Abstract
BACKGROUND
Some observational studies have reported that transfusion of red-cell units that have been stored for more than 2 to 3 weeks is associated with serious, even fatal, adverse events. Patients undergoingcardiac surgery may be especially vulnerable to the adverse effects of transfusion.
METHODS
We conducted a randomized trial at multiple sites from 2010 to 2014. Participants 12 years of age or older who were undergoing complex cardiac surgery and were likely to undergo transfusion of red cells were randomly assigned to receive leukocyte-reduced red cells stored for 10 days or less (shorter-term storage group) or for 21 days or more (longer-term storage group) for all intraoperative and postoperative transfusions. The primary outcome was the change in Multiple Organ Dysfunction Score (MODS; range, 0 to 24, with higher scores indicating more severe organ dysfunction) from the preoperative score to the highest composite score through day 7 or the time of death or discharge.
RESULTS
The median storage time of red-cell units provided to the 1098 participants who received red-cell transfusion was 7 days in the shorter-term storage group and 28 days in the longer-term storage group. The mean change in MODS was an increase of 8.5 and 8.7 points, respectively (95% confidence interval for the difference, −0.6 to 0.3; P = 0.44). The 7-day mortality was 2.8% in the shorter-term storage group and 2.0% in the longer-term storage group (P = 0.43); 28-day mortality was 4.4% and 5.3%, respectively (P = 0.57). Adverse events did not differ significantly between groups except that hyperbilirubinemia was more common in the longer-term storage group.
CONCLUSIONS
The duration of red-cell storage was not associated with significant differences in the change in MODS. We did not find that the transfusion of red cells stored for 10 days or less was superior to the transfusion of red cells stored for 21 days or more among patients 12 years of age or older who were undergoing complex cardiac surgery. (Funded by the National Heart, Lung, and Blood Institute; RECESS ClinicalTrials.gov number, NCT00991341.)
THE OBJECTIVE OF RED-CELL TRANSFUSION is to increase oxygen delivery and improve clinical outcomes. However, in the United States, storage systems are licensed for up to 42 days on the basis of the estimated in vivo recovery of transfused red cells rather than on the basis of the clinical effectiveness of the transfusion.1 During storage, red cells undergo numerous changes.2,3 Some laboratory data suggest that red cells stored for longer periods may not traverse the microcirculation or deliver oxygen as effectively as those stored for shorter periods.4,5
Several observational studies have assessed the association between the duration of red-cell storage and patient outcomes, with differing results.6–10 A few randomized trials have been conducted, but most were not designed to detect differences in clinical end points.11,12 The largest randomized study published to date, Age of Red Blood Cells in Premature Infants, which included 377 low-birth-weight neonates randomly assigned to receive transfusions of red cells stored for no more than 8 days or to receive the standard of care, showed no significant between-group differences in adverse outcomes.13
Patients who have cardiac surgery often receive multiple units of red cells and may be especially vulnerable to end-organ injury because of compromised cardiac output or the proinflammatory state that follows cardiopulmonary bypass.14 A single-center retrospective study involving 6002 patients undergoing cardiac surgery showed that patients receiving red cells stored for more than 14 days, as compared with those receiving cells stored for 14 days or less, had an increased incidence of several adverse outcomes.15 Three other retrospective studies of the effect of the duration of red-cell storage in patients undergoing cardiac surgery showed no significant differences in outcomes.16–18 We designed the Red-Cell Storage Duration Study (RECESS) to compare clinical outcomes after cardiac surgery in patients who received transfused red cells stored for 10 days or less or for 21 days or more.
Methods
Oversight
RECESS was a multicenter, prospective, randomized clinical trial conducted by the Transfusion Medicine and Hemostasis Clinical Trials Network (TMHCTN) without commercial support. A TMHCTN subcommittee designed the study; the design and rationale have been described previously.19 The study was approved by the institutional review board at each participating hospital. Study participants or the parents or guardians of minors provided written informed consent, and minors provided assent. Site coordinators gathered data and submitted results electronically to a data-coordinating center. A data and safety monitoring board conducted a quarterly review of the primary outcome, mortality, and data on adverse events (see the Supplementary Appendix, available with the full text of this article at NEJM.org, for interim monitoring boundaries). The lead author and the TMHCTN subcommittee wrote the first draft of the manuscript, which was reviewed by all the authors. The lead author vouches for the completeness and accuracy of the data and adherence to the study protocol. The protocol, which includes the statistical analysis plan, is available at NEJM.org.
Study Patients
Participants were required to be 12 years of age or older, weigh 40 kg or more, and be scheduled for complex cardiac surgery with planned median sternotomy. Patients 18 years of age or older were also required to have a score of 3 or higher on the Transfusion Risk Understanding Scoring Tool (TRUST), which corresponds to a likelihood of receiving a red-cell transfusion during surgery or on the first day after surgery of 60% or more.20 TRUST scores range from 0 to 8 points, with higher scores indicating a greater probability of red-cell transfusion (see Table S1 in the Supplementary Appendix, available at NEJM.org). Key criteria for exclusion included planned use of autologous or directed donations, washed or volume-reduced blood components, or blood components from which additive solution had been removed; severe renal dysfunction; use of an intraaortic balloon pump for treatment of cardiogenic shock; planned deep hypothermic circulatory arrest; previous red-cell transfusion during the current admission; or concurrent or recent enrollment in another clinical study. Detailed eligibility criteria are listed in the Supplementary Appendix.
Study Design and Treatment Protocols
Participants were randomly assigned, in a 1:1 ratio, to receive red-cell units stored for 10 days or less or red-cell units stored for 21 days or more for all transfusions from randomization through postoperative day 28, hospital discharge, or death, whichever occurred first. Randomization occurred no earlier than 1 day before scheduled surgery and was performed only if the transfusion service had enough units of red cells stored for both durations to meet the cross-match request. Thus, participants who were assigned to the group receiving units with a longer storage period received the same units they would have received under the standard inventory practice of first-in–first-out, in which the oldest units are used first. If units that had been stored for the assigned duration were unavailable for any of the transfusions, units that had been stored for a period that matched the assigned storage duration as closely as possible were selected. All units were leukoreduced before storage and were stored in additive solutions AS1, AS3, or AS5 (see Table S2 in the Supplementary Appendix for details).21 The decision of whether to provide a red-cell transfusion was made at the discretion of the clinician.
Randomization was performed with the use of permuted blocks of varying sizes, with stratification according to age (<18 years vs. ≥18 years) and status with respect to admission to the intensive care unit (ICU) at the time of randomization and was balanced by site with the use of a centralized computer system.22 Although only the transfusion service was aware of each participant’s assigned treatment, the expiration dates on the red-cell units were not obscured, in accordance with regulatory requirements.
Assessments, Monitoring, and Outcome Measures
The primary outcome was the change in the Multiple Organ Dysfunction Score (MODS; range, 0 to 24 points, with higher scores indicating more severe organ dysfunction)23 from the preoperative baseline through postoperative day 7, hospital discharge, or death, whichever occurred first (referred to as the 7-day change in MODS), among patients who met the criteria for evaluation (defined as patients who underwent cardiac surgery within 30 days after randomization and received at least one red-cell transfusion between randomization and postoperative hour 96). Participants who did not receive red cells within this time frame were excluded from further follow-up. The use of MODS has been validated in patients in the ICU and was used in a previous trial of red-cell transfusion in patients with cardiovascular disease who were in the ICU.24 The score is sensitive to minor changes in clinical status and incorporates death. The 7-day MODS was calculated as the sum of the worst postoperative scores from each of the six organ systems included, through postoperative day 7 (or hospital discharge or death) (see Table S3 in the Supplementary Appendix for the organ systems included and the way in which function is scored). These worst component scores could occur on different days. The maximum score of 24 was assigned to participants who died in the hospital by day 7. Data were collected on all serious adverse events and on prespecified types of nonserious adverse events.
Statistical Analyses
As specified in the protocol, all planned analyses were limited to participants who met the criteria for evaluation. Unless otherwise specified, an intention-to-treat analysis was conducted with data from all participants who met the criteria for evaluation, with data analyzed according to the participants’ assigned treatment groups even if some or all red-cell transfusions were not performed as assigned or if the surgery performed deviated from that originally planned. An additional intention-to-treat analysis of the 4-day change in MODS included all participants who underwent randomization and underwent cardiac surgery, even if they did not receive a transfusion. Analyses were performed at New England Research Institutes (the TMHCTN data-coordinating center) with the use of SAS software, version 9.3 (SAS Institute).
The target sample size of 1170 participants who met the criteria for evaluation was intended to achieve a two-sided 95% confidence interval of approximately ±0.85 MODS points for the between-group difference in the 7-day change in MODS if the standard deviation in each group was 7.3 points, which is the standard deviation observed in a previous study of ICU patients with cardiovascular disease.24 This sample size allowed for 3% inflation for interim monitoring.
Data on the primary outcome were compared with the use of an analysis of covariance (ANCOVA), with adjustment for the baseline MODS. A planned secondary per-protocol analysis excluded participants who received any red-cell unit between randomization and postoperative day 7 that did not meet the assigned criterion for storage duration. Subgroup analyses of the primary outcome were performed for seven prespecified subgroups and four additional subgroups by means of interaction tests.
Between-group differences in binary and categorical variables were compared with the use of Fisher’s exact test. Ordinal variables were compared with the use of Mantel–Haenzel tests. Continuous variables were compared with the use of Kruskal–Wallis tests for unadjusted analyses and ANCOVA for adjusted analyses. Owing to skewness, changes in serum bilirubin levels were compared in two ways: with ANCOVA to adjust for baseline values and with the Kruskal–Wallis test. Time-to-event outcomes were compared with the use of Cox regression.
Results
Patients and Treatment Assignments
From January 2010 through January 2014, a total of 1709 patients at 33 U.S. hospitals consented to participate in the study, and 1481 underwent randomization; 538 participants who met the criteria for evaluation were included in the group receiving units stored for 10 days or less (shorter-term storage group) and 560 participants who met the criteria for evaluation were included in the group receiving units stored for 21 days or more (longer-term storage group) (Fig. S1 in the Supplementary Appendix). There were 383 participants who did not meet the criteria for evaluation, in most cases because they did not receive red-cell transfusions. Study enrollment was halted before 1170 participants who met the criteria for evaluation were included owing to time constraints on the funding of the study.
The baseline characteristics of the participants and the characteristics of the surgical procedures performed were similar in the two treatment groups (Table 1). Male participants made up 43% of the study population. The median age was 72 years (range, 14 to 93). The mean baseline MODS was 0.7 points.
Table 1.
Baseline Characteristics and Surgical Procedures of the Study Participants.*
| Characteristic | Red-Cell Storage ≤10 Days (N = 538) |
Red-Cell Storage ≥21 Days (N = 560) |
|---|---|---|
| Demographic data | ||
|
| ||
| Age — yr | ||
|
| ||
| Median | 73 | 72 |
|
| ||
| Interquartile range | 66–80 | 65–79 |
|
| ||
| Male sex — no. (%) | 228 (42) | 247 (44) |
|
| ||
| White race — no./total no. (%)† | 485/534 (91) | 493/545 (90) |
|
| ||
| Clinical data | ||
|
| ||
| Weight — kg | ||
|
| ||
| Median | 75 | 74 |
|
| ||
| Interquartile range | 65–89 | 66–86 |
|
| ||
| Hemoglobin — g/liter‡ | ||
|
| ||
| Median | 11.7 | 12.0 |
|
| ||
| Interquartile range | 10.7–12.8 | 10.8–13.0 |
|
| ||
| Creatinine — mg/dl§ | ||
|
| ||
| Median | 1.1 | 1.0 |
|
| ||
| Interquartile range | 0.8–1.4 | 0.8–1.4 |
|
| ||
| Platelet count — per mm3¶ | ||
|
| ||
| Median | 207,000 | 209,000 |
|
| ||
| Interquartile range | 166,000–254,000 | 163,000–258,000 |
|
| ||
| Bilirubin — mg/dl‖ | ||
|
| ||
| Median | 0.6 | 0.6 |
|
| ||
| Interquartile range | 0.4–0.9 | 0.4–0.8 |
|
| ||
| ABO blood group — no. (%) | ||
|
| ||
| Group O | 209 (39) | 235 (42) |
|
| ||
| Group A | 240 (45) | 241 (43) |
|
| ||
| Group B | 64 (12) | 63 (11) |
|
| ||
| Group AB | 25 (5) | 21 (4) |
|
| ||
| Minimum TRUST score** | ||
|
| ||
| Median | 4 | 4 |
|
| ||
| Interquartile range | 3–5 | 3–5 |
|
| ||
| Baseline MODS | 0.7±0.8 | 0.6±0.8 |
|
| ||
| In ICU at randomization — no. (%)†† | 33 (6) | 34 (6) |
|
| ||
| Surgical characteristics | ||
|
| ||
| Procedure — no. (%) | ||
|
| ||
| CABG plus valve repair or replacement plus other | 69 (13) | 76 (14) |
|
| ||
| CABG plus valve repair or replacement 110 (20) 119 (21) | ||
|
| ||
| CABG plus other | 46 (9) | 49 (9) |
|
| ||
| CABG | 126 (23) | 127 (23) |
|
| ||
| Valve repair or replacement plus other | 91 (17) | 95 (17) |
|
| ||
| Valve repair or replacement | 91 (17) | 81 (14) |
|
| ||
| Other | 5 (1) | 13 (2) |
|
| ||
| Type of surgical access — no. (%) | ||
|
| ||
| Primary sternotomy | 384 (71) | 410 (73) |
|
| ||
| Repeat sternotomy | 150 (28) | 143 (26) |
|
| ||
| Thoractomy | 3 (1) | 2 (<1) |
|
| ||
| Minimally invasive | 1 (<1) | 5 (1) |
|
| ||
| Cardiopulmonary bypass — no. (%) | 516 (96) | 535 (96) |
|
| ||
| Duration of cardiopulmonary bypass — min‡‡ | ||
|
| ||
| Median | 141 | 139 |
|
| ||
| Interquartile range | 99–187 | 105–194 |
Plus–minus values are means ±SD. P values for all comparisons were greater than 0.05 unless noted otherwise. To convert the values for bilirubin to micromoles per liter, multiply by 17.1; to convert the values for creatinine to micromoles per liter, multiply by 88.4. CABG denotes coronary-artery bypass grafting.
Race was self-reported.
Data on hemoglobin level were not available for three participants in the group receiving red cells stored for 10 days or less.
Data on creatinine level were not available for five participants in the group receiving red cells stored for 10 days or less and for two participants in the group receiving red cells stored for 21 days or more.
Data on platelet count level were not available for three participants in the group receiving red cells stored for 10 days or less.
Data on bilirubin level were not available for 19 participants in the group receiving red cells stored for 10 days or less and for 10 participants in the group receiving red cells stored for 21 days or more.
If data on any components of the Transfusion Risk Understanding Scoring Tool (TRUST) score were not available, the score for that component was assumed to be 0. Data on the duration of cardiopulmonary bypass were not available for one participant in the group receiving red cells stored for 10 days or less. To be eligible for study participation, patients 18 years of age or older were required to have a TRUST score of 3 or higher, which corresponds to a likelihood of receiving a red-cell transfusion during surgery or on the first day after surgery of 60% or more. P = 0.01 by the Kruskal–Wallis test for the between-group comparison of the distribution of TRUST scores.
No P value was calculated for the variable of presence in the intensive care unit (ICU) because randomization was stratified according to this factor.
Data on the duration of cardiopulmonary bypass applies to participants for whom the surgery was performed with the use of this technique.
Blood Components Received
The number of red-cell units transfused per patient did not differ significantly between the treatment groups, nor did the proportion of patients who received a large number of units (≥8 units) (Fig. S2 in the Supplementary Appendix). The 25th, 50th, and 75th percentiles for the number of units received were 2, 4, and 6 in the shorter-term storage group and 2, 3, and 6 in the longer-term storage group (P = 0.80). Through postoperative day 7, the median number of units transfused in each group was 3 (P = 0.86).
The percentage of transfused red-cell units that had the assigned storage duration is shown in Figure 1. The mean (±SD) durations of storage were 7.8±4.8 days in the shorter-term storage group and 28.3±6.7 days in the longer-term storage group. The percentage of patients who underwent transfusion of red-cell units with the assigned storage duration is shown in Table S4 and Figure S3 in the Supplementary Appendix. A total of 478 participants (89%) in the shorter-term storage group and 488 participants (87%) in the longer-term storage group received only units of the assigned storage duration.
Figure 1. Storage Duration of Red-Cell Units, According to Study Group.
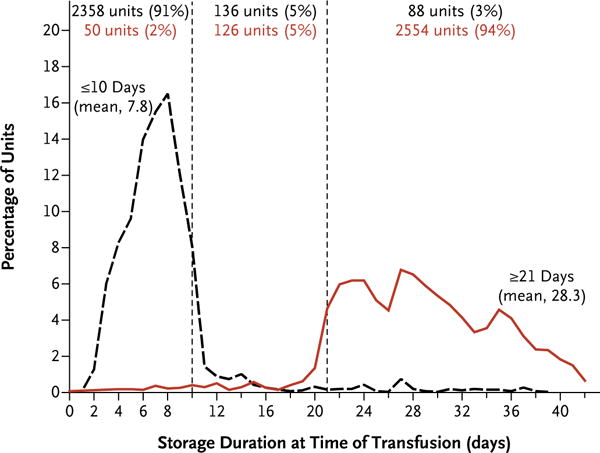
The black dashed line indicates the distribution of storage durations for red-cell units transfused in participants who were randomly assigned to receive blood stored for 10 days or less, and the red line indicates the distribution of storage durations for red-cell units transfused in participants assigned to receive blood stored for 21 days or longer.
There was no significant between-group difference with respect to receipt of blood components other than red cells. In the shorter-term storage group, 49% of the patients received platelets, 47% received plasma, and 20% received cryoprecipitate; the corresponding proportions in the longer-term storage group were 53%, 50%, and 21% (P≥0.20 for all comparisons).
Primary Outcome
Among the 1087 participants for whom there were complete data for the calculation of the 7-day change in MODS, the mean 7-day change was 8.5 points in the shorter-term storage group and 8.7 points in the longer-term storage group. In an analysis adjusted for the baseline MODS, the difference in the means was −0.2 points — that is, a difference of 0.2 points in favor of shorter-term storage (95% confidence interval [CI] for difference, −0.6 to 0.3; P = 0.44) (Table 2). When the change in MODS was restricted to post-transfusion measurements, there was still no significant between-group difference (with a mean change in MODS of 7.7 points in the shorter-term storage group and 7.6 points in the longer-term storage group; adjusted difference, 0.1; 95% CI, −0.4 to 0.6; P = 0.76).
Table 2.
Primary and Secondary Outcomes.*
| Outcome | Red-Cell Storage ≤10 Days (N = 538) |
Red-Cell Storage ≥21 Days Effect (N = 560) |
Estimated Treatment | P Value |
|---|---|---|---|---|
| Primary outcome: ΔMODS at 7 days† | 8.5±3.6 | 8.7±3.6 | −0.2 (−0.6 to 0.3) | 0.44 |
|
| ||||
| Secondary outcomes‡ | ||||
|
| ||||
| ΔMODS at 28 days | 8.7±4.0 | 9.1±4.2 | −0.3 (−0.8 to 0.2) | 0.20 |
|
| ||||
| All-cause mortality — no. (%) | ||||
|
| ||||
| 7 Days | 15 (2.8) | 11 (2.0) | 0.8 (−1.0 to 2.7) | 0.43 |
|
| ||||
| 28 Days | 23 (4.4) | 29 (5.3) | −0.9 (−3.4 to 1.7) | 0.57 |
|
| ||||
| Median stay in ICU — days§ | 3 | 3 | 1.07 (0.95 to 1.21) | 0.27 |
|
| ||||
| Median stay in hospital — days§ | 8 | 8 | 0.99 (0.88 to 1.13) | 0.92 |
Plus–minus values are unadjusted means ±SD. Unless otherwise noted, all outcomes were assessed through postoperative day 7, hospital discharge, study withdrawal, or death, whichever occurred first. The group receiving red cells stored for 21 days or more is the reference group. Analysis of covariance was adjusted for baseline value.
For the change in MODS at 7 days, data were unavailable for four participants in the group assigned to receive red cells stored for 10 days or less and for seven in the group assigned to receive red cells stored for 21 days or more.
Data on the change in MODS at 28 days were unavailable for 7 participants in the group assigned to receive red cells stored for 10 days or less and for 5 in the group assigned to receive red cells stored for 21 days or more. Data on all-cause mortality through 7 days were unavailable for 7 participants in the group assigned to receive red cells stored for 10 days or less and for 4 in the group assigned to receive red cells stored for 21 days or more; data on all-cause mortality through 28 days were unavailable for 14 participants in the group assigned to receive red cells stored for 10 days or less and for 9 in the group assigned to receive red cells stored for 21 days or more.
Length of stay was measured from date of surgery through day 28±3, death, hospital discharge, or the end of the study, whichever occurred first. For these outcomes, the estimated treatment effect was calculated as a hazard ratio with the use of a Cox model.
The only component of the 7-day change in MODS for which there was a significant between-group difference was the hepatic component, which is based on total serum bilirubin level (Table S5 in the Supplementary Appendix). The mean changes in MODS for this component were 0.5 points in the shorter-term storage group and 0.7 points in the longer-term storage group (P<0.001).
In the secondary per-protocol analysis, which was limited to the 967 participants who received only units that were stored within the assigned storage duration window and for whom data on the 7-day change in MODS were available, the mean 7-day change in MODS was 8.2 points in the shorter-term storage group and 8.5 points in the longer-term storage group. After adjustment for baseline MODS, the difference in means was −0.3 points (95% CI, −0.7 to 0.1; P = 0.17).
All subgroup analyses had P values greater than 0.05 for interaction, indicating that the effects of red-cell storage duration did not differ significantly on the basis of any of these patient characteristics (Fig. 2). An analysis of all participants who underwent surgery, regardless of whether they received a transfusion, showed that the mean 4-day change in MODS was 7.8 points in the shorter-term storage group and 8.0 points in the longer-term storage group; the adjusted difference in means was −0.2 points (95% CI, −0.6 to 0.1; P = 0.20).
Figure 2. Between-Group Differences in 7-Day Change in MODS.
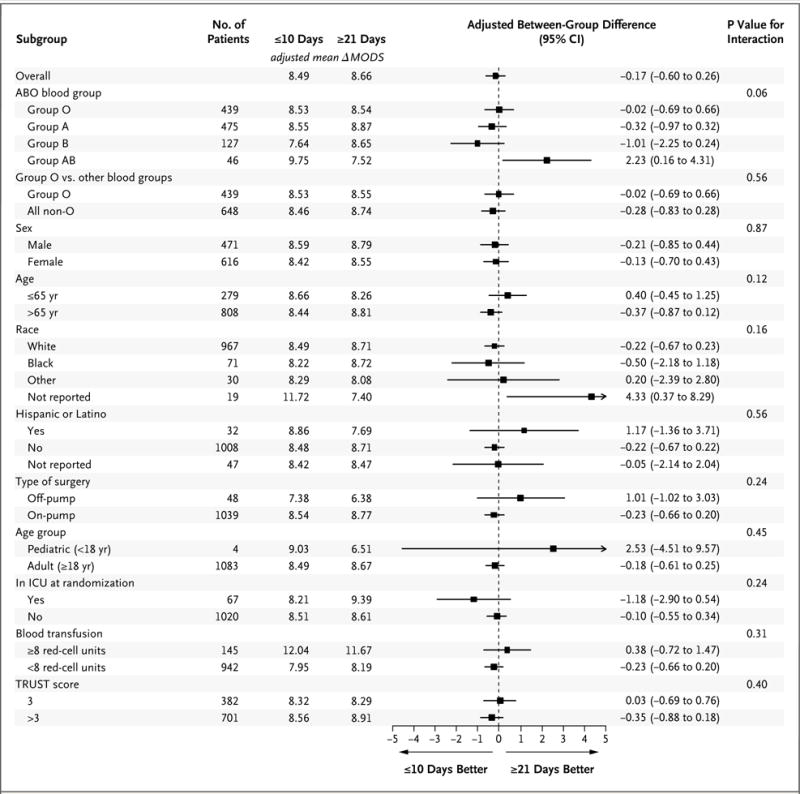
All subgroup analyses, except for the analysis of group O versus other blood groups, age (≤65 years vs. >65 years), receipt of 8 or more units of red cells versus less than 8 units, and score on the Transfusion Risk Understanding Scoring Tool (TRUST) were prespecified in the protocol. Scores on the Multiple Organ Dysfunction Score (MODS) range from 0 to 24 points, with higher scores indicating more severe organ dysfunction. ΔMODS denotes the change in MODS. Race and ethnic group were self-reported. TRUST scores range from 0 to 8, with higher scores indicating a greater probability of red-cell transfusion. A TRUST score of 3 or higher corresponds to a likelihood of receiving a red-cell transfusion during surgery or on the first day after surgery of 60% or more.
Secondary Outcomes
All-cause mortality was similar in the two groups (Table 2). Among the 1087 participants for whom there were complete data on 7-day mortality, 15 participants in the shorter-term storage group (2.8%) and 11 in the longer-term storage group (2.0%) died by postoperative day 7 (P = 0.43). Among the 1075 participants for whom there were complete data on 28-day mortality, 23 in the shorter-term storage group (4.4%) and 29 in the longer-term storage group (5.3%) died (P = 0.57). In a time-to-event analysis of all 1098 participants, the time to death did not differ significantly between the two groups (hazard ratio, 0.83; 95% CI, 0.48 to 1.44; P = 0.50) (Fig. S4 in the Supplementary Appendix).
There were no significant between-group differences in the 28-day change in MODS, the length of hospital stay, or the length of ICU stay. The change in total serum bilirubin level was significantly higher in the longer-term storage group than in the shorter-term storage group (1.5 mg per deciliter vs. 0.8 mg per deciliter [26 μmol per liter vs. 14 μmol per liter]; P<0.01 by both the Kruskal-Wallis test and ANCOVA).
Adverse Events
The mean number of serious adverse events per participant was 1.6 in both groups (P = 0.75). Participants in the shorter-term storage group were less likely than those in the longer-term storage group to have a serious adverse event related to a hepatobiliary disorder (5% vs. 9%, P = 0.02), and this finding was due entirely to the fact that fewer participants in the shorter-term storage group had hyperbilirubinemia. There was no significant difference in the numbers of participants in the two groups with serious or nonserious adverse events related to any other Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (Table 3, and Table S6 in the Supplementary Appendix).
Table 3.
Adverse Events Occurring in at Least 50 Participants.*
| Adverse Event | Red-Cell Storage ≤10 Days (N = 538) |
Red-Cell Storage ≥21 Days (N = 560) |
P Value |
|---|---|---|---|
| no. of patients (%) | |||
|
| |||
| Serious event | |||
|
| |||
| At least one event | 283 (53) | 288 (51) | 0.72 |
|
| |||
| Cardiac disorders | 111 (21) | 132 (24) | 0.25 |
|
| |||
| Hepatobiliary disorders | 29 (5) | 51 (9) | 0.02 |
|
| |||
| Infections and infestations | 42 (8) | 49 (9) | 0.59 |
|
| |||
| Injury, poisoning, and procedural complications | 29 (5) | 41 (7) | 0.22 |
|
| |||
| Investigations | 40 (7) | 39 (7) | 0.82 |
|
| |||
| Metabolism and nutrition disorders | 72 (13) | 67 (12) | 0.53 |
|
| |||
| Renal and urinary disorders | 38 (7) | 36 (6) | 0.72 |
|
| |||
| Respiratory, thoracic, and mediastinal disorders | 106 (20) | 109 (19) | 0.94 |
|
| |||
| Vascular disorders | 124 (23) | 116 (21) | 0.38 |
|
| |||
| Other event | |||
|
| |||
| At least one event | 324 (60) | 334 (60) | 0.85 |
|
| |||
| Cardiac disorder | 57 (11) | 61 (11) | 0.92 |
|
| |||
| Hepatobiliary disorder | 78 (14) | 99 (18) | 0.16 |
|
| |||
| Infection or infestation | 22 (4) | 22 (4) | 1.00 |
|
| |||
| Injury, poisoning, or procedural complication | 13 (2) | 18 (3) | 0.47 |
|
| |||
| Investigations | 38 (7) | 30 (5) | 0.26 |
|
| |||
| Metabolism and nutrition disorder | 209 (39) | 210 (38) | 0.66 |
|
| |||
| Renal and urinary disorder | 25 (5) | 22 (4) | 0.66 |
|
| |||
| Respiratory, thoracic, and mediastinal disorder | 53 (10) | 53 (9) | 0.84 |
|
| |||
| Vascular disorder | 98 (18) | 99 (18) | 0.88 |
Adverse events were categorized with the use of Medical Dictionary for Regulatory Activities (MedDRA) System Organ Classes. Information on adverse events in all organ-system classes is available in Table S6 in the Supplementary Appendix.
Discussion
RECESS was a randomized trial that focused on the clinical effect of the duration of red-cell storage on patients undergoing cardiac surgery. The change in MODS was chosen as the primary outcome for the study since it is based on objective measurements, reflects even small physiologic changes, and has been validated. We used a composite of the worst scores for each organ system to address the possibility that the six organ systems might exhibit dyssynchronous changes.
A between-group difference of 1 point or less in the change in MODS is unlikely to be clinically significant or to warrant a major change in the practice of blood banking.11 In this study, the estimated between-group difference in the change in MODS was −0.2 points (with a confidence interval of −0.6 to 0.3), which is clearly not a difference of any clinical importance. Only the hepatic component of MODS, assessed by means of total serum bilirubin level, differed significantly between the groups. This finding is not unexpected, because red cells hemolyze during storage. Once transfused, stored red cells also have higher rates of in vivo hemolysis because of increased fragility.25 Similarly, no significant between-group differences were detected for any secondary outcomes except those related to hyperbilirubinemia.
There was minimal overlap in the duration of red-cell storage between the two groups, and there was a difference of 20 days in the mean duration of storage. In the United States, the average storage duration of transfused red cells is 17.9 days.26 The mean storage durations of the red-cell units used in the two study groups bracketed this average but were within the range of the storage duration for red cells commonly available for transfusion, and there were no significant between-group differences in adverse outcomes. The number of red-cell units transfused per participant and the proportion of participants who received a high number of units (≥8) were similar in the two groups. These findings are important because a greater number of transfused units is associated with a higher rate of adverse outcomes.27 The characteristics of the surgical procedures performed in the two groups were also similar.
One limitation of our study was that regulations prevented us from obscuring the expiration date of each red-cell unit. However, storage duration is not included on the unit labels, and adherence to the assignment in both study groups was excellent. In addition, the components of MODS are objective physiological measurements that would not be affected by awareness of group assignment. Another limitation was that it was not feasible to power the study to detect differences in mortality or other infrequent clinical events. However, the differences observed for these outcomes, including mortality, were very small and were not uniformly in favor of one group.
RECESS was not designed to compare the effects of red cells transfused near the end of the allowed storage period (e.g., 35 to 42 days) with the effects of those transfused after being stored for 10 days or less. Thus, we cannot exclude the possibility that transfusion of the oldest red cells would have an adverse effect. However, in our study, very few units stored for 35 days or more were transfused, a practice that is representative of the distribution of the U.S. inventory.26
Another large randomized trial of the effect of storage duration on red cells (Age of Red Blood Cells in Premature Infants)13 also showed no significant differences in any of the measured clinical outcomes among premature, low-birth-weight infants. The results of randomized clinical trials in different patient groups do not support restricting red-cell transfusion to units stored for a shorter period than that indicated by the current licensed expiration dates. However, there may be other patient populations in which red-cell storage duration does have a clinical effect, a possibility that is being investigated in the Age of Blood Evaluation trial28 and the Age of Blood in Children in Pediatric Intensive Care Units trial (ClinicalTrials.gov number, NCT01977547). In any case, the results of our study do not support the preferential transfusion of red cells with shorter storage periods in patients 12 years of age or older who are undergoing complex cardiac surgery.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute.
APPENDIX
The authors’ full names and academic degrees are as follows: Marie E. Steiner, M.D., Paul M. Ness, M.D., Susan F. Assmann, Ph.D., Darrell J. Triulzi, M.D., Steven R. Sloan, M.D., Ph.D., Meghan Delaney, D.O., M.P.H., Suzanne Granger, M.S., Elliott Bennett-Guerrero, M.D., Morris A. Blajchman, M.D., Vincent Scavo, M.D., Jeffrey L. Carson, M.D., Jerrold H. Levy, M.D., Glenn Whitman, M.D., Pamela D’Andrea, R.N., Shelley Pulkrabek, M.T., C.C.R.C., Thomas L. Ortel, M.D., Ph.D., Larissa Bornikova, M.D., Thomas Raife, M.D., Kathleen E. Puca, M.D., Richard M. Kaufman, M.D., Gregory A. Nuttall, M.D., Pampee P. Young, M.D., Ph.D., Samuel Youssef, M.D., Richard Engelman, M.D., Philip E. Greilich, M.D., Ronald Miles, M.D., Cassandra D. Josephson, M.D., Arthur Bracey, M.D., Rhonda Cooke, M.D., Jeffrey McCullough, M.D., Robert Hunsaker, M.D., Lynne Uhl, M.D., Janice G. McFarland, M.D., Yara Park, M.D., Melissa M. Cushing, M.D., Charles T. Klodell, M.D., Ravindra Karanam, M.D., Pamela R. Roberts, M.D., Cornelius Dyke, M.D., Eldad A. Hod, M.D., and Christopher P. Stowell, M.D., Ph.D.
The authors’ affiliations are as follows: Fairview–University Medical Center, Minneapolis (M.E.S., S.P., J.M.), and Mayo Clinic, Rochester (G.A.N.) — both in Minnesota; Johns Hopkins University (P.M.N., G.W.) and University of Maryland (R.C.) — both in Baltimore; New England Research Institutes, Data Coordinating Center, Watertown (S.F.A., S.G.), Boston Children’s Hospital (S.R.S.), Massachusetts General Hospital (L.B., C.P.S.), Brigham and Women’s Hospital (R.M.K.), Tufts University (R.E.), St. Elizabeth’s Medical Center (R.H.), and Beth Israel Deaconess Medical Center (L.U.), Boston, and Baystate Medical Center, Springfield (R.E.) — all in Massachusetts; University of Pittsburgh and University of Pittsburgh–Mercy Hospital, Pittsburgh (D.J.T., P.D.); Puget Sound Blood Center and University of Washington (M.D.) and Swedish Medical Center (S.Y.) — all in Seattle; Duke University, Durham (E.B.-G., J.H.L., T.L.O.), and University of North Carolina, Chapel Hill (Y.P.) — both in North Carolina; McMaster University, Hamilton, ON, Canada (M.A.B.); Indiana–Ohio Heart and St. Joseph Hospital (V.S.) — both in Fort Wayne, IN; Rutgers Robert Wood Johnson Medical School, New Brunswick (J.L.C.), and Newark Beth Israel Medical Center, Newark (R.K.) — both in New Jersey; University of Iowa, Iowa City (T.R.); Aurora St. Luke’s Medical Center (K.E.P.) and Froedert Memorial Lutheran Hospital (J.G.M.), Milwaukee, and Aspirus Heart and Vascular Institute, Wausau (R.M.) — all in Wisconsin; Vanderbilt University, Nashville (P.P.Y.); University of Texas Southwestern Medical Center, Dallas (P.E.G.); Children’s Healthcare of Atlanta, Emory University, and Emory University Hospital, Atlanta (C.D.J.); St. Luke’s–Texas Heart Institute, Houston (A.B.); Weill Cornell Medical College (M.M.C.) and Columbia University Medical Center (E.A.H.) — both in New York; University of Florida, Gainesville (C.T.K.); University of Oklahoma, Oklahoma City (P.R.R.); and University of North Dakota School of Medicine and Health Sciences, Fargo (C.D.).
Footnotes
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
The investigators and staff who participated in the Red-Cell Storage Duration Study (RECESS) are listed in the Supplementary Appendix, available at NEJM.org.
A Quick Take summary is available at NEJM.org
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91:13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 2.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somani A, Steiner ME, Hebbel RP. The dynamic regulation of microcirculatory conduit function: features relevant to transfusion medicine. Transfus Apher Sci. 2010;43:61–8. doi: 10.1016/j.transci.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai AG, Hofmann A, Cabrales P, Intaglietta M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: the experimental evidence. Transfus Apher Sci. 2010;43:69–78. doi: 10.1016/j.transci.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triulzi DJ, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Apher Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 8.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–94. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 9.Vamvakas EC. Meta-analysis of clinical studies of the purported deleterious effects of “old” (versus “fresh”) red blood cells: are we at equipoise? Transfusion. 2010;50:600–10. doi: 10.1111/j.1537-2995.2009.02465.x. [DOI] [PubMed] [Google Scholar]
- 10.van de Watering L. Red cell storage and prognosis. Vox Sang. 2011;100:36–45. doi: 10.1111/j.1423-0410.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 11.Hébert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 12.Bennett-Guerrero E, Stafford-Smith M, Waweru PM, et al. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49:1375–83. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. JAMA. 2012;308:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 14.Elahi MM, Yii M, Matata BM. Significance of oxidants and inflammatory mediators in blood of patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:455–67. doi: 10.1053/j.jvca.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Koch CGH, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 16.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 17.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–9. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Edgren G, Kamper-Jørgensen M, Eloranta S, et al. Duration of red blood cell storage and survival of transfused patients. Transfusion. 2010;50:1185–95. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner ME, Assmann SF, Levy JH, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 2010;43:107–16. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alghamdi AA, Davis A, Brister S, Corey P, Logan A. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion. 2006;46:1120–9. doi: 10.1111/j.1537-2995.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 21.Roback JD, Grossman BJ, Harris T, Hillyer CD, editors. Technical manual. 17th. Bethesda, MD: AABB Press; 2011. p. 192. [Google Scholar]
- 22.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 23.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hébert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–34. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitaker BI, Hinkins S. The 2011 National blood collection and utilization survey report. Washington, DC: Department of Health and Human Services; 2013. http://www.aabb.org/research/hemovigilance/bloodsurvey/Documents/11-nbcus-report.pdf. [Google Scholar]
- 27.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix J, Hébert P, Fergusson D, et al. The Age of Blood Evaluation (ABLE) randomized controlled trial: study design. Transfus Med Rev. 2011;25:197–205. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


