Abstract
Acute calculous cholecystitis (ACC) is the most frequent complication of cholelithiasis and represents one-third of all surgical emergency hospital admissions, many aspects of the disease are still a matter of debate. Knowledge of the current evidence may allow the surgical team to develop practical bedside decision-making strategies, aiming at a less demanding procedure and lower frequency of complications. In this regard, recommendations on the diagnosis supported by specific criteria and severity scores are being implemented, to prioritize patients eligible for urgency surgery. Laparoscopic cholecystectomy is the best treatment for ACC and the procedure should ideally be performed within 72 h. Early surgery is associated with better results in comparison to delayed surgery. In addition, when to suspect associated common bile duct stones and how to treat them when found are still debated. The antimicrobial agents are indicated for high-risk patients and especially in the presence of gallbladder necrosis. The use of broad-spectrum antibiotics and in some cases with antifungal agents is related to better prognosis. Moreover, an emerging strategy of not converting to open, a difficult laparoscopic cholecystectomy and performing a subtotal cholecystectomy is recommended by adept surgical teams. Some authors support the use of percutaneous cholecystostomy as an alternative emergency treatment for acute Cholecystitis for patients with severe comorbidities.
Keywords: Cholecystitis, Cholelithiasis, Biliary stones, Cholecystectomy, Laparoscopy
Core tip: This paper presented herein is a practical and comprehensive review of the acute cholecystitis. This common intra-abdominal infection can proceed to severe complications due to its natural history and requires operative treatment. Surgeons should keep in mind some basic concepts to allow them to make correct decisions about ideal operative strategy including timing.
INTRODUCTION
Acute calculous cholecystitis (ACC) represents the second source of complicated intra-abdominal infection (18.5%), according to the World Society of Emergency Surgery complicated intra-abdominal infections Score study[1]. Biliary stones are the main etiology and are present in 6.5% of men and 10.5% of women[2]. The risk of complications, like ACC, gallstone pancreatitis, and choledocholithiasis is 1% to 4% per year. Furthermore, it is recognized that patients with symptomatic cholecystolithiasis will develop ACC more frequently than their asymptomatic counterparts; thereby, effectively raising the risk of complications to five times higher (i.e., 20%)[3].
ACC is the most common complication of cholecystolithiasis accounting for 14% to 30% of cholecystectomies performed in many countries[4]. The disease can be diagnosed at any grade of severity including wall inflammation, local complication and systemic organ dysfunction. Moreover, complicated grades of the disease increase with age, with a peak between 70 and 75 years[5].
The aim is of this manuscript is to provide a practical and comprehensive review of the most important aspects of ACC and its complications. In parallel, to highlight the current evidence that helps the surgeons bedside decision making, on how best to manage the disease, to improve outcomes.
PATHOPHYSIOLOGY
ACC is caused by an inflammatory/infectious process involving the gallbladder wall, in many cases due to an impacted gallstone in the infundibulum or in the cystic duct[2]. The continued mucin production from epithelium and the gallbladder distention, results in micro and macro circulatory perfusion deficits. The subsequent events are serosa edema, mucosal sloughing, venous and lymphatic congestion, ischemia and necrosis with regional or diffuse peritonitis. Acute inflammation may be complicated by secondary bacterial infection, from the bile duct, via the portal lymphatic or vascular system. The microorganisms present in the gastrointestinal tract are the most common pathogens[5].
CLINICAL DIAGNOSIS
There is no unique marker capable of definitively indicating the diagnosis of ACC with high accuracy. The key aspects for diagnosis are upper left side signs of inflammation (pain and tenderness) and positive Murphy’s sign, as well as clinical and biochemical indicators of systemic inflammatory response. These data must be nowadays supported with positive imaging such as abdominal ultrasound (AUS)[6,7].
Acute cholecystitis severity
The Tokyo Guidelines (TG13) is practical and in accordance with the pathophysiological aspects involved in the inflammation progression from gallbladder wall to regional and systemic complications. Therefore, the grade I represents a mild disease with only wall inflammation. The grade II is associated with local sign of complications such as palpable mass, pericholeystic fluid; onset of symptoms > 72 h; laboratory data showing leukocytosis > 18000/mm3 and elevated C-reactive protein level. Finally, grade III is associated with organ dysfunction: Cardiovascular (refractory hypotension to volemic resuscitation at 30 mL/kg per hour), decrease of consciousness, respiratory failure (PaO2/FiO2: < 300), oliguria (creatinine: > 2.0 mg/dL), PTT/INR > 1.5 and platelets count below 100.000/mm3[6].
The American Association of Surgery of Trauma proposes a uniform grading system for eight intra-abdominal infectious diseases including ACC. The grades range from I to V, considering the progressive anatomic inflammation severity (from mild to serious widespread complications)[8].
Yacoub et al[9] have developed five parameters to score and stratify patients under risk of gangrenous ACC (Figure 1). They are age > 45 years, heart beat > 90/min and gallbladder thickness > 4.5 mm (1 point for each parameter), leukocyte count > 13000 mm3 (1.5 points) and male (2 points). Among their patients with ACC, 13% received 0-2 points (low probability), 33% received 2-4.5 points (intermediate probability) and 87% received > 4.5 points (high probability). The authors concluded that this fast bedside checklist could schedule patients for emergency cholecystectomy[9].
Figure 1.
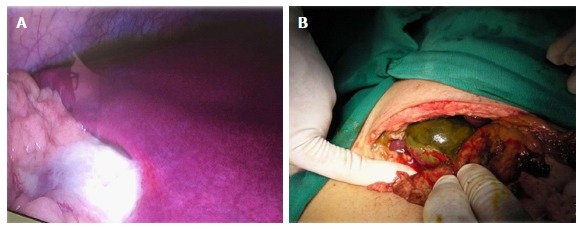
Complicated acute cholecystitis. A: Laparoscopic approach; B: Laparotomic approach.
Currently the WSES is in the process of validating a new acute cholecystitis severity score. It takes into account the patient’s clinical state, previous surgical intervention and intra-abdominal adhesions, degree of sepsis and regional inflammation[10]. While the paper highlights the initial operative severity score during laparoscopic cholecystectomy to help standardize reporting results of one of the most commonly performed surgeries worldwide, the score also assesses disease severity in the perioperative period and not exclusively in the preoperative period.
IMAGING DIAGNOSIS
Planar radiography is not so effective in the context of gallstones diagnosis, because they are radiolucent in the majority of cases (80%-85%)[11]. Instead, AUS is the first-line imaging requested in suggestive cases of ACC. It allows easy and practical bedside diagnosis due its compelling findings such as: Gallstones, lumen distension, three-phase wall thickening (Figure 2), sonographic Murphy’s, perivisceral fluid and hyperemia on Color Dopller[12-15]. However, Kiewiet et al[12] have shown that AUS does not have the same accuracy in the diagnosis of ACC as it has in diagnosing cholecystolithiasis. The findings of gallstones, gallbladder wall thickness and Murphy’s signal on AUS show high predictive value for ACC diagnosis (95%)[16]. However, not always all signals are present at the same time and gallbladder wall thickening may be observed in other systemic diseases, such as liver, renal and heart failure, probably because portal hypertension[17].
Figure 2.

Transabdominal ultrasound in acute cholecystitis.
Computed tomography (CT) is useful for the diagnosis of complicated forms of ACC (emphysematous and gangrenous cholecystitis)[18,19], besides it is value in the differential diagnosis with other intra-abdominal diseases, especially in obese patients or when gaseous distention limits the use of AUS. In addition, CT cholangiography (when not jaundiced) in diagnosing common bile duct stones (CBDS) is less employed, with a reported sensitivity from 50% to 90%[20-22].
Cholescintigraphy is an excellent method to diagnose ACC, but it is limited to some centers. It uses the principle that radiopharmaceuticals (diisopropyl iminodiacetic acid) should fulfill the gallbladder content in half an hour. Therefore, if gallbladder is not contrasted, few hours later, the diagnosis of ACC is highly probable, because there is cystic duct obstruction. Shea et al[23] showed in their meta-analysis that cholescintigraphy is the imaging of choice in difficult cases and has the highest diagnostic accuracy (Figure 3).
Figure 3.

Cholescintigraphy in acute calculous cholecystitis.
ASSESSING ASSOCIATED CBDS
The presence of associated CBDS should be stratified in all cases of cholecystectomy into low, moderate and high risk. The American Society of Gastrointestinal Endoscopy, has recently confirmed that the presence of choledocholithiasis on AUS and/or bilirubin > 4 mg/dL + dilated CBD criteria had higher specificity (more than 50%) for the CBDS diagnosis[24]. Padda et al[25] found in a cohort study that patients with ACC and CBDS present changes in liver function tests. So, the alkaline phosphatase is increased in 77% of the times, bilirubin in 60% and aminotransferase levels in 90%.
In fact, the enzymes could be affected by gallbladder inflammation secondary the acute transient hepatocellular injury, and even their use alone is of limited value[26]. Patients of moderate risk for choledocholithiasis should be underwent a magnetic resonance cholangiopancreatography (MRCP) or endoscopic ultrasound (EUS) in the preoperative period. The use of intra-operative cholangiography (IOC), and/or laparoscopic ultrasound are effective alternative for decrease the incidence of missing CBDS during cholecystectomy too. Therefore, the use of endoscopic retrograde cholangiopancreatography (ERCP) should be reserved for patients that are stratified into the high-risk groups[24,27].
Giljaca et al[28], in the recent Cochrane meta-analysis, compared the level of diagnostic accuracy between MRCP and EUS and concluded that both tests are highly accurate and able to exclude the presence of CBDS with high sensibility and specificity (95%). They therefore recommend routinely avoiding the use of the more invasive ERCP, when possible, and instead reserving it for patients already graded as high risk for CBDS[24,28].
Amouyal et al[29] have shown that EUS is an excellent approach for detecting CBDS and could replace ERCP in many instances. It prevents the risk of overlooking them, when there are normal biochemical predictors and an absence of CBD enlargement on AUS. The exam is less invasive than ERCP, and has excellent sensitivity and specificity for the detection of CBDS including small stones (< 5 mm)[29].
HOW TO MANAGE ASSOCIATED COMMON BILE DUCT STONE
Patients with symptomatic ACC and CBDS detected during preoperative and/or intraoperative studies should be candidates to undergo CBDS extraction. The choice of treatment depends on the level of surgical expertise, equipment, and the availability of multidisciplinary facilities at each hospital[30]. The options include: open cholecystectomy (OC) with open common bile duct exploration; laparoscopic cholecystectomy (LC) with laparoscopic common bile duct extraction (LCBDE); and LC with endoscopic stone extraction (ESE) performed either preoperatively, intraoperative or postoperatively[31,32]. A systematic review of randomized controlled trials has shown that OC with open CBDE has the lowest incidence of retained stones, but is associated with high morbidity and mortality, especially in elderly patients[30,32]. In addition, there was no difference in the retained CBDS among preoperative or intra-operative ERCP and LCBDSE[30,31]. The procedure, either via the transcystic duct (more than 50% success), or via choledochotomy (considered to be the more difficult group) is safe and effective to perform in units that are set up for this type of intervention[33,34]. Therefore, LCBDE is a safe and effective approach for managing option CBDS, has been demonstrated to shorten the hospital stay and should be encouraged as a possible salvage procedure following cases of ESE failure[34].
As a rule, however, operations for severe ACC should focus on dealing with the problem at hand, as CBDS can be removed later. The severity of the local inflammatory process near the bile duct can mean that LCBDE would be difficult to perform. A temporary fenestrated transcystic catheter, inserted via the cystic duct into the duodenum (antegrade stent) is an option. Should this be considered, the definite treatment of CBDS would be postponed until the patient recovers and the catheter in the duodenum favors the ERCP. Nonetheless, this approach has not been tested yet prospectively and for coincidental CBDS that are not actively causing obstruction; critics have suggested it seems to be over-treatment, and complications from this technique have been known to occur.
LAPAROSCOPIC OR OPEN APPROACH
Laparoscopy has significant advantages over open surgery in managing septic patients. The immune response and the levels cytokines yielded, which are associated with systemic inflammatory response severity, are smaller and influence the clinical outcomes[35].
Recent systematic reviews and meta-analyses from the WSES concluded that in the setting of ACC post-operative morbidity, mortality, and hospital stay were significantly decreased after LC, as was the incidence of pneumonia and wound infection. Severe haemorrhage, bile leakage rates, and/or operative times were not significantly different between patients undergoing OC and LC. The group of experts concluded that cholecystectomy in ACC should be preferably managed by laparoscopy in the first instance[36]. Though other relevant treatment modalities include mini-cholecystectomy, reduced-port cholecystectomy, single-port cholecystectomy and robotic cholecystectomy, these were determined to be neither practical nor cost-effective in severe cases of ACC.
Because the surgeon’s commitment is primarily to their patient and not to the laparoscopy procedure itself, the operation cannot be performed if the “critical view of safety” (CVS) is not obtained during cholecystic pedicle dissection, regardless of the chosen approach (i.e., laparoscopy vs laparotomy). Failure to identify the CVS is a strong indication of IOC for the complete understanding of the biliary anatomy (Figure 4). The reported incidence of bile duct injury (CBDI) during LC ranges from 0.16% to 1.5%, and has not decreased over time. Stefanidis et al[37] studied how often surgeons resort to the consideration of the CVS during LC and their results were disappointed. Only 20% of observed surgeons achieved adequately the CVS during LC; that is, CVS criterion was not routinely used by majority of surgeons. Furthermore, one-fourth of those who claimed to obtain the CVS did so inadequately[37].
Figure 4.
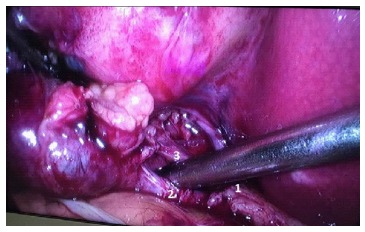
Laparoscopic cholecystectomy showing the critical view of safety. 1: Common hepatic duct; 2: Cystic duct; 3: Cystic artery.
Retrograde laparoscopic cholecystectomy (RLC) or “fundus first” laparoscopic cholecystectomy, a procedure that sometimes utilizes a liver retractor, does have a role in cases in which the standard technique (i.e., cephalad fundic traction and antegrade dissection) fails to provide good exposure[38]. Another emerging strategy that refrains from the need to convert to opening a difficult LC and performing a subtotal cholecystectomy (SCL) is also underway. There is increasing evidence about the feasibility and safety of this procedure, which employs a strategy of “calculated retreat is not defeat[39]. SCL procedures are nominated “fenestrating” and “reconstituting” types and are good alternative in difficult cases. Laparoscopic subtotal cholecystectomy has its advantages but may require advanced laparoscopic skills[39].
An alternative approach aimed at preventing bile duct injury (BDI) is laparoscopic partial cholecystectomy (LPC). A recent systematic review concluded that, when a difficult gallbladder is encountered during LC, LPC is a safe alternative to conversion and closing of the cystic duct, gallbladder remnant, or both seems to be preferable[40]. Currò et al[41] (2017) conducted a prospective randomized study comparing three-dimensional vs two-dimensional imaging for LC and, despite their small sample, concluded that three-dimensional approach does not improve the performance time of LC in experienced hands. Further study is necessary, however, to verify if it can reduce biliary complications[41].
TIMING OF SURGICAL TREATMENT
Gurusamy et al[42] (2010) in their meta-analysis compared early laparoscopic cholecystectomy (ELC - 1 wk of onset of symptoms) X delayed laparoscopic cholecystectomy (DLC - at least 6 wk after symptoms free) in patients with ACC. They concluded that the two groups presented similar results regarding bile duct injury and conversion rate, but the hospital stay was shorter by 4 d for ELC and recommend the approach[42].
Cao et al[43] (2015) in their meta-analyses studied if ELC is superior to DLC for ACC management. They showed that ELC group has presented reductions in mortality, bile duct complications and improvement in many other parameters analyzed.
Although the procedure should be performed within the first 72 h, patients still benefit from early surgery compared to delayed surgery. Therefore, the period of onset of symptoms should not influence the surgeons’ willingness to perform an ELC. They suggest that ELC is the standard of care in the treatment of ACC[43].
According to TG13, for patients with grade I disease, cholecystectomy at an early stage (e.g., within 72 h of onset of symptoms) is recommended. If non-operative treatment (antimicrobial therapy) is chosen and no improvement is observed within 24-48 h, reconsider ELC first. For patients classified as grade II (i.e., they demonstrate local complications), emergency surgery must be expedited (via laparotomy or laparoscopy) and in the absence of adequate facilities, skilled personnel or technical equipment, patient transfer should be considered. For patients with grade III and/or those unfit to undergo an emergency cholecystectomy, gallbladder drainage may be an attractive alternative. This therapy is typically complemented with antibiotics and intensive care; an interval cholecystectomy may also be performed at three months, following improvement in the patient’s health status[6]. However, Amirthalingam et al[44] (2016) suggested that these recommendations are too restrictive, stating instead that patients with moderate and severe ACC can be managed by ELC and sometimes, even those that fall into the category of grade I should be managed using percutaneous drainage because of potential underlying.
In addition, the 2016 WSES guidelines on ACC identify two important aspects in the management. First of all, they conclude that “surgery is superior to observation of ACC in the clinical outcome and shows some cost-effectiveness advantages due to the gallstone-related complications (33% in relapse) and to the high rate of readmission and surgery in the observation group”. Second, they confirm that “cholecystectomy is the gold standard for treatment of ACC”[45].
ANTIMICROBIAL TREATMENT
The role of therapeutic antibiotics in ACC is controversial, but seems appropriate in non-operative treatment, which should be reserved for patients with mild disease[6]. The use of preoperative prophylactic antibiotics is not suitable for low-risk patients undergoing LC. The main purpose of starting antibiotics in surgically managed cases of ACC is to prevent perioperative infectious complications[46], however, according to van Dijk et al[47] in recent systematic review, which assessed its effect in the course of ACC conclude: They are not effective for patients undergone to non-operative treatment neither in those one selected for cholecystectomy.
When antibiotics are indicated, the choice of antimicrobial agent is guided by the likely type of pathogen being targeted, taking into consideration whether it was acquired in the community or a healthcare setting, whether it is extended spectrum β-lactamase (ESBL) producing, the presence of sepsis, as well as the agent’s pharmacodynamics and pharmacokinetics. Blood cultures are not always positive and many times the prescription is based on empiric approach. As we know, critically-ill patients need acute care measures and the intravenous antibiotics administration within the first hour. Microbiological data take at least 48 h for the identification of the microorganisms. In addition, the Hospital based Antibiotic Stewardship Programs should be involved to provide the most frequent pathogens and their susceptibility/resistance profiles[48].
The most important pathogens in ACC originate in the patient’s indigenous flora and include Enterobacteriaceae: E. coli and Klebsiella sp, Streptococcus sp, and anaerobes such as Bacteroides fragilis group. In these cases, narrower spectrum activity antimicrobials targeting the previously mentioned pathogens are the best option. However, in patients with ESBL-producing Enterobacteriaceae infections, agents against ESBL-producing bacteria need to be warranted[48]. Campanile et al[49] (2014) recommend the use of antibiotics and antifungal agents in high-risk patients with gangrenous cholecystitis as their use is tied to lower incidence of infection at the surgical site and better prognosis. The Table 1 illustrates more clearly their antimicrobial recommendations[49].
Table 1.
The choice of antibiotics for treatment of acute calculous cholecystitis according the WSES proposal in two different scenarious
| Community acquired | Health care associated | ||
| Infections situations | Drug | Infections situations | Drug |
| No severe Sepse ESBL - | Amoxicilin Clavulanate | No severe sepse | Piperacilin Tazobactan + Tigecicline + - Fluconazol |
| No severe Sepse ESBL + | Tigecicline | ||
| Severe Sepse ESBL - | Piperacilin Tazobactan | Severe sepse | Piperacilin Tazobactan + Tigecicline + Echinocandin or Carbapenen + Teiclopanin + Echinocandin |
| Severe Sepse ESBL + | Piperacilin Tazobactan + Tigecicline + Fluconazole | ||
From: Campaline et al[47], 2014. WSES. ESBL: Extended spectrum β-lactamase.
COMPLICATIONS
Bile leak from a duct of Luschka is more common than true bile duct injury and occurs in 0.1%-0.5% of patients after cholecystectomy. Other complications include peritonitis (0.2%), hemorrhage and surgical site infection including spaces and organs. Operative complication rates are comparable between the laparoscopic and laparotomic approaches. In addition, there is less concern for contamination and lower rates of wound infection when the gallbladder is taken out in a retrieval bag during laparoscopic cholecystectomy[50-53].
A recent systematic review assessed the associated factors linked to the conversion of LC to OC. The results showed that male patients, age 60-65 years, sclerotic gallbladder or wall thickness (4-5 mm) and acute cholecystitis, were significant risk factors for conversion[54].
WHEN TO PERFORM CHOLECYSTOSTOMY
Percutaneous cholecystostomy (PC) is an alternative to emergency cholecystectomy in complicated cases of high risk patients, however, there are yet no evidences supporting this claim[55,56]. Gurusamy et al[56] (2013) in a Cochrane Database systematic review included two trials with 156 participants. The first trial compared PC followed by ELC vs DLC (70 participants). The results showed that the mortality, morbidity and conversion rate were the same among the two groups[56].
The second trial (86 participants), compared PC vs conservative treatment (86 participants). Again, the result of the study showed no difference in the same parameters[56].
It has been difficult to establish the role of percutaneous gallbladder drainage because of the different existing definitions for the “high-risk patient”[42,54]. In an attempt to clarify the conflicting evidences, Yeo et al[57] 2017 in a retrospective review, studied 103 aged patients (median: 80 years), who had undergone PC procedures. The study results showed that the patients with higher APACHE II scores, higher Charlson index, delay in diagnosis and carrying out the procedure had higher in-hospital mortality. On the other, the absence of these findings was associated with eventual cholecystectomy[57].
CONCLUSION
Presented herein is a practical and comprehensive review of the ACC. This common intra-abdominal infection can proceed to severe complications due to its natural history and requires operative treatment. Surgeons should keep in mind some basic concepts to allow them to make correct decisions about ideal operative strategy including timing.
The clinical diagnosis should be based on strictly criteria and the patient should be stratified according grade and the possibility of local and systemic complications. Laparoscopy is the suggested first approach for cholecystectomy guaranteeing significant advantages over open surgery. In select cases, percutaneous cholecystostomy may be used as a lifesaving manoeuvre. In addition, the possibility of choledocholithiasis should be kept in mind and its therapeutic alternatives considered. Finally, to recognize the basic principles that guide the antimicrobial use for prophylactic and therapeutic proposes.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: January 23, 2017
First decision: February 17, 2017
Article in press: April 10, 2017
P- Reviewer: Bandyopadhyay SK, Li W, Shelat VG, Tomazic A, Zhu H S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, Ordoñez CA, Leppaniemi A, Fraga GP, Coccolini F, et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study) World J Emerg Surg. 2015;10:61. doi: 10.1186/s13017-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health Consensus Development Conference Statement on Gallstones and Laparoscopic Cholecystectomy. Am J Surg. 1993;165:390–398. doi: 10.1016/s0002-9610(05)80929-8. [DOI] [PubMed] [Google Scholar]
- 4.Orlando R, Russell JC, Lynch J, Mattie A. Laparoscopic cholecystectomy. A statewide experience. The Connecticut Laparoscopic Cholecystectomy Registry. Arch Surg. 1993;128:494–498; discussion 498-499. doi: 10.1001/archsurg.1993.01420170024002. [DOI] [PubMed] [Google Scholar]
- 5.Riall TS, Zhang D, Townsend CM, Kuo YF, Goodwin JS. Failure to perform cholecystectomy for acute cholecystitis in elderly patients is associated with increased morbidity, mortality, and cost. J Am Coll Surg. 2010;210:668–677, 677-679. doi: 10.1016/j.jamcollsurg.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Garden OJ, Kiriyama S, Hata J, et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:35–46. doi: 10.1007/s00534-012-0568-9. [DOI] [PubMed] [Google Scholar]
- 7.Duncan CB, Riall TS. Evidence-based current surgical practice: calculous gallbladder disease. J Gastrointest Surg. 2012;16:2011–2025. doi: 10.1007/s11605-012-2024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafi S, Aboutanos M, Brown CV, Ciesla D, Cohen MJ, Crandall ML, Inaba K, Miller PR, Mowery NT. Measuring anatomic severity of disease in emergency general surgery. J Trauma Acute Care Surg. 2014;76:884–887. doi: 10.1097/TA.0b013e3182aafdba. [DOI] [PubMed] [Google Scholar]
- 9.Yacoub WN, Petrosyan M, Sehgal I, Ma Y, Chandrasoma P, Mason RJ. Prediction of patients with acute cholecystitis requiring emergent cholecystectomy: a simple score. Gastroenterol Res Pract. 2010;2010:901739. doi: 10.1155/2010/901739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugrue M, Sahebally SM, Ansaloni L, Zielinski MD. Grading operative findings at laparoscopic cholecystectomy- a new scoring system. World J Emerg Surg. 2015;10:14. doi: 10.1186/s13017-015-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright SL, Knudson MP. Evaluation of acute abdominal pain in adults. Am Fam Physician. 2008;77:971–978. [PubMed] [Google Scholar]
- 12.Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264:708–720. doi: 10.1148/radiol.12111561. [DOI] [PubMed] [Google Scholar]
- 13.Nino-Murcia M, Jeffrey RB. Imaging the patient with right upper quadrant pain. Semin Roentgenol. 2001;36:81–91. doi: 10.1053/sroe.2001.22825. [DOI] [PubMed] [Google Scholar]
- 14.Schiller VL, Turner RR, Sarti DA. Color doppler imaging of the gallbladder wall in acute cholecystitis: sonographic-pathologic correlation. Abdom Imaging. 1996;21:233–237. doi: 10.1007/s002619900053. [DOI] [PubMed] [Google Scholar]
- 15.Paulson EK, Kliewer MA, Hertzberg BS, Paine SS, Carroll BA. Diagnosis of acute cholecystitis with color Doppler sonography: significance of arterial flow in thickened gallbladder wall. AJR Am J Roentgenol. 1994;162:1105–1108. doi: 10.2214/ajr.162.5.8165991. [DOI] [PubMed] [Google Scholar]
- 16.Ralls PW, Colletti PM, Lapin SA, Chandrasoma P, Boswell WD, Ngo C, Radin DR, Halls JM. Real-time sonography in suspected acute cholecystitis. Prospective evaluation of primary and secondary signs. Radiology. 1985;155:767–771. doi: 10.1148/radiology.155.3.3890007. [DOI] [PubMed] [Google Scholar]
- 17.van Breda Vriesman AC, Engelbrecht MR, Smithuis RH, Puylaert JB. Diffuse gallbladder wall thickening: differential diagnosis. AJR Am J Roentgenol. 2007;188:495–501. doi: 10.2214/AJR.05.1712. [DOI] [PubMed] [Google Scholar]
- 18.Reginelli A, Mandato Y, Solazzo A, Berritto D, Iacobellis F, Grassi R. Errors in the radiological evaluation of the alimentary tract: part II. Semin Ultrasound CT MR. 2012;33:308–317. doi: 10.1053/j.sult.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Buonamico P, Suppressa P, Lenato GM, Pasculli G, D’Ovidio F, Memeo M, Scardapane A, Sabbà C. Liver involvement in a large cohort of patients with hereditary hemorrhagic telangiectasia: echo-color-Doppler vs multislice computed tomography study. J Hepatol. 2008;48:811–820. doi: 10.1016/j.jhep.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Neitlich JD, Topazian M, Smith RC, Gupta A, Burrell MI, Rosenfield AT. Detection of choledocholithiasis: comparison of unenhanced helical CT and endoscopic retrograde cholangiopancreatography. Radiology. 1997;203:753–757. doi: 10.1148/radiology.203.3.9169700. [DOI] [PubMed] [Google Scholar]
- 21.Baron RL. Diagnosing choledocholithiasis: how far can we push helical CT? Radiology. 1997;203:601–603. doi: 10.1148/radiology.203.3.9169674. [DOI] [PubMed] [Google Scholar]
- 22.Brink JA, Kammer B, Mueller PR, Balfe DM, Prien EL, Ferrucci JT. Prediction of gallstone composition: synthesis of CT and radiographic features in vitro. Radiology. 1994;190:69–75. doi: 10.1148/radiology.190.1.8259431. [DOI] [PubMed] [Google Scholar]
- 23.Shea JA, Berlin JA, Escarce JJ, Clarke JR, Kinosian BP, Cabana MD, Tsai WW, Horangic N, Malet PF, Schwartz JS. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med. 1994;154:2573–2581. [PubMed] [Google Scholar]
- 24.He H, Tan C, Wu J, Dai N, Hu W, Zhang Y, Laine L, Scheiman J, Kim JJ. Accuracy of ASGE high-risk criteria in evaluation of patients with suspected common bile duct stones. Gastrointest Endosc. 2017;pii:S0016–5107(17)30083-4. doi: 10.1016/j.gie.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Padda MS, Singh S, Tang SJ, Rockey DC. Liver test patterns in patients with acute calculous cholecystitis and/or choledocholithiasis. Aliment Pharmacol Ther. 2009;29:1011–1018. doi: 10.1111/j.1365-2036.2009.03956.x. [DOI] [PubMed] [Google Scholar]
- 26.Chang CW, Chang WH, Lin CC, Chu CH, Wang TE, Shih SC. Acute transient hepatocellular injury in cholelithiasis and cholecystitis without evidence of choledocholithiasis. World J Gastroenterol. 2009;15:3788–3792. doi: 10.3748/wjg.15.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gwinn EC, Daly S, Deziel DJ. The use of laparoscopic ultrasound in difficult cholecystectomy cases significantly decreases morbidity. Surgery. 2013;154:909–915; discussion 915-917. doi: 10.1016/j.surg.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Giljaca V, Gurusamy KS, Takwoingi Y, Higgie D, Poropat G, Štimac D, Davidson BR. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev. 2015;(2):CD011549. doi: 10.1002/14651858.CD011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amouyal P, Palazzo L, Amouyal G, Ponsot P, Mompoint D, Vilgrain V, Gayet B, Fléjou JF, Paolaggi JA. Endosonography: promising method for diagnosis of extrahepatic cholestasis. Lancet. 1989;2:1195–1198. doi: 10.1016/s0140-6736(89)91801-1. [DOI] [PubMed] [Google Scholar]
- 30.Rábago LR, Ortega A, Chico I, Collado D, Olivares A, Castro JL, Quintanilla E. Intraoperative ERCP: What role does it have in the era of laparoscopic cholecystectomy? World J Gastrointest Endosc. 2011;3:248–255. doi: 10.4253/wjge.v3.i12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dasari BV, Tan CJ, Gurusamy KS, Martin DJ, Kirk G, McKie L, Diamond T, Taylor MA. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. 2013;(12):CD003327. doi: 10.1002/14651858.CD003327.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong DF, Xin Y, Chen DW. Comparison of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and laparoscopic exploration of the common bile duct for cholecystocholedocholithiasis. Surg Endosc. 2006;20:424–427. doi: 10.1007/s00464-004-8248-8. [DOI] [PubMed] [Google Scholar]
- 33.Kelly MD. Results of laparoscopic bile duct exploration via choledochotomy. ANZ J Surg. 2010;80:694–698. doi: 10.1111/j.1445-2197.2010.05269.x. [DOI] [PubMed] [Google Scholar]
- 34.Shelat VG, Chan CY, Liau KH, Ho CK. Laparoscopic exploration can salvage failed endoscopic bile duct stone extraction. Singapore Med J. 2012;53:313–317. [PubMed] [Google Scholar]
- 35.Di Saverio S. Emergency laparoscopy: a new emerging discipline for treating abdominal emergencies attempting to minimize costs and invasiveness and maximize outcomes and patients’ comfort. J Trauma Acute Care Surg. 2014;77:338–350. doi: 10.1097/TA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 36.Coccolini F, Catena F, Pisano M, Gheza F, Fagiuoli S, Di Saverio S, Leandro G, Montori G, Ceresoli M, Corbella D, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg. 2015;18:196–204. doi: 10.1016/j.ijsu.2015.04.083. [DOI] [PubMed] [Google Scholar]
- 37.Stefanidis D, Chintalapudi N, Anderson-Montoya B, Oommen B, Tobben D, Pimentel M. How often do surgeons obtain the critical view of safety during laparoscopic cholecystectomy? Surg Endosc. 2017;31:142–146. doi: 10.1007/s00464-016-4943-5. [DOI] [PubMed] [Google Scholar]
- 38.Kelly MD. Laparoscopic retrograde (fundus first) cholecystectomy. BMC Surg. 2009;9:19. doi: 10.1186/1471-2482-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strasberg SM, Pucci MJ, Brunt LM, Deziel DJ. Subtotal Cholecystectomy-”Fenestrating” vs “Reconstituting” Subtypes and the Prevention of Bile Duct Injury: Definition of the Optimal Procedure in Difficult Operative Conditions. J Am Coll Surg. 2016;222:89–96. doi: 10.1016/j.jamcollsurg.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Henneman D, da Costa DW, Vrouenraets BC, van Wagensveld BA, Lagarde SM. Laparoscopic partial cholecystectomy for the difficult gallbladder: a systematic review. Surg Endosc. 2013;27:351–358. doi: 10.1007/s00464-012-2458-2. [DOI] [PubMed] [Google Scholar]
- 41.Currò G, La Malfa G, Lazzara S, Caizzone A, Fortugno A, Navarra G. Three-Dimensional Versus Two-Dimensional Laparoscopic Cholecystectomy: Is Surgeon Experience Relevant? J Laparoendosc Adv Surg Tech A. 2015;25:566–570. doi: 10.1089/lap.2014.0641. [DOI] [PubMed] [Google Scholar]
- 42.Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–150. doi: 10.1002/bjs.6870. [DOI] [PubMed] [Google Scholar]
- 43.Cao AM, Eslick GD, Cox MR. Early laparoscopic cholecystectomy is superior to delayed acute cholecystitis: a meta-analysis of case-control studies. Surg Endosc. 2016;30:1172–1182. doi: 10.1007/s00464-015-4325-4. [DOI] [PubMed] [Google Scholar]
- 44.Amirthalingam V, Low JK, Woon W, Shelat V. Tokyo Guidelines 2013 may be too restrictive and patients with moderate and severe acute cholecystitis can be managed by early cholecystectomy too. Surg Endosc. 2016 doi: 10.1007/s00464-016-5300-4. Nov 1; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Ansaloni L, Pisano M, Coccolini F, Peitzmann AB, Fingerhut A, Catena F, Agresta F, Allegri A, Bailey I, Balogh ZJ, et al. 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg. 2016;11:25. doi: 10.1186/s13017-016-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galili O, Eldar S, Matter I, Madi H, Brodsky A, Galis I, Eldar S. The effect of bactibilia on the course and outcome of laparoscopic cholecystectomy. Eur J Clin Microbiol Infect Dis. 2008;27:797–803. doi: 10.1007/s10096-008-0504-8. [DOI] [PubMed] [Google Scholar]
- 47.van Dijk AH, de Reuver PR, Tasma TN, van Dieren S, Hugh TJ, Boermeester MA. Systematic review of antibiotic treatment for acute calculous cholecystitis. Br J Surg. 2016;103:797–811. doi: 10.1002/bjs.10146. [DOI] [PubMed] [Google Scholar]
- 48.Sartelli M, Weber DG, Ruppé E, Bassetti M, Wright BJ, Ansaloni L, Catena F, Coccolini F, Abu-Zidan FM, Coimbra R, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA) World J Emerg Surg. 2016;11:33. doi: 10.1186/s13017-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campanile FC, Pisano M, Coccolini F, Catena F, Agresta F, Ansaloni L. Acute cholecystitis: WSES position statement. World J Emerg Surg. 2014;9:58. doi: 10.1186/1749-7922-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livingston EH, Rege RV. A nationwide study of conversion from laparoscopic to open cholecystectomy. Am J Surg. 2004;188:205–211. doi: 10.1016/j.amjsurg.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 51.Nair RG, Dunn DC, Fowler S, McCloy RF. Progress with cholecystectomy: improving results in England and Wales. Br J Surg. 1997;84:1396–1398. [PubMed] [Google Scholar]
- 52.David GG, Al-Sarira AA, Willmott S, Deakin M, Corless DJ, Slavin JP. Management of acute gallbladder disease in England. Br J Surg. 2008;95:472–476. doi: 10.1002/bjs.5984. [DOI] [PubMed] [Google Scholar]
- 53.Lawrentschuk N, Hewitt PM, Pritchard MG. Elective laparoscopic cholecystectomy: implications of prolonged waiting times for surgery. ANZ J Surg. 2003;73:890–893. doi: 10.1046/j.1445-2197.2003.02826.x. [DOI] [PubMed] [Google Scholar]
- 54.Philip Rothman J, Burcharth J, Pommergaard HC, Viereck S, Rosenberg J. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery - A Systematic Review and Meta-Analysis of Observational Studies. Dig Surg. 2016;33:414–423. doi: 10.1159/000445505. [DOI] [PubMed] [Google Scholar]
- 55.Winbladh A, Gullstrand P, Svanvik J, Sandström P. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford) 2009;11:183–193. doi: 10.1111/j.1477-2574.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gurusamy KS, Rossi M, Davidson BR. Percutaneous cholecystostomy for high-risk surgical patients with acute calculous cholecystitis. Cochrane Database Syst Rev. 2013;(8):CD007088. doi: 10.1002/14651858.CD007088.pub2. [DOI] [PubMed] [Google Scholar]
- 57.Yeo CS, Tay VW, Low JK, Woon WW, Punamiya SJ, Shelat VG. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepatobiliary Pancreat Sci. 2016;23:65–73. doi: 10.1002/jhbp.304. [DOI] [PubMed] [Google Scholar]


