Brain Res. 2016;296(4):61–69. doi: 10.1016/j.bbr.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Braunschweig D., Ashwood P., Krakowiak P., et al Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer HS., Morris C., Gause C., Pollard M., Zimmerman AW., Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. 2009;211(1-2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Khandaker GM., Cousins L., Deakin J., Lennox BR., Yolken R., Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikasova L., De Rossi P., Bouchet D., et al Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135(pt 5):1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 13.Dalmau J., Lancaster E., Martinez-Hernandez E., Rosenfeld MR., Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiani R., Lawden M., Eames P., et al Anti-NMDA-receptor encephalitis presenting with catatonia and neuroleptic malignant syndrome in patients with intellectual disability and autism. BJPsych Bull. 2015;39(1):32–35. doi: 10.1192/pb.bp.112.041954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creten C., Van Der Zwaan S., Blankespoor RJ., et al Late onset autism and anti-NMDA-receptor encephalitis. Lancet. 2011;378(9785):98. doi: 10.1016/S0140-6736(11)60548-5. [DOI] [PubMed] [Google Scholar]
- 16.Titulaer MJ., McCracken L., Gabilondo I., et al Late-onset anti-NMDA receptor encephalitis. Neurology. 2013;81(12):1058–1063. doi: 10.1212/WNL.0b013e3182a4a49c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox-Edmiston E., Van de Water J. Maternal anti-fetal brain IgG autoantibodies and autism spectrum disorder: current knowledge and its implications for potential therapeutics. CNS Drugs. 2015;29(9):715–724. doi: 10.1007/s40263-015-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]