Abstract
A newly isolated rod-shaped, gram-negative anaerobic bacterium from human feces, named Julong 732, was found to be capable of metabolizing the isoflavone dihydrodaidzein to S-equol under anaerobic conditions. The metabolite, equol, was identified by using electron impact ionization mass spectrometry, 1H and 13C nuclear magnetic resonance spectroscopy, and UV spectral analyses. However, strain Julong 732 was not able to produce equol from daidzein, and tetrahydrodaidzein and dehydroequol, which are most likely intermediates in the anaerobic metabolism of dihydrodaidzein, were not detected in bacterial culture medium containing dihydrodaidzein. Chiral stationary-phase high-performance liquid chromatography eluted only one metabolite, S-equol, which was produced from a bacterial culture containing a racemic mixture of dihydrodaidzein. Strain Julong 732 did not show racemase activity to transform R-equol to S-equol and vice versa. Its full 16S rRNA gene sequence (1,429 bp) had 92.8% similarity to that of Eggerthella hongkongenis HKU10. This is the first report of a single bacterium capable of converting a racemic mixture of dihydrodaidzein to enantiomeric pure S-equol.
There has been growing interest in the effects of dietary phytoestrogenic isoflavones on human health because isoflavones possess diverse physiological activities, including anticarcinogenic (1, 13), antimutagenic (12), and antioxidant (17) activities, as well as antiproliferative effects against tumor cells (14). The main components of dietary phytoestrogenic isoflavones are daidzein, genistein, and their glycosides daidzein and genistein, which are distributed predominantly in leguminous plants, such as soybeans.
After ingestion, isoflavones are subjected to diverse metabolic processes in the intestinal tract prior to absorption. The metabolites of daidzein include dihydrodaidzein, tetrahydrodaidzein, equol, and O-desmethylangolensin, and these metabolites have been detected in human urine (9, 18, 19). The crucial role of gut microflora in the metabolism of isoflavones in human beings has also been previously explored (9). In vivo studies proved that bacteria in the gastrointestinal tract play an important role in determining the magnitude and pattern of isoflavone bioavailability (30). Setchell et al. (28) demonstrated that incubation of textured vegetable protein with human fecal bacteria resulted in the formation of equol, which among isoflavone metabolites was known to be the most effective in stimulating an estrogenic response (6, 25). However, only 30 to 40% of the population can produce equol from daidzein (25, 27). In vitro studies proved interindividual differences in the bacteria responsible for equol production. Equol is a chiral molecule that can exist in two enantiomeric forms with potentially different biological activities (22). A recent study demonstrated that the binding affinity of a natural-form S-equol to estrogen receptor β is 13 times higher than that of R-equol and 2 times higher than that of (±)equol, whereas R-equol showed a preference for binding to estrogen receptor α which is 4 times higher than that of S-equol and 2 times higher than that of (±)equol (23).
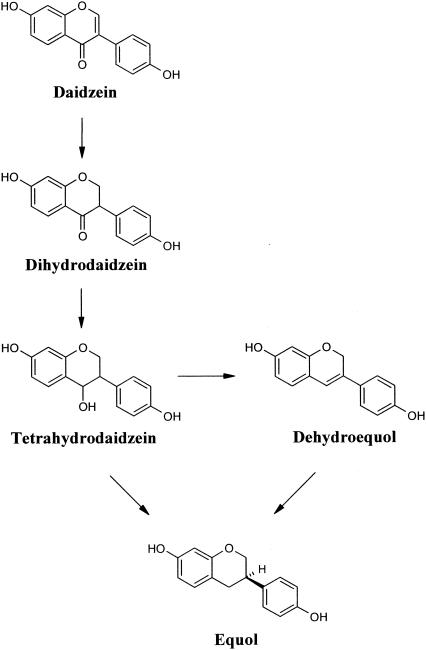
In recent years, there have been attempts to isolate bacterial strains from human feces that are able to metabolize (iso)flavones under anaerobic conditions (7, 15, 16, 26). Hur et al. (16) isolated an intestinal bacterium, Clostridium sp. strain HGH6, that is capable of reducing daidzein to dihydrodaidzein, but further metabolism to tetrahydrodaidzein or equol did not occur. Although extensive research has been performed to search for a single bacterium capable of producing equol from isoflavones, there have been no reports to date which describe a specific bacterium in relation to production of equol from tetrahydrodaidzein or dehydroequol (18) (Fig. 1).
FIG. 1.
Proposed metabolic pathway leading to formation of equol from daidzein in a urine sample (modified from reference 18).
In this study, chemically synthesized dihydrodaidzein was used as the substrate administered to a newly isolated rod-shaped, gram-negative, anaerobic bacterium from human feces which can convert dihydrodaidzein to S-equol.
MATERIALS AND METHODS
Chemicals.
Daidzein and equol in a racemic mixture were purchased from Indofine Co. (Somerville, N.J.). Brain heart infusion (BHI) was from Difco Co. (Detroit, Mich.). Pd/C (10% palladium on activated charcoal), dioxane, and sodium borohydride (NaBH4) were purchased from Aldrich Co. (St. Louis, Mo.). Ammonium formate was purchased from Junsei Co. (Tokyo, Japan). Acetonitrile, ethyl acetate, methanol, and acetic acid were of high-performance liquid chromatography (HPLC) grade.
Chemical synthesis of dihydrodaidzein and tetrahydrodaidzein.
Catalytic transfer hydrogenation was performed as described by Krishnamurty and Sathyanarayana (20) with minor modifications. Daidzein (0.26 g) in 20 ml of methanol was prepared in a round flask containing 0.255 g of Pd/C and 0.252 g of ammonium formate. The reaction mixture was refluxed for 2 h at 65°C. The solutions were monitored for the production of dihydrodaidzein and tetrahydrodaidzein by HPLC. After reaction completion, the mixture was cooled down and the Pd/C was filtered off via a 0.45 μm-pore-size filter, followed by evaporation with a SpeedVac AES1010 (ThermoSavant, New York, N.Y.). The residue was redissolved in 100% methanol and purified with Sephadex LH20 (Amersham Pharmacia Biotech, Amersham, United Kingdom). The samples purified with the LH20 column were evaporated to dryness with the SpeedVac AES1010. The residue was redissolved in methanol to separate and isolate products by using a Thermo Prep-HPLC (ThermoSeparation Products, Riviera Beach, Fla.). The reaction products were subjected to analysis by electron impact ionization mass spectrometry (EI-MS) and nuclear magnetic resonance (NMR) spectroscopy. The EI-MS and 1H and 13C NMR spectra of a racemic mixture of dihydrodaidzein and tetrahydrodaidzein were identical to those reported previously (9, 29).
Bacterial isolation and identification.
A human fecal sample from a healthy female volunteer was collected in 5 ml of BHI liquid medium which was covered with 2 ml of sterilized mineral oil. The fecal sample was diluted serially from 10−1 to 10−8 in BHI liquid medium. One hundred microliters of each diluted solution was spread on BHI agar plates and incubated in an anaerobic chamber (5% CO2, 10% H2, and 85% N2) at 37°C for 24 h. More than 1,000 colonies were isolated and maintained on BHI agar plates. Over 100 pools, which were composed of 10 randomly combined single colonies, were incubated in BHI liquid medium containing 100 μM daidzein and 100 μM dihydrodaidzein to find positive pools capable of metabolizing the substrates. From the positive pools, each single colony was individually incubated with daidzein or dihydrodaidzein to find specific the bacterial colony(ies) responsible for the conversion of daidzein or dihydrodaidzein. Only one bacterial strain, named strain Julong 732, showed the capacity to convert dihydrodaidzein to equol under anaerobic conditions. 16S rRNA gene sequence analysis was used to identify strain Julong 732. Strain Julong 732 was grown in the 5 ml of BHI liquid medium in an anaerobic chamber for 2 days. One milliliter of the culture was centrifuged at 16,000 × g (centrifuge 5415D; Eppendorf, Hamburg, Germany) for 5 min. The supernatant was discarded, and 30 μl of NaOH (0.05 N) was added to the tubes. The tubes were incubated in boiling water for 15 min for extraction of genomic DNA. The 16S rRNA gene from chromosomal DNA of Julong 732 was amplified by PCR with the universal primers 27F (AGAGTTTGATCMTGGCTCAG) and 1492R (GGYTACCTTGTTACGACTT) (20). The PCR program used for amplification was as follows: 94°C for 5 min; followed by 29 cycles consisting of 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min 30 s; and a single final extension step consisting of 72°C for 10 min. The PCR products amplified from genomic DNA were purified with an AccuPrep gel purification kit (Accurate Chemical Science Corp., Westbury, N.Y.). The ToPo-cloning kit (Invitrogen, Carlsbad, Calif.) was used for ligation and transformation. Complete nucleotide sequences (1,429 bp) were obtained by using an ABI 377 XL upgrade DNA sequencer (Perkin-Elmer, Boston, Mass.) and software provided by the manufacturer. Sequence similarity searches were performed with the BLAST program. The sequence information was then imported into the PHYDIT program (10) for assembly and alignment. The sequence of the 16S rRNA gene (1,429 bp) for strain Julong 732 was compared to sequences from type strains held in the GenBank database. Nucleotide substitution rates were calculated, and phylogenetic trees were constructed by the neighbor-joining method (24). This bacterial strain has been deposited in the Korean Culture Center of Microorganisms with deposit number KCCM-10490.
Production and purification of the metabolite produced from a racemic mixture of dihydrodaidzein by strain Julong 732.
For preparation of the metabolite in large quantities, bacterial strain Julong 732 was grown in a 500-ml flask containing 200 ml of BHI liquid medium under anaerobic conditions for 2 days. A dihydrodaidzein stock solution (40 mM in N,N-dimethyl formamide) was added to the 2-day-old culture broth at a final concentration of 0.8 mM. The bacterial culture was then continuously incubated under anaerobic conditions for 1 week. The culture medium was extracted with an equal volume of ethyl acetate three times, followed by evaporation to dryness with an NE New rotary vacuum evaporator (Eyela, Tokyo, Japan). The dried metabolite was redissolved in 100% methanol and purified on an LC-918 recycling preparative HPLC (Analytical Industry Co., Tokyo, Japan).
HPLC.
Analytical HPLC profiles were obtained with a Varian ProStar HPLC (Varian, Walnut Creek, Calif.) equipped with a photodiode array detector and a C18 column (5-μm particle size, 4.6 by 250 mm; Waters, Fullerton, Calif.). The mobile phase was composed of 10% acetonitrile solution in water (solution A) and 90% acetonitrile solution in water (solution B), which were buffered with 0.1% acetic acid. The elution program was as follows: solution B was run at 30% for 15 min, linearly increased to 50% for 10 min, and then linearly increased to 70% for 5 min. The flow rate was 1 ml/min. All samples were monitored at 270 nm. UV spectra of the peaks were recorded from 200 to 400 nm. For the analysis of enantioisomers, a Sumi Chiral OA-7000 (5-μm particle size, 4.6 by 250 mm; Sumika Chemicals, Osaka, Japan) was used with a mobile phase composed of 40% acetonitrile in 20 mM potassium phosphate buffer (pH 3.0). The isocratic elution program with the mobile phase lasted for 35 min. The flow rate was 1 ml/min, and UV spectra of the peaks were recorded from 200 to 400 nm. For the purification of chemically synthesized dihydrodaidzein and tetrahydrodaidzein, a Thermo HPLC P2000 instrument (ThermoSeparation Products) equipped with an UV photodiode array 100 detector (Dionex Corp., Sunnyvale, Calif.) and a Spherisorb C18 reverse-phase column (5-μm particle size, 10 by 250 mm; Waters) was used. The mobile phase, composed of 60% methanol in 0.1% acetic acid solution, was used in an isocratic mode, running for 40 min. The injection volume was 50 to 100 μl, based on the concentration of the products, and the flow rate was 2 ml/min. The responding peak solutions were collected and evaporated to dryness with the AES1010 automated environmental SpeedVac (ThermoSeparation Products). For preparative separation of the enantiomeric R- and S-equols, 200 milligrams of a racemic mixture of equol was dissolved in 2 ml of acetonitrile (100%). Thirty microliters of the equol solution was injected into a Thermo HPLC P2000 instrument (ThermoSeparation Products) equipped with a Prep-Sumi-Chiral OA-7000 column (8 × 250 mm; Sumica Chemicals, Osaka, Japan). The mobile phase in the isocratic mode consisted of 40% acetonitrile in 20 mM potassium buffer (pH 3.0) at a flow rate of 2.0 ml/min. The peak solutions for R- and S-equols were collected individually and evaporated to dryness with the AES1010 automated environmental SpeedVac. As a result, 86.9 mg of R-equol and 82.6 mg of S-equol, respectively, were obtained. For the preparative purification of the biological metabolite produced from dihydrodaidzein by strain Julong 732, an LC918 recycling preparative HPLC equipped with a JAIGEL-W251 polymeric gel filtration column (20 by 500 mm; Analytical Industry) and an RI-50 detector (Analytical Industry) was used. The mobile phase in the isocratic mode was 100% methanol at a flow rate of 3 ml/min, and the injection volume was 3 ml. The corresponding peak was collected and evaporated to dryness with the AES1010 automated environmental SpeedVac (ThermoSeparation Products).
EI-MS.
EI-MS was done with a JMS-AX50510A mass spectrometer (JEOL Co. Ltd., Tokyo, Japan) in positive mode. The source temperature was 250°C, and the ionization voltage was 70 eV. An electron multiplier of 1.2 kV (regular spectrum type) was used.
NMR Spectrometry.
1H and 13C NMR spectra in acetone-d6 were obtained at 400 MHz on a Jnm-La NMR spectrometer (JEOL Co. Ltd., Akishima, Japan) at a temperature of 296 K. For 1H NMR analysis, 16 transients were acquired with a 3-s relaxation delay, using 32,000 data points. The 90° pulse was 11.2 μs, with a spectral width of 100 ppm. The 13C NMR was obtained with a spectral width of 200 ppm, collecting 64,000 data points. The 90° pulse was 10.0 μs.
Chirality studies of biosynthesized equol.
The specific rotation ([α]D) in ethanol was obtained with a polarimeter (Autopol III; Rudolph Research Analytical, Flanders, N.J.). Measurements of circular dichroism (CD) in ethanol were recorded on a J-715 spectrometer (Jasco Corp., Tokyo, Japan). The melting point was obtained with a differential scanning calorimeter (DSC Q1000; TA Instruments, New Castle, Del.). Differential scanning calorimeter analysis was done from −70 to 300°C at a heating rate of 10°C/min.
Kinetics of biotransformation of dihydrodaidzein and its derivatives by strain Julong 732.
Strain Julong 732 was tested for biotransformation activity with the isoflavones daidzein, dihydrodaidzein, tetrahydrodaidzein, S- and R-equol, and genistein while the strain was growing in a test tube containing 2 ml of BHI. Strain Julong 732 (optical density at 600 nm [OD600], 0.03) was inoculated into medium containing each compound at 400 μM. The anaerobic bacterial culture of strain Julong 732 containing dihydrodaidzein was sampled daily for 5 days to study biotransformation kinetics. Biotransformation of the rest of the compounds was analyzed one time after 4 days of incubation. All experiments were performed in triplicate.
Nucleotide sequence accession number.
The full sequence (1,429 bp) of the 16S rRNA gene of strain Julong 732 has been deposited in GenBank (accession no. AY310748).
RESULTS
Identification of human intestinal bacterial strain Julong 732.
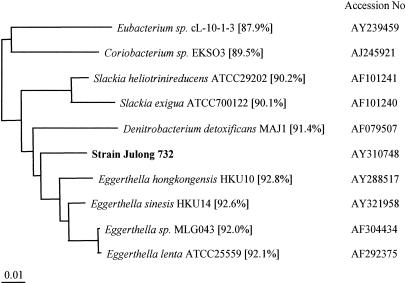
A newly isolated bacterial strain, Julong 732, from the human intestinal tract was determined to be a rod-shaped, gram-negative, anaerobic bacterium. Its full 16S rRNA gene sequence (1,429 bp) had 92.8% similarity to that of Eggerthella hongkongenis HKU10 (accession no. AY288517) (Fig. 2), so strain Julong 732 could be a new species of Eggerthella.
FIG. 2.
Dendrogram showing the phylogenetic affiliation of strain Julong 732.
Identification of the metabolite of dihydrodaidzein produced by strain Julong 732.
An elution profile of the analytical HPLC chromatography showed that a single metabolite was produced from dihydrodaidzein by strain Julong 732. The metabolite of dihydrodaidzein gave a maximum absorbance at 280 nm (Fig. 3). An EI-MS spectrum for the metabolite of dihydrodaidzein gave an (M + H)+ molecular ion peak at m/z 242 (relative intensity, 86%) (Fig. 3), consistent with the molecular formula for equol (C15H14O3). The other daughter ion peaks (m/z) of the metabolite were 120 (100), 123 (82), 135 (27), 107 (28), and 91 (14) (numbers in parentheses are percent relative intensities), which were identical to the data previously reported for equol (4). For further structural identification, the metabolite of dihydrodaidzein was subjected to 1H and 13C NMR analyses. Both the 1H and 13C NMR data were identical to those for equol reported previously (2, 4, 9): equol 1H-NMR [(CD3)2CO, 400 MHz], 2.91 (m, 2H, H-4), 3.06 (m, 1H, H-3), 3.92 (m, 1H, H-2), 4.17 (m, 1H, H-2), 6.27 (d, 1H, J = 2.4 Hz, H-8), 6.35 (dd, 1H, J = 8.3Hz, H-5), 6.82 (d, 2H, J = 8.5Hz, H-3′), 7.14 (d, 2H, J = 8.5Hz, H-6′), 8.08 (s, 1H, OH), 8.22 (s, 1H, OH); equol 13C-NMR [(CD3)2CO, 100 MHz], 32.65 (C-4), 38.79 (C-3), 71.57 (C-2), 103.59 (C-8), 108.77 (C-6), 114.04 (C-4a), 116.20 (C-3′,5′), 129.21 (C-2′,6′), 130.99 (C-5), 133.42 (C-1′), 155.97 (C-8a), 157.13 (C-7), 157.51 (C-4′). Therefore, based on the EI-MS and 1H and 13C NMR spectral analyses, the metabolite of dihydrodaidzein produced by strain Julong 732 was unambiguously determined to be equol.
FIG. 3.
HPLC elution profile of metabolism of dihydrodaidzein by strain Julong 732 in BHI liquid medium under anaerobic conditions. The insets show UV spectra of dihydrodaidzein (DHD) and a metabolite of dihydrodaidzein and the EI-MS (m/z) spectrum of the metabolite of dihydrodaidzein. AU, absorbance units.
Chirality studies of biosynthesized equol.
Chiral stationary-phase HPLC analysis of the incubation mixture containing dihydrodaidzein detected only one peak, illustrating 100% enantiomeric excess (e.e.) of biologically synthesized equol (Fig. 4). The specific rotation ([α]24D) of biosynthesized equol was −23.0°. Since S-form equol was reported to have a negative [α]D value ([α]548 = −21.5° in chloroform) (8), the equol biosynthesized by strain Julong 732 was determined to be that of the S form. The CD spectrum of S-form equol (Fig. 4) in ethanol exhibited positive and negative cotton effects in the informative region of 200 to 300 nm. S-form equol displayed a high-amplitude positive cotton effect at 237 nm and a negative cotton effect at 285 nm. The melting point of S-form equol was 190.8°C, consistent with previously reported data for S-form equol (189 to 190.5°C) which was isolated from heartwood of Millettia pendula (8). The melting points of R- and S-equols which were separated from racemic-mixture equol were 188.9 and 190.3°C, respectively.
FIG. 4.
HPLC elution profiles on a Sumi Chiral OA-7000 column and CD spectra of racemic-mixture equol (A) and S-equol (B) produced from dihydrodaidzein by strain Julong 732 under anaerobic conditions. AU, absorbance units.
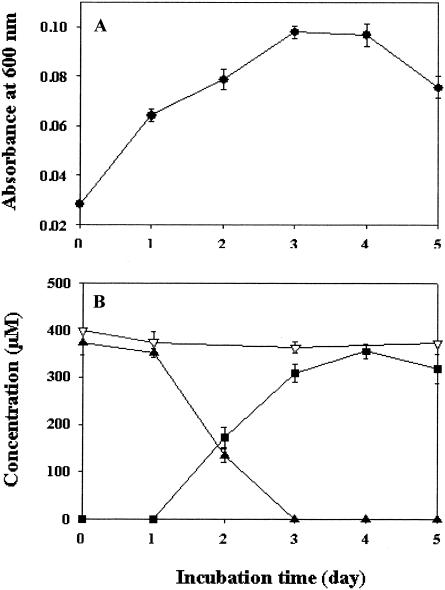
Kinetics of biotransformation of dihydrodaidzein and its derivatives by strain Julong 732.
Figure 5 shows the kinetics of biotransformation of dihydrodaidzein by strain Julong 732 growing in BHI medium containing 400 μM dihydrodaidzein. Strain Julong 732 was grown to an optical density at 600 nm of 0.1 under anaerobic conditions for 4 days. During bacterial growth, production of equol with a concomitant decrease of dihydrodaidzein was observed after 1 day of incubation and reached the largest amount by day 4. When a resting cell culture of strain Julong 732 (optical density at 600 nm of 0.10) was incubated with the isoflavones daidzein, dihydrodaidzein, tetrahydrodaidzein, R-equol, S-equol, dehydroequol, and genistein for 4 days, dihydrodaidzein and tetrahydrodaidzein were biotransformed in the amounts of 390 and 20 μM, respectively, to equol. However, strain Julong 732 did not produce metabolites from daidzein, R-equol, S-equol, dehydroequol, and genistein.
FIG. 5.
Bacterial growth (A) and biotransformation kinetics of dihydrodaidzein while strain Julong 732 was growing in BHI medium (B). ▴, dihydrodaidzein with strain; ▪, equol produced from dihydrodaidzein by strain; ▿, dihydrodaidzein without strain. Error bars indicate standard deviations.
DISCUSSION
Equol, which is found in various animals, is not a natural isoflavone occurring in leguminous plants; rather, it is a urinary metabolite produced from daidzein (2, 5, 11, 21, 22). Joannou et al. (18) reported a urinary metabolic profile of isoflavones in which daidzein was biologically reduced to dihydrodaidzein, to tetrahydrodaidzein, and to equol in sequential reactions or was reduced to dehydroequol (7,4′-dihydroxyisoflav-3-ene) (18). Among the intestinal metabolites of dietary daidzein, equol has been known as the most phytoestrogenic compound, lowering the incidence of hormone-dependent diseases such as breast cancer and prostate cancer (3, 23, 25).
Strain Julong 732, which was isolated from a human intestine, was able to convert the starting compounds dihydrodaidzein and tetrahydrodaidzein to S-equol under anaerobic conditions. However, it could not reduce daidzein to the corresponding dihydrodaidzein and tetrahydrodaidzein or to dehydroequol under anaerobic conditions as shown in Fig. 1. In addition, strain Julong 732 could not cleave the C ring of daidzein, which would result in the formation of O-desmethylangolensin or 2-(4-hydroxyphenyl)propionic acid (24, 27). Strain Julong 732 stereoselectively produced S-equol (100% e.e.) from enantiomeric mixtures of dihydrodaidzein. Enantioselectivity of strain Julong 732 toward the parent compound R- or S-dihydrodaidzein could not been determined because R- or S-dihydrodaidzein was abiotically equilibrated in the reaction medium or water at pH 7.0, most likely through a tautomerization reaction. Therefore, there are two possibilities to explain S-equol (100% e.e.) production from dihydrodaidzein. One is that strain Julong 732 has an enantioselective preference to reduce S-dihydrodaidzein to S-equol. The other is that strain Julong 732 has an enantioselective preference to reduce R-dihydrodaidzein to R-equol, which in turn is converted to S-equol by racemase activity. However, strain Julong 732 did not show racemase activity to transform S-equol to R-equol or vice versa. Considering these reactions, stereoselective production of S-equol might be caused by the enantioselective preference of strain Julong 732 for the starting compound S-dihydrodaidzein. Additionally, strain Julong 732 reduced tetrahydrodaidzein to S-equol but not to dehydroequol, which was found in urine samples and was most likely a metabolite produced from tetrahydrodaidzein. However, the amount of S-equol produced from tetrahydrodaidzein was significantly small compared to that produced from dihydrodaidzein. As previously reported by Hur et al. (16), who isolated an intestinal bacterial strain, Clostridium sp. strain HGH6, that partially converted daidzein to dihydrodaidzein which was not further reduced to tetrahydrodaidzein or equol, strain Julong 732 did not show the whole intestinal pathway of daidzein as suggested by Joannou et al. (18). This suggests that more than one bacterial species may be involved in the completion of the metabolic cycle of daidzein to S-equol. Therefore, it may be possible to produce more phytoestrogenic and natural S-equol from daidzein when the intestinal microbial community consists of Clostridium sp. strain HGH6 and strain Julong 732. This is the first report of an isolated human intestinal bacterium capable of metabolizing a racemic mixture of dihydrodaidzein to S-equol with 100% enantiomeric excess.
Acknowledgments
This work was partially supported by the BK21 program of the Ministry of Education in Korea.
We are grateful to Min Ye, Department of Pharmacognosy and Biotechnology, Peking University, for helpful discussions. We thank R. A. Kanaly at the UNU and GIST Joint Program on Science and Technology for Sustainability, Gwangju, Korea, for his comments on the manuscript.
REFERENCES
- 1.Adlercreutz, H., H. Hamalainen, S. Gorbach, and S. Goldin. 1992. Dietary phyto-oestrogens and the menopause in Japan. Lancet 339:1233. [DOI] [PubMed] [Google Scholar]
- 2.Adlercreutz, H., P. I. Musey, T. Fosis, C. Bannwart, K. Wahala, T. Makela, G. Brunow, and T. Hase. 1986. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin. Chim. Acta 158:147-154. [DOI] [PubMed] [Google Scholar]
- 3.Akaza, H., N. Miyanaga, N. Takashima, S. Naito, Y. Hirao, T. Tsukamoto, T. Fujioka, M. Mori, W. J. Kim, J. M. Song, and A. J. Pantuck. 2004. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 34:86-89. [DOI] [PubMed] [Google Scholar]
- 4.Alda, J. O., J. A. Mayoral, M. Lou, I. Gimenez, R. M. Martinez, and R. P. Garay. 1996. Purification and chemical characterization of a potent inhibitor of the Na-K-Cl cotransport system in rat urine. Biochem. Biophys. Res. Commun. 221:279-285. [DOI] [PubMed] [Google Scholar]
- 5.Axelson, M., D. N. Kirk, R. D. Farrant, G. Cooley, A. M. Lawson, and K. D. R. Setchell. 1982. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl) chroman] in human urine. Biochem. J. 201:353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borriello, S. P., K. D. R. Setchell, M. Axelson, and A. M. Lawson. 1985. Production and metabolism of lignans by the human fecal flora. J. Appl. Bacteriol. 58:37-43. [DOI] [PubMed] [Google Scholar]
- 7.Braune, A., M. Gutschow, W. Engst, and M. Blaut. 2001. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 67:5558-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckingham, J. 1994. Dictionary of natural products, 1st ed. Chapman and Hall, London, United Kingdom.
- 9.Chang, Y. C., and M. G. Nair. 1995. Metabolism of daidzein and genistein by intestinal bacteria. J. Nutr. Proc. 58:1892-1896. [DOI] [PubMed] [Google Scholar]
- 10.Chun, J. 1995. Computer-assisted classification and identification of actinomycetes. Ph.D. thesis. University of Newcastle, Newcastle upon Tyne, United Kingdom.
- 11.Common, R., and L. Aimsworth. 1961. Identification of equol in the urine of the domestic fowl. Biochim. Biophys. Acta 53:403-404. [DOI] [PubMed] [Google Scholar]
- 12.Hartman, P. E., and D. M. Shankel. 1990. Antimutagens and anticarcinogens: a survey of putative interceptor molecules. Environ. Mol. Mutagen. 15:145-182. [DOI] [PubMed] [Google Scholar]
- 13.Hirano, T., M. Gotoh, and K. Oka. 1994. Natural flavonoids and lignans one potent cyto-static agents against human leukemic HL-60 cells. Life Sci. 55:1061-1069. [DOI] [PubMed] [Google Scholar]
- 14.Hirano, T., K. Oka, and M. Akiba. 1989. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75-1. Res. Commun. Chem. Pathol. Pharmacol. 64:69-78. [PubMed] [Google Scholar]
- 15.Hur, H. G., R. D. Beger, T. M. Heinze, J. O. J. Lay, J. P. Freeman, and F. Rafii. 2002. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of isoflavonoid daidzein. Arch. Microbiol. 178:8-12. [DOI] [PubMed] [Google Scholar]
- 16.Hur, H. G., J. O. J. Lay, R. D. Beger, J. P. Freeman, and F. Rafii. 2000. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 174:422-428. [DOI] [PubMed] [Google Scholar]
- 17.Jha, H. C., G. V. Recklinghausen, and F. Zilliken. 1985. Inhibition of in vitro microsomal lipid peroxidation by isoflavonoids. Biochem. Pharmacol. 34:1367-1369. [DOI] [PubMed] [Google Scholar]
- 18.Joannou, G. E., G. E. Kelly, A. Y. Reeder, M. Waring, and C. Nelson. 1995. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J. Steroid Biochem. Mol. Biol. 54:167-184. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, G. E., C. Nelson, M. A. Waring, G. E. Joannou, and A. Y. Reeder. 1993. Metabolites of dietary (soya) isoflavones in human urine. Clin. Chim. Acta 223:9-12. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamurty, H. G., and S. Sathyanarayana. 1986. Catalytic transfer hydrogenation, a chemo-selective reduction of isoflavones to isoflavanones. Synth. Commun. 16:1657-1663. [Google Scholar]
- 21.Luk, K. C., L. Stern, and M. Weigele. 1983. Isolation and identification of “diazepam-like” compounds from bovine urine. J. Nutr. Proc. 46:852-861. [DOI] [PubMed] [Google Scholar]
- 22.Marrian, G. F., and G. A. D. Haselwood. 1932. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares urine. Biochem. J. 26:1226-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muthyala, R. S., Y. H. Ju, S. Sheng, L. D. Williams, D. R. Doerge, B. S. Katzenellenbogen, W. G. Helferich, and J. A. Katzenellenbogen. 2004. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 12:1559-1567. [DOI] [PubMed] [Google Scholar]
- 24.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 25.Sathyamoorthy, N., and T. T. Y. Wang. 1997. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur. J. Cancer 33:2384-2389. [DOI] [PubMed] [Google Scholar]
- 26.Schoefer, L., R. Mohan, A. Schwiertz, A. Braune, and M. Blaut. 2003. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 69:5849-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setchell, K. D. R., and H. Adlercreutz. 1988. Mammalian lignans and phytoestrogens: recent studies on their formation, metabolism, and biological role in health and disease, p. 315-345. In I. R. Rowland (ed.), Role of the gut flora in toxicity and cancer. Academic Press, London, United Kingdom.
- 28.Setchell, K. D. R., S. P. Borriello, P. Hulme, D. N. Kirk, and M. Axelson. 1984. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 40:569-578. [DOI] [PubMed] [Google Scholar]
- 29.Wahala, K., A. Salakka, and H. Adlercreutz. 1998. Synthesis of novel mammalian metabolites of the isoflavonoid phytoestrogens daidzein and genistein. Proc. Soc. Exp. Biol. Med. 217:293-299. [DOI] [PubMed] [Google Scholar]
- 30.Xu, X., K. S. Harris, H. J. Wang, P. A. Murphy, and S. Hendrich. 1995. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 125:2307-2315. [DOI] [PubMed] [Google Scholar]