Abstract
Human disease caused by Escherichia coli O157:H7 is a function of the number of cells that are present at potential sites of infection and host susceptibility. Such infectious doses are a result, in part, of the quantity of cells that are ingested and that survive human host defenses, such as the low-pH environment of the stomach. To more fully understand the kinetics of E. coli O157:H7 survival in gastric fluid, individual E. coli O157:H7 strains were suspended in various media (i.e., saline, cooked ground beef [CGB], and CGB containing a commercial antacid product [CGB+A]), mixed at various proportions with simulated human gastric fluid (SGF), and then incubated at 37°C for up to 4 h. The highest inactivation rate among nine E. coli O157:H7 strains was observed in saline. Specifically, the average survival rates in 100:1 and 10:1 proportions of SGF-saline were −1.344 ± 0.564 and −0.997 ± 0.388 log10 CFU/h, respectively. In contrast, the average inactivation rate for 10 E. coli O157:H7 strains suspended in 10:1 SGF-CGB was −0.081 ± 0.068, a rate that was 12-fold lower than that observed for SGF-saline. In comparison, the average inactivation rate for Shigella flexneri strain 5348 in 100:1 and 10:1 SGF-saline was −8.784 and −17.310, respectively. These latter inactivation rates were 7- to 17-fold higher than those for E. coli O157:H7 strains in SGF-saline and were 4-fold higher than those for E. coli O157:H7 strains in SGF-CGB. The survival rate of E. coli O157:H7 strain GFP80EC increased as the dose of antacid increased from one-half to twice the prescribed dose. A similar trend was observed for the matrix pH over the range of pH 1.6 to 5.7, indicating that pH is a primary factor affecting E. coli O157:H7 survival in SGF-CGB+A. These results can be used in risk assessment to define dose-response relationships for E. coli O157:H7 and to evaluate potential surrogate organisms.
The extremely low pH of the stomach is one of the early defenses against infections caused by food-borne bacteria. This has been recognized for various bacterial pathogens, such as Vibrio, Salmonella, and Campylobacter spp., where reduced gastric acidity via bicarbonate, gastrectomy, or proton pump inhibitor medication increases human susceptibility to infection (9, 16, 23, 27). The degree of bacterial susceptibility to low pH varies among bacterial pathogens, and it has been suggested that pH susceptibility is associated with infectious dose (8, 15, 20, 21, 25, 31, 32).
Escherichia coli O157:H7 contaminates raw ground beef and occasionally causes serious human food-borne illness. Its infectious dose may be less than 1,000 cells (2, 22). Numerous reports document the relative degree of acid resistance for E. coli O157:H7 in a variety of foods and synthetic media; however, few studies have examined the survival of E. coli O157:H7 in ground beef exposed to gastric fluid, including the effects of strain variation, different proportions of ground beef-gastric fluid, and the effects of antacid treatment.
This information is relevant to the development of dose-response models in risk assessment, because many data are collected from human volunteer experiments where stomach pH is neutralized with bicarbonate and where the influence of food matrices and gastric fluid are not typically modeled (7). In addition, various risk assessments for E. coli O157:H7 in ground beef have used surrogate dose-response data for Shigella flexneri and Shigella dysenteriae without knowing the relative survival rates for each type of pathogen in gastric fluid (10). This report provides additional information about interactions between E. coli O157:H7 and the gastric environment, including comparisons with Shigella spp.
MATERIALS AND METHODS
Bacteria and culture conditions.
Ten E. coli O157:H7 strains (OB1340, OB90520A, OB141412, OB1525C, OB1423C, OB1514C1, OB1680G, OB1533A, DB1538, and GFP80EC) were used. A complete description of the strains and the methods for storing and culturing the bacteria strains has been reported previously (30). Five clinical Shigella isolates (S. flexneri [#1B, #2], S. boydii [#3], S. sonnei [#1], and S. dysenteriae [#3]) were provided by Petra Sanchez and Maria Inez Sato of Companhia de Tecnologia de Saneamento Ambiental, São Paulo, Brasil. A sixth clinical isolate, S. flexneri strain 5348, was obtained from the Microbial Food Safety Research Unit culture collection (33). On the day prior to experimentation, bacteria were transferred from frozen stock cultures to 10 ml of brain heart infusion broth (BHI; Difco Laboratories, Detroit, Mich.) and were cultured without agitation for 4 to 6 h at 37°C. Next, approximately 10 μl was transferred to 10 ml of fresh BHI and was incubated without agitation for approximately 16 to 18 h at 37°C. The broth cultures were diluted in 0.1% peptone (Difco) water (PW) or 0.85% NaCl (saline), and then dilutions were added to various suspension media (saline, cooked ground beef [CGB], or CBG containing a commercial antacid [CGB+A]). CFU per milliliter were measured at predetermined time intervals.
Ground beef preparation.
Ground beef (∼5 to 7% fat) was purchased from a local retail store. Ground beef was formed into 100-g patties and cooked on a clam-style commercial grill (George Foreman Grilling Machine; Salton Inc., Mt. Prospect, Ill.) until the internal temperature measured 72°C according to a calibrated thermocouple (series 396; Atkins Technical Co., Gainesville, Fla.). The cooked product was cooled to room temperature and processed with a household-size grinder (Model K5SSWH; KitchenAid, St. Joseph, Mich.) equipped with a die containing 20 4.5-mm-diameter holes. Ninety-gram portions were placed in 400-ml-capacity stomacher bags (Koch Industries, Kansas City, Mo.), vacuum sealed, and stored at −20°C until experimentation. On the day of experimentation, cooked, frozen ground beef was warmed to 37°C, and then portions of ground beef were transferred to 100-ml-capacity stomacher bags.
SGF studies.
The overall experimental design involved growing individual strains of E. coli O157:H7 in BHI broth, diluting cultures in PW or saline to predetermined test concentrations, adding the diluted bacteria to different media, and then mixing these media with 37°C simulated gastric fluid (SGF) at different proportions of SGF to medium (SGF-medium). Experimental variables included the suspension medium, the proportion of SGF to medium, individual E. coli O157:H7 and Shigella spp. isolates, dose of antacid, and incubation time. One-hundred microliters of the samples were plated on tryptic soy agar (TSA; Difco) at selected time intervals, and CFU were enumerated.
Preparation of simulated gastric fluid.
The SGF was produced following the formulation reported by Beumer et al. (6). The following constituents were mixed in deionized water: proteose peptone (8.3 g/liter; Difco), d-glucose (3.5 g/liter; Mallinckrodt, Paris, Ky.), NaCl (2.05 g/liter; Mallinckrodt), KH2PO4 (0.6 g/liter; Sigma Chemical Company, St. Louis, Mo.), CaCl2 (0.11 g/liter; Fisher, Fair Lawn, N.J.), KCl (0.37 g/liter; Sigma), porcine bile (0.05 g/liter; Sigma), lysozyme (0.10 g/liter; Sigma), and pepsin (0.0133 g/liter; Sigma). The SGF was adjusted to pH 1.5 with HCl, filter sterilized through a 0.22-μm-pore-size membrane, and stored at 4°C until use. Previous experiments showed that storage of the SGF at 4°C for up to 7 days did not result in significant changes in bactericidal properties (data not shown).
Rate as a function of the proportion of SGF-medium.
A single E. coli strain, OB90520A, was prepared as described above and diluted in saline to approximately 106 CFU/ml, and then various volumes were added to saline and mixed with 37°C SGF to achieve proportions of SGF-saline (vol/vol) of 1 part SGF per 100 parts saline (1:100) to 100 parts SGF per 1 part saline (100:1). The final volume of the mixtures ranged from 40 to 50 ml. Samples were removed at predetermined time intervals, and E. coli O157:H7 cells were enumerated.
Rate as a function of the concentration of E. coli O157:H7.
E. coli OB90520A was prepared as described above and diluted in saline to predetermined concentrations, and then a 100:1 proportion of SGF-saline was made by mixing 0.5 ml of the diluted inoculum with 49.5 ml of SGF. Final concentrations of E. coli O157:H7 in saline ranged from 102 to 106 CFU/ml. At the 102 inoculum level, measurement sensitivity was increased by plating four 250-μl aliquots on TSA per time interval.
Rate as a function of E. coli O157:H7 strain.
The 10 individual E. coli O157:H7 and 6 Shigella isolates were prepared as described above and added to CGB, and then SGF was mixed with CGB at a 100:1 or 10:1 proportion for final E. coli O157:H7 concentrations of 105 to 106 CFU/ml. This same procedure was used to study strain variation in SGF-saline, except that E. coli O157:H7 strain GFP80EC was not used and the Shigella isolate used was S. flexneri 5348. The 100:1 and 10:1 SGF-saline or SGF-CGB mixtures were made in a final volume of 50 ml.
Rate as a function of antacid dose.
E. coli O157:H7 strain GFP80EC was prepared as described above and added to CGB. Tablets of a commercial antacid (Tums; Smithkline Beecham, Pittsburg, Pa.) were crushed with a mortar and pestle. The manufacturer's recommended dose was two tablets, which contained a total of 1,000 mg of calcium carbonate. Weighed quantities of antacid were added to CGB to simulate single-, half-, and double-prescribed doses. Negative controls did not receive antacid. The diluted E. coli O157:H7 culture and CGB+A were added to a stomacher bag and stomached for 2 min. Various volumes of SGF were mixed with the CGB+A medium at proportions of 1:1, 10:1, and 100:1, and then they were stomached for 1 min. In separate experiments, purified calcium carbonate (Fisher) was used in place of antacid at quantities equivalent to the amount of calcium carbonate in single-, half-, and double-prescribed doses of the commercial antacid.
Data analyses.
The surviving numbers of E. coli O157:H7 were enumerated on TSA, the counts were converted to log10 CFU/milliliter, and the data were recorded in an Excel spreadsheet (Microsoft Corp., Redmond, Wash.). Each experiment consisted of a minimum of two trials, two replicates, and duplicate platings per time interval. Primary curves were fit to data using DMFit software (courtesy of J. Baranyi, Institute of Food Research, Norwich, United Kingdom) and the dynamic D-model (4). The D-model can generate parameter values for lag time, inactivation or growth rate, and maximum population density. Linear and nonlinear regression analyses were performed using Excel software. Student's t test was used to measure differences among the means at a 95% confidence level. Means separations were performed using the pair-wise least significant difference (LSD) method.
RESULTS AND DISCUSSION
Inactivation of E. coli O157:H7 as a function of the proportion of SGF-saline.
A variety of physiological signals are involved in stimulating the stomach to produce as much as 1 to 2 liters of gastric fluid per day (17, 28). We tested the hypothesis that the inactivation rate of E. coli O157:H7 would be greater in higher proportions of SGF to medium.
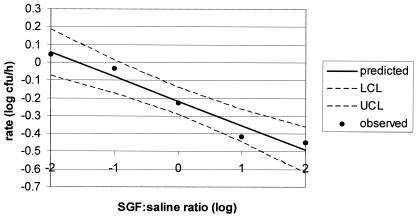
The change in E. coli O157:H7 strain OB90520A CFU over time (rate) in various proportions of SGF-saline followed a linear relationship: y = −0.137 × x + −0.217, where y is the rate (μ) and x is the log10 proportion of SGF to saline (R2 = 0.958) (Fig. 1). E. coli O157:H7 strain OB90520A was inactivated at all proportions of SGF-saline, except in 1:100 SGF-medium, where the growth rate was 0.043.
FIG. 1.
Inactivation of E. coli O157:H7 OB90520A as a function of the SGF-saline log ratio. UCL and LCL are the upper and lower confidence levels, respectively.
Inactivation of E. coli O157:H7 as a function of the concentration of E. coli O157:H7 in SGF-saline.
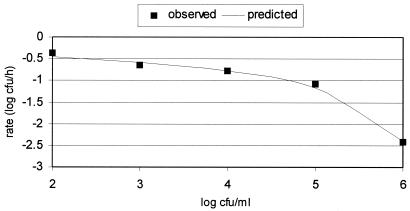
At a 100:1 proportion of SGF-saline, E. coli O157:H7 OB90520A was inactivated at an increasing rate as the concentration of E. coli O157:H7 in saline increased from 102 to 106 CFU/per ml (Fig. 2). This effect displayed a linear form from 102 to 105 CFU/ml, and then the inactivation rate sharply increased as cell numbers increased from 105 to 106 CFU/ml. This relationship could be represented with the following equation: y = 1/(a + b × x), where y is the rate (μ), x is the log concentration of E. coli O157:H7, and the coefficients a and b are −3.072 and 0.443, respectively.
FIG. 2.
Inactivation of E. coli O157:H7 OB90520A as a function of the concentration of E. coli O157:H7 in 100:1 SGF-saline.
I am not able to explain the basis of an increasing inactivation rate with increasing concentrations of E. coli O157:H7 in SGF-saline. One hypothesis is that a bactericidal factor(s) is released from lysed or whole cells that then exerts a concentration-dependent toxic effect on other cells, hence greater inactivation at higher cell concentrations. This finding deserves further study.
Variation in inactivation rate of E. coli O157:H7 strains and Shigella isolates in SGF-saline and SGF-CGB. (i) SGF-saline.
At a 100:1 proportion of SGF-saline, six of the nine E. coli O157:H7 strains were not significantly different among the E. coli O157:H7 strains tested (Table 1). The inactivation rate for strain OB1340 (mean value [X̄ μ] = −0.264) was significantly lower than that for strains OB90520A (X̄ μ = −1.807) and OB1514C1 (X̄ μ = −1.917). At a 10:1 proportion of SGF-saline, six of seven E. coli O157:H7 strains were not significantly different. The inactivation rate for strain OB1340 (X̄ μ = −1.739) was significantly higher among the seven strains. The average inactivation rate for all E. coli O157:H7 strains at a 100:1 proportion of SGF-saline was −1.344 ± 0.564 compared to −0.997 ± 0.388 at a 10:1 proportion of SGF-saline. These two mean values were not significantly different. In marked contrast to E. coli O157:H7 survival, the mean inactivation rate for S. flexneri strain 5348 at both 100:1 (X̄ μ = −8.784) and 10:1 (X̄ μ = −17.310) proportions of SGF to saline was 7-fold and 17-fold higher, respectively, than that for the mean of the nine E. coli O157:H7 strains.
TABLE 1.
Variation in inactivation rates of E. coli O157:H7 strains and S. flexneri strain 5348 in SGF-saline
| Strain | Ratio | Ratea | Significance |
|---|---|---|---|
| E. coli O157:H7 OB1340 | 100:1 | −0.264 (0.036) | Ab (2)c |
| E. coli O157:H7 OB90520A | 100:1 | −1.807 (1.316) | B (12) |
| 10:1 | −0.933 (0.354) | X (6) | |
| E. coli O157:H7 OB141412 | 100:1 | −1.563 (0.314) | A,B (2) |
| E. coli O157:H7 OB1525C | 100:1 | −1.749 (1.309) | A,B (7) |
| 10:1 | −1.176 (0.059) | X,Y (2) | |
| E. coli O157:H7 OB1423C | 100:1 | −1.738 (0.919) | A,B (6) |
| 10:1 | −0.947 (0.127) | X (2) | |
| E. coli O157:H7 OB1514C1 | 100:1 | −1.917 (0.721) | B (4) |
| 10:1 | −1.739 (0.147) | Y (2) | |
| E. coli O157:H7 OB1680G | 100:1 | −1.101 (0.435) | A,B (4) |
| 10:1 | −0.627 (0.007) | X (2) | |
| E. coli O157:H7 OB1533A | 100:1 | −0.708 (0.477) | A,B (4) |
| 10:1 | −0.575 (0.424) | X (2) | |
| E. coli O157:H7 DB1538 | 100:1 | −1.248 (0.428) | A,B (4) |
| 10:1 | −0.986 (0.305) | X (2) | |
| S. flexneri 5348 | 100:1 | −8.784 (3.133) | C (6) |
| 10:1 | −17.310 (0.000) | Z (2) |
Mean inactivation rate (standard deviation).
Means with the same letter are not significantly different. A, B, and C are for the 100:1 proportion, and X, Y and Z are for the 10:1 proportion.
Numbers in parentheses are the number of replicate experiments.
Roering et al. (24) studied the survival of three-strain mixtures of Salmonella enterica serovar Typhimurium DT104, Listeria monocytogenes, and E. coli O157:H7 in SGF. They observed a 1.7 to 2.8 log reduction in E. coli O157:H7 levels at 2 h of incubation compared to a 5.5 to 6.0 log reduction for S. enterica serovar Typhimurium DT104 and L. monocytogenes, respectively. Using an approximate 100:1 proportion of SGF-PW, their data show a mean inactivation rate for E. coli O157:H7 of −1.036 (±0.423), which was very similar to my reported mean of −1.344 (±0.564) in a similar ratio of SGF-saline. Similarly, Arnold and Kaspar (3) measured the percentage of survivors in 100 ml of SGF and described an average inactivation rate for five E. coli O157:H7 strains of −1.498. Koo et al. (19) examined the inactivation of Vibrio vulnificus strains in a different formulation of SGF (pH 3) than that reported by Beumer et al. (6) and observed an inactivation rate of −0.769.
(ii) SGF-CGB.
The average inactivation rate (−0.081 ± 0.068) for 10 E. coli O157:H7 strains in a 10:1 proportion of SGF-CGB was approximately 12-fold lower than that observed for E. coli O157:H7 in the same proportion of SGF-saline, indicating that CGB reduced the bactericidal effects of SGF (Table 2). This effect could have been due, in part, to the lower pH of SGF-saline (pH 1.5) compared to that of SGF-CGB (pH 2.4). Others have reported that the stomach pH may rise to 6.7 after ingesting a meal, but that the pH remains at 2.5 for at least 2 h, similar to the time intervals and pH used in my 10:1 proportion (11, 13, 26). Factors other than the pH of CGB may have inhibited bactericidal components of SGF, such as porcine bile, lysozyme, and pepsin.
TABLE 2.
Variation in inactivation and growth rates of E. coli O157:H7 and Shigella spp. strains in a 10:1 proportion of SGF-CGB
| Strain | Ratea | Significance |
|---|---|---|
| E. coli O157:H7 OB1340 | −0.019 (0.036) | A,Bb (6)c |
| E. coli O157:H7 OB90520A | 0.001 (0.073) | A (6) |
| E. coli O157:H7 OB141412 | −0.070 (0.053) | A,B,C (6) |
| E. coli O157:H7 OB1525C | −0.022 (0.042) | A,B (6) |
| E. coli O157:H7 OB1423C | −0.091 (0.032) | A,B,C (4) |
| E. coli O157:H7 OB1514C1 | −0.064 (0.054) | A,B,C (6) |
| E. coli O157:H7 OB1680G | −0.200 (0.198) | D,E (6) |
| E. coli O157:H7 OB1533A | −0.088 (0.054) | A,B,C (6) |
| E. coli O157:H7 DB1538 | −0.131 (0.073) | C,D (6) |
| E. coli O157:H7 GFP 80EC | −0.130 (0.071) | C,D (4) |
| Shigella spp.d | −0.300 (0.138) | E (16) |
Mean inactivation rate (standard deviation).
Means with the same letter are not significantly different.
Numbers in parentheses are the number of replicate experiments.
Mean rate for separate strains is given.
The mean inactivation rate (−0.300) for six Shigella isolates in SGF-CGB was approximately fourfold higher than that for the average of the 10 E. coli O157:H7 strains. These observations indicate that dose-response models developed for E. coli O157:H7 and based on human volunteer studies using non-E. coli O157:H7 species, where the pathogen is administered without food and/or with a pH neutralizing solution, should consider these differences and their effect on surrogate species data in E. coli O157:H7 risk assessment.
Waterman and Small (32) reported that 79% of a single E. coli O157:H7 strain survived when mixed with raw ground beef and Luria-Bertani broth at a 100:1 ratio for 2 h at 37°C and pH 2.5. In addition, they observed 41 and 95% survival for two S. flexneri strains. For an equivalent 2-h exposure in 10:1 SGF-CGB, the present studies showed an average of 69% survival for the nine E. coli O157:H7 strains and an average of 25% survival for the six Shigella isolates.
E. coli O157:H7 survival in SGF-CGB containing antacid.
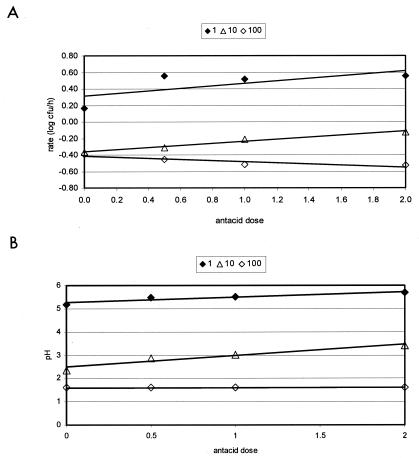
Linear regression models were used to compare the effects of SGF, antacid dose, and the proportion of SGF-CGB on the rate of E. coli O157:H7 GFP80EC survival (Fig. 3A and B). Overall, at the different proportions of SGF-CGB tested, the inactivation rate increased as the dose of antacid increased (Fig. 3A). Specifically, at 1:1, 10:1, and 100:1 proportions of SGF-CGB, the slope of the change in rate was 0.153, 0.126, and −0.064, respectively, as antacid increased from 0.5 to 2 times the prescribed dose. These same trends were observed for the matrix pH (Fig. 3B), indicating that a primary factor affecting E. coli O157:H7 survival in SGF-CGB is likely pH. Giannella et al. (14) have also suggested that the bactericidal activity of gastric fluid is primarily a function of HCl-mediated pH. However, Alm (1) proposed that the bactericidal effect of human gastric fluid is greater than that of saline adjusted to the same pH.
FIG. 3.
Effect of antacid dose on the (A) inactivation rate of E. coli O157:H7 GFP80EC and (B) matrix pH, with various proportions of SGF-CGB (♦, 1:1; triangle, 10:1; ⋄; 100:1).
In the commercial antacid product, calcium carbonate is indicated as the active ingredient that increases gastric pH. In separate experiments, equivalent amounts of calcium carbonate were used to simulate the quantity of calcium carbonate contained in 0, 0.5, 1, and 2 times the prescribed dose of antacid in a 10:1 SGF-CGB mixture. As the dose of calcium carbonate increased, the inactivation rate increased from −0.396 to −0.211, 0.027, and 0.196, respectively (data not shown). This rate of change (slope = 0.297) was approximately twofold higher than that for the comparable doses of the commercial antacid product (slope = 0.126), indicating that other undefined components of the commercial product may affect the growth and/or inactivation rate.
A number of reports show that previous bacterial growth environments have a pronounced effect on inducing resistance to subsequent acidic environments (5, 20, 21, 31). For example, Arnold and Kaspar (3) show that stationary-phase cells have greater acid resistance than logarithmic-phase cells. We inoculated SGF-medium with late-stationary-phase cells; therefore, our results likely estimate moderate acid resistance but possibly not the degree of resistance that would be produced under more challenging pH environments.
Takumi et al. (29) examined the inactivation of a single E. coli O157:H− strain and developed human exposure models as a function of published dynamic changes in stomach pH and food residence time. Using morpholineethanesulfonic acid as a surrogate for low gastric pH, E. coli O157 cells were challenged at static pH levels of 1.5, 2.0, 2.5, and 3.0. They reported that 2.2 and 0% of the cells survived after 1 and 2 h, respectively, at pH 1.5. In contrast, we observed an average of 75 and 50% survival among nine E. coli O157:H7 strains at 1 and 2 h, respectively, at pH 1.5 in SGF-saline.
The survival of E. coli O157 in the stomach is a function of a changing pH environment, where the fasting pH ranges from 1.3 to 1.7 and 6.2 to 6.7 after ingesting the meal and then to fasting pH values within an additional 2 to 4 h (13, 26). In addition, the gastric emptying time affects the exposure of bacteria to low pH. Clarkston et al. (11) reported that approximately 80% of a meal is transported through the stomach within 3 h of ingestion. Gordon and Small (15) estimated that bacteria in food would be exposed to a pH of 2.5 for approximately 2 h. Our experimental design included this time interval.
Takumi et al. (29) simulated a changing pH environment by using a pH-controlled fermentor and found that ∼50% of the bacteria survived for 120 to 150 min and that 4 h was required to eliminate all detectable quantities of the E. coli O157:H− strain. They suggest that modeling the effects of pH is adequate to model the effects of the stomach gastric fluid, even though other factors could play a role in killing. However, our data show that significant numbers of E. coli O157:H7 could remain in gastric fluid, even when assuming that the fluid would remain at pH 2.5 for 4 h.
de Jonge et al. (12) reported that there were no differences between acid-sensitive and acid-resistant E. coli O157 strains when they were added to a pH-controlled fermentor that gradually decreased pH to levels similar to that observed for gastric fluid in human volunteers after they ate a solid meal. In this environment, they found that acid resistance was acquired within 17 min.
The survival of any bacterial pathogen in the stomach cannot be modeled with 100% confidence due to the complex nature of diverse food matrices and individual human physiological conditions. However, it is possible to estimate pathogen behavior under controlled conditions that approximate different types of gastric conditions. We believe that these experiments provide more information than was previously available about the response of E. coli O157:H7 strains to conditions that may be present in the human stomach and which directly affect the numbers of pathogenic E. coli O157:H7 that gain access to sites of infection.
Acknowledgments
I thank Dustin Paul, Amy Slater, and Benne Marmer for technical assistance and James Smith for helpful discussions.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation of or endorsement by the U.S. Department of Agriculture.
REFERENCES
- 1.Alm, L. 1983. Survival rate of Salmonella and Shigella in fermented milk products with and without added human gastric juice: an in vitro study. Prog. Food Nutr. Sci. 7:19-28. [PubMed] [Google Scholar]
- 2.American Gastroenterological Association. 1995. Consensus conference statement: Escherichia coli O157:H7 infections-an emerging national health crisis, July 11-13, 1994. Gastroenterology 108:1923-1934. [PubMed] [Google Scholar]
- 3.Arnold, K. W., and C. W. Kaspar. 1995. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:2037-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranyi, J., and T. A. Roberts. 1994. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 23:277-294. [DOI] [PubMed] [Google Scholar]
- 5.Bearson, S., B. Bearson, and J. W. Foster. 1997. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 147:173-180. [DOI] [PubMed] [Google Scholar]
- 6.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 7.Bieber, D., S. W. Ramer, C.-Y. Wu, W. J. Murray, T. Tobe, R. R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. L., T. Ross, T. A. McMeekin, and P. D. Nichols. 1997. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int. J. Food Microbiol. 37:163-173. [DOI] [PubMed] [Google Scholar]
- 9.Cash, R. A., S. I. Music, J. P. Libonati, M. J. Snyder, R. P. Wenzel, and R. B. Hornick. 1974. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic response to a known inoculum. J. Infect. Dis. 129:45-52. [DOI] [PubMed] [Google Scholar]
- 10.Cassin, M. H., A. M. Lammerding, E. C. D. Todd, W. Ross, and R. S. McColl. 1998. Quantitative risk assessment for Escherichia coli O157:H7 in ground beef hamburgers. Int. J. Food Microbiol. 41:21-44. [DOI] [PubMed] [Google Scholar]
- 11.Clarkston, W. K., M. M. Pantano, J. E. Morley, M. Horowitz, J. M. Littlefield, and F. R. Burton. 1997. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am. J. Physiol. 272:R243-R248. [DOI] [PubMed] [Google Scholar]
- 12.de Jonge, R., K. Takumi, W. S. Ritmeester, and F. M. van Leusden. 2003. The adaptive response of Escherichia coli O157 in an environment with changing pH. J. Appl. Microbiol. 94:555-560. [DOI] [PubMed] [Google Scholar]
- 13.Dressman, J. B., R. R. Berardi, L. C. Dermentzoglou, T. L. Russell, S. P. Schmaltz, J. L. Barnett, and K. M. Jarvenpaa. 1990. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm. Res. 7:756-761. [DOI] [PubMed] [Google Scholar]
- 14.Giannella, R. A., S. A. Broitman, and N. Zamcheck. 1972. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 13:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, J., and P. L. Small. 1993. Acid resistance in enteric bacteria. Infect. Immun. 61:364-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt, P. 1985. Severe Salmonella infections in patients with reduced gastric acidity. Practitioner 229:1027-1030. [PubMed] [Google Scholar]
- 17.Johnson, L. R. 2001. Gastric secretion, p. 75-94. In L. R. Johnson (ed.), Gastrointestinal physiology, 6th ed. Mosby, St. Louis, Mo.
- 18.Just, J. R., and M. A. Daeschel. 2003. Antimicrobial effects of wine on Escherichia coli O157:H7 and Salmonella typhimurium in a model stomach system. J. Food Sci. 68:285-290. [Google Scholar]
- 19.Koo, J., A. DePaola, and D. L. Marshall. 2000. Effect of simulated gastric fluid and bile on survival of Vibrio vulnificus and Vibrio vulnificus phage. J. Food. Prot. 63:1665-1669. [DOI] [PubMed] [Google Scholar]
- 20.Lin, J., I. S. Lee, J. Frey, J. L. Slonczewski, and J. W. Foster. 1995. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 177:4097-4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, J., M. P. Smith, K. C. Chapin, H. S. Baik, G. N. Bennett, and J. W. Foster. 1996. Mechanisms of extreme acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neal, R. K., H. M. Scott, R. C. Slack, and R. R. A. Logan. 1996. Omeprazole as a risk factor for Campylobacter gastroenteritis: case control study. Br. Med. J. 312:414-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roering, A. M., J. B. Luchansky, A. M. Ihnot, S. E. Ansay, C. W. Kaspar, and S. C. Ingham. 1999. Comparative survival of Salmonella typhimurium DT104, Listeria monocytogenes, and Escherichia coli O157:H7 in preservative-free apple cider and simulated gastric fluid. Int. J. Food Microbiol. 46:263-269. [DOI] [PubMed] [Google Scholar]
- 25.Rotimi, V. O., L. Egwari, and B. Akande. 1990. Acidity and intestinal bacteria: an in-vitro assessment of the bactericidal activity of hydrochloric acid on intestinal pathogens. African J. Medic. Med. Sci. 19:275-280. [PubMed] [Google Scholar]
- 26.Russell, T. L., R. R. Berardi, J. L. Barnett, L. C. Dermentzoglou, K. M. Jarvenpaa, S. P. Schmaltz, and J. B. Dressman. 1993. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 10:187-196. [DOI] [PubMed] [Google Scholar]
- 27.Schiraldi, O., V. Benvestito, C. di Bari, R. Moschetta, and G. Pastore. 1974. Gastric abnormalities in cholera: epidemiological and clinical considerations. Bull. W. H. O. 51:349-352. [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, J. L. 2003. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J. Food Prot. 66:1292-1303. [DOI] [PubMed] [Google Scholar]
- 29.Takumi, K., R. de Jonge, and A. Havelaar. 2000. Modelling inactivation of Escherichia coli by low pH: application to passage through the stomach of young and elderly people. J. Appl. Microbiol. 89:935-943. [DOI] [PubMed] [Google Scholar]
- 30.Tamplin, M. L. 2002. Growth of Escherichia coli O157:H7 in raw ground beef stored at 10°C and the influence of competitive bacterial flora, strain variation, and fat level. J. Food Prot. 65:1535-1540. [DOI] [PubMed] [Google Scholar]
- 31.Uljas, H. E., and S. C. Ingham. 1998. Survival of Escherichia coli O157:H7 in synthetic gastric fluid after cold and acid habituation in apple juice or tryptic soy broth acidified with hydrochloric acid or organic acids. J. Food Prot. 61:939-947. [DOI] [PubMed] [Google Scholar]
- 32.Waterman, S. R., and P. L. Small. 1998. Acid-sensitive enteric pathogens are protected from killing under extreme acidic conditions of pH 2.5 when they are inoculated onto certain solid food sources. Appl. Environ. Microbiol. 64:3882-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaika, L. L., A. H. Kim, and L. Ford. 1991. Effect of sodium nitrite on growth of Shigella flexneri. J. Food Prot. 54:424-428. [DOI] [PubMed] [Google Scholar]