Abstract
In the thymus, strongly self-reactive T cells may undergo apoptotic deletion or differentiate into Foxp3+ T-regulatory (T-reg) cells. Mechanisms that partition T cells into these two fates are unclear. Here, we show that IL-2 signalling is required to prevent deletion of CD4+ CD8– CCR7+ Helios+ thymocytes poised to upregulate Foxp3. The deletion prevented by IL-2 signalling is Foxp3 independent and occurs later in thymocyte development than the deletion that is prevented by Card11 signalling. Our results distinguish two bottlenecks at which strongly self-reactive thymocytes undergo deletion or progress to the next stage of T-reg differentiation; Card11 regulates the first bottleneck and IL-2 regulates the second.
During T-cell development in the thymus, TCR signalling strength plays a critical role in determining T-cell fate. While weak TCR signalling induces thymocytes to develop into naïve T cells, strong TCR signalling induces alternative fates, including apoptotic deletion or Foxp3+ T-reg differentiation. Whereas deletion can occur at any stage of thymocyte development,1, 2, 3 upregulation of Foxp3 during T-reg differentiation occurs mainly in mature CD4+ CD8– (CD4 single positive, CD4SP) thymocytes that express the chemokine receptor, CCR7.4, 5 The strongly TCR signalled CD4SP CCR7+ thymocyte population thus contains cells that are poised for deletion or poised for Foxp3+ T-reg differentiation (i.e. T-reg precursor), but the signals and steps that determine this cell fate decision are only partially understood.
Helios is the only molecular marker known to be upregulated by strong TCR signalling and downregulated by weak TCR signalling in thymocytes.6 Mice with defective apoptosis have an increased number of CD4SP CCR7+ Helios+ Foxp3– cells and Foxp3+ T-reg cells in the thymus, while these populations are diminished in mice that lack Card11 (also called CARMA1) or c-Rel.6, 7, 8, 9, 10 Card11-deficient mice with defective apoptosis have a substantial CD4SP CCR7+ Helios+ Foxp3– thymocyte population6 but Foxp3+ cells are still absent.9 These data reveal two essential and distinct functions of Card11 in T-reg differentiation: to prevent apoptotic deletion of T-reg precursors and to mediate Foxp3 upregulation in T-reg precursors.
Thymic T-reg differentiation has been characterised as a two-step process consisting of strong TCR signalling followed by cytokine-induced Foxp3 upregulation.11 IL-2 and IL-15 are members of a cytokine family that signals via the cytokine receptor, CD132 (the common γ-chain).12 Mice lacking CD132, or the α or β subunits of the IL-2 receptor, have very few Foxp3+ T-reg cells in the thymus.13, 14 However, CD132-deficient mice with a defective apoptosis pathway have a substantial population of thymic Foxp3+ T-reg cells.8 It has been postulated that cytokine signalling is required to prevent deletion induced by strong TCR signalling.15 A competing hypothesis proposes that cytokine signalling is required to counteract a proapoptotic protein signature, which is induced in developing T-reg cells by Foxp3.8 Distinguishing between these possibilities is essential to advance our understanding of how thymocytes partition into deletion versus T-reg differentiation fates.
To address this, we examined the stage(s) at which various genetic defects impinge on the development of strongly TCR signalled thymocytes. We found that IL-2 signalling is essential to prevent deletion of CD4SP CCR7+ Helios+ thymocytes at a later developmental stage than Card11 is required to prevent deletion. The deletion prevented by IL-2 signalling occurs in a Foxp3-independent manner. We propose that variation in Card11 and IL-2 signalling determines whether CD4SP CCR7+ thymocytes undergo deletion or progress to the next stage of Foxp3+ T-reg differentiation.
Results
CD4SP thymocytes able to respond to IL-2 express CCR7 and Helios
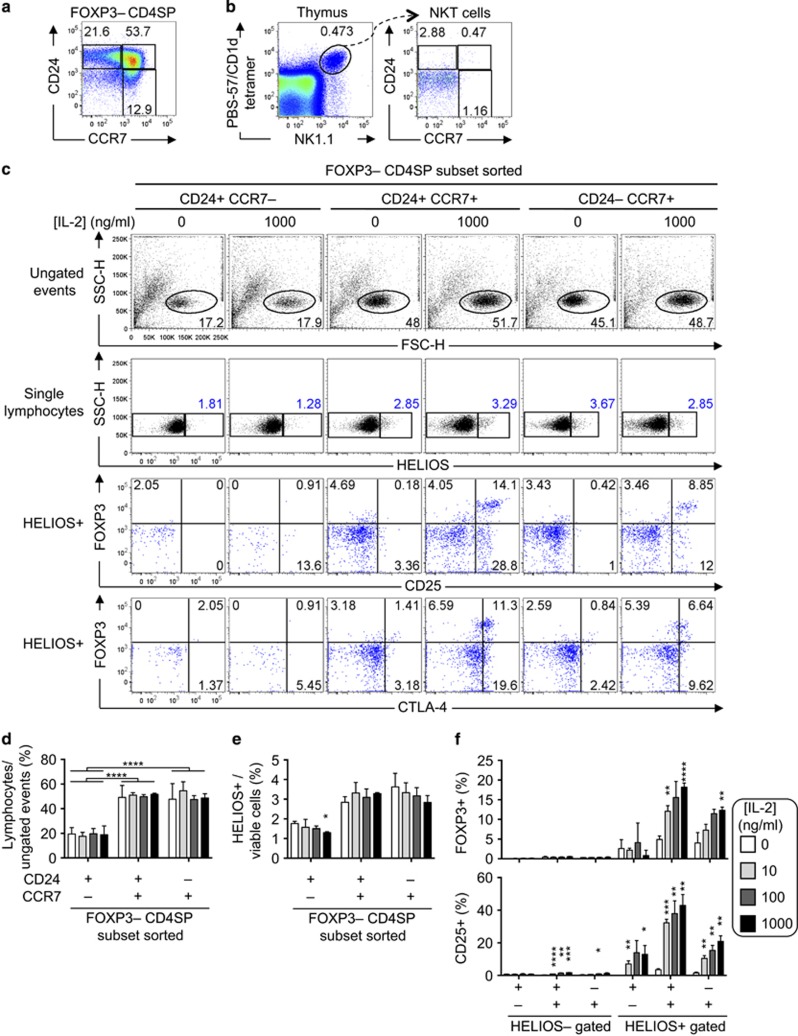
To test whether CD4SP thymocytes at distinct developmental stages are differentially responsive to IL-2, thymocytes from Foxp3GFP mice were sorted into three subsets of CD4SP Foxp3– cells: the least mature CCR7– CD24+ cells, semi-mature CCR7+ CD24+ cells and most mature CCR7+ CD24– cells6 (Figure 1a). This sorting strategy was used, in part, to exclude NKT cells and non-nascent T-reg precursor cells, which have a CCR7– CD24– phenotype (Figure 1b and ref. 16). After 20 h, the frequency of lymphocytes among ungated events was significantly lower in CCR7– CD24+ cultures compared with the other subsets (Figures 1c, top row and d), consistent with reduced survival of CCR7– CD24+ cells. The addition of IL-2 to the cultures had no significant effect on the frequency of lymphocytes detected, nor the frequency of Helios+ cells, at any maturation stage (Figures 1c, second row and d and e). These data provided no evidence that IL-2 induced preferential survival of Helios– or Helios+ thymocytes, nor was there evidence that IL-2 induced Helios expression, during these short-term cultures. The Foxp3+ cell frequency among CCR7+ Helios+ cells was significantly higher in the presence of 1000 ng/ml IL-2 than in cultures with no IL-2 added, but this was not observed in Helios+ cells at the least mature CCR7– CD24+ stage, nor in Helios– cells at any maturation stage (Figures 1c and f). Most Foxp3+ cells expressed CD25, but Foxp3+ CD25– and Foxp3– CD25+ populations were also detectable, with the highest CD25+ cell frequencies observed among CCR7+ Helios+ cells (Figures 1c and f). CTLA-4 was also detectable in a subset of the Foxp3+ cells (Figure 1c). These results show that the CCR7+ Helios+ subset of CD4SP thymocytes is enriched in cells capable of responding to IL-2 by expressing one or more of the T-reg-associated proteins, Foxp3, CD25 and CTLA-4.
Figure 1.
The CCR7+ Helios+ subset of CD4SP thymocytes is enriched in cells that can respond to IL-2. (a) CD24/CCR7 phenotype of Foxp3– CD4SP thymocytes from B6 mice showing gating for CD24– CCR7+, CD24+ CCR7+ and CD24– CCR7+ subsets. (b) CD24– CCR7– phenotype of thymic NKT cells. Left plot shows total thymocytes from a B6 mouse stained with anti-NK1.1 plus a tetramer of mouse CD1d loaded with the α-galactosylceramide analogue, PBS-57. Thymic NKT (PBS-57/CD1d+ NK1.1+) cells are analysed for CD24/CCR7 phenotype in the right plot. Results are representative of two experiments. (c) Foxp3-GFP– CD4SP thymocytes were FACS sorted into three subsets based on CCR7 and CD24 expression and cultured for 20 h in the presence or absence of IL-2 (denoted above plots). Top row shows side-scattered light amplitude (SSC-H) versus forward-scattered light amplitude (FSC-H) for all events in the flow cytometry datafiles and the gate used to define lymphocytes. After excluding doublets based on FSC width and SSC width (gates not shown), single lymphocytes were analysed for Helios expression in the second row. Gated Helios+ events were analysed for Foxp3/CD25 (third row) and Foxp3/CTLA-4 (fourth row) phenotypes. (d) Lymphocyte frequency among ungated events as shown in top row of (c). (e) Helios+ cell frequency among single lymphocytes as shown in second row of (c). (f) Foxp3+ (top) or CD25+ (bottom) cell frequency among Helios– or Helios+ cells after 20 h in culture in the presence or absence of graded concentrations of IL-2 as gated in third and fourth rows of (c). Columns and error bars represent mean and s.e.m. of data obtained from three mice. Similar data were obtained in a repeat experiment. Statistical analyses used were (d) two-way ANOVA with Sidak's post-test or (e and f) multiple t-tests comparing each concentration of exogenous IL-2 to the 0 ng/ml IL-2 condition for each T-cell subset, without assuming a consistent standard deviation, with Holm–Sidak post-tests. Multiplicity adjusted P-value symbols: * 0.01–0.05, ** 0.001–0.01, **** <0.0001
Foxp3 is not required for CTLA-4 induction in CD4SP CCR7+ Helios+ thymocytes
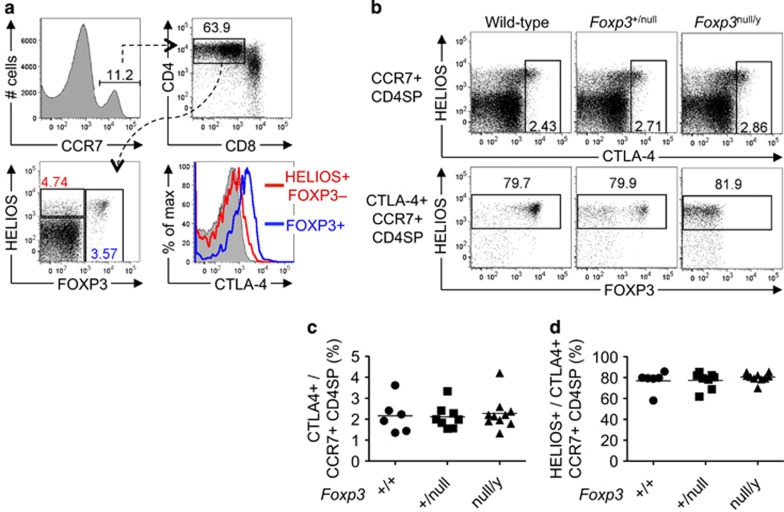
Among CD4SP CCR7+ thymocytes, we noted that the Helios+ Foxp3– subset expressed less CTLA-4 than the Foxp3+ subset (Figure 2a). As CTLA-4 and Foxp3 expression were correlated, and Foxp3 binds to the Ctla4 gene,17 we tested whether Foxp3 is required for CTLA-4 expression by examining mice bearing a Foxp3null allele.18 Normal frequencies of CTLA-4+ cells were detected among CD4SP CCR7+ thymocytes from Foxp3+/null and Foxp3null/y mice (Figures 2b and c). Approximately 80% of CD4SP CCR7+ CTLA-4+ thymocytes were Helios+, independent of Foxp3 genotype (Figures 2b and d). Thus, CTLA-4 upregulation occurs independently of Foxp3 in CD4SP CCR7+ thymocytes, predominantly in cells that express high levels of Helios.
Figure 2.
CTLA-4 upregulation in CD4SP CCR7+ Helios+ cells is Foxp3 independent. (a) Gating strategy used to distinguish three subsets within the CCR7+ CD4SP thymocyte population based on Helios/Foxp3 phenotype, and CTLA-4 expression on these three subsets. (b) Helios/CTLA-4 phenotype (upper panel) and Helios/Foxp3 phenotype on the CTLA-4+ subset (lower panel) of CCR7+ CD4SP thymocytes from B6 mice ('Wild-type') or female B6.Foxp3+/null and male B6.Foxp3null/y littermates aged 6–20 days. (c, d) Graphs show the frequencies of (c) CTLA-4+ cells or (d) Helios+ cells, as gated in (b), with each symbol representing one mouse
IL-2 is required for Foxp3 and CTLA-4 expression in CD4SP CCR7+ Helios+ thymocytes
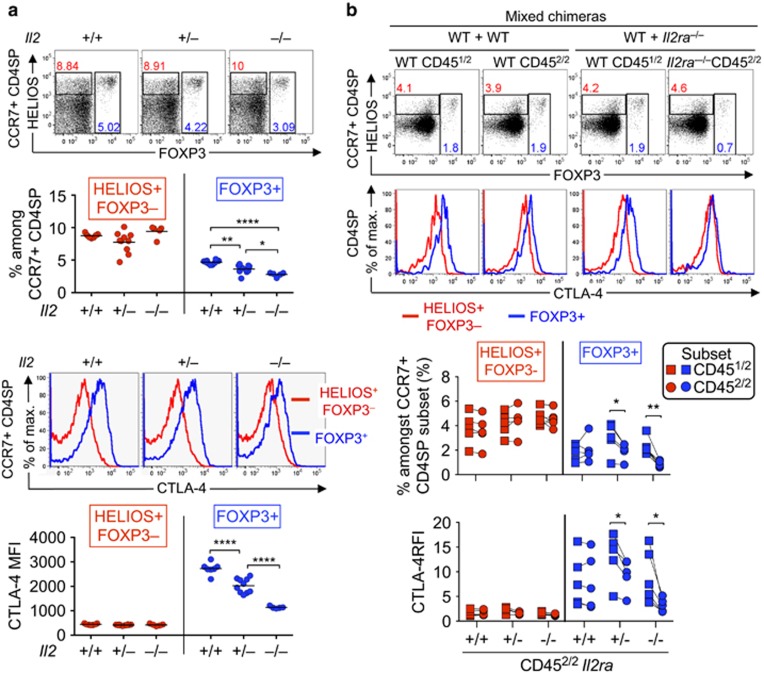
To investigate the effects of IL-2 on strongly TCR signalled thymocytes in vivo, we first compared CD4SP CCR7+ thymocytes in IL-2-sufficient and IL-2-deficient mice. To avoid potential effects of systemic immune dysregulation in Il2–/– mice,19 we examined the pups from three litters at 14–15 days after birth, before the onset of overt disease. The Foxp3+ T-reg cell frequency among CD4SP CCR7+ thymocytes was significantly decreased in Il2–/– mice (Figure 3a). In contrast, there were no significant differences in Helios+ Foxp3– cell frequencies within CD4SP CCR7+ thymocytes (Figure 3a).
Figure 3.
Foxp3 and CTLA-4 upregulation in CD4SP CCR7+ Helios+ cells is IL-2 dependent. (a) Upper plots show Helios/Foxp3 phenotype of CCR7+ CD4SP thymocytes from Il2+/+, Il2+/– and Il2–/– littermates on the B10.BR background examined at 14–15 days after birth. Lower histograms show CTLA-4 expression on Helios+ Foxp3– (red) and Foxp3+ (blue) subsets of CCR7+ CD4SP thymocytes from the same mice. Summaries (below) show the frequencies, or CTLA-4 MFI, of Helios+ Foxp3– or Foxp3+ cells as gated in the plots, with each symbol representing one mouse. (b) Helios/Foxp3 phenotype of CD451/2 and CD452/2 subsets of CCR7+ CD4SP thymocytes (top row), and CTLA-4 expression on their Helios+ Foxp3– and Foxp3+ subsets (bottom row), from CD451/1 mice that were irradiated and reconstituted 6–10 weeks earlier with BM mixtures from CD451/2 wild-type mice plus CD452/2 mice that were either Il2ra+/+, Il2ra+/– or Il2ra–/–. Graphs show the frequencies of populations gated in plots (top row) or the CTLA-4 relative fluorescence intensity (RFI) (bottom row, normalised to the CCR7+ CD4SP Helios– Foxp3– subset in the same sample). Lines join measurements from an individual mouse. Data in (a) and (b) were compiled from two and three experiments, respectively. Two-tailed Student's t-tests (unpaired in a; paired in b) P-value symbols: * 0.01–0.05, ** 0.001–0.01, **** <0.0001
To examine the consequences of cell-autonomous deficiency in IL-2 signalling, we used mice with an inactivating mutation in the Il2ra gene, which encodes CD25. Irradiated mice were reconstituted with wild-type bone marrow (BM) mixed with Il2ra+/+, Il2ra+/– or Il2ra–/– BM. At 10 weeks after BM reconstitution, there was a significant decrease in the Foxp3+ T-reg cell frequency among CD4SP CCR7+ thymocytes derived from Il2ra+/– or Il2ra–/– BM (Figure 3b). However, there were no significant differences in Helios+ Foxp3– cells (Figure 3b).
As IL-2 was sufficient to induce CTLA-4 in vitro (Figure 1), we examined whether IL-2 is necessary for CTLA-4 induction in vivo. CTLA-4 expression on Foxp3+ cells was decreased in a manner dependent on the Il2 or Il2ra gene dose (Figures 3a and b). Although dispensable for the generation of CD4SP CCR7+ Helios+ Foxp3– thymocytes, IL-2 signalling is sensitively required for the transition to the next stage of T-reg differentiation, which is characterised by upregulation of Foxp3 and/or CTLA-4.
IL-15 can partially substitute for IL-2 in thymic Foxp3+ T-reg differentiation
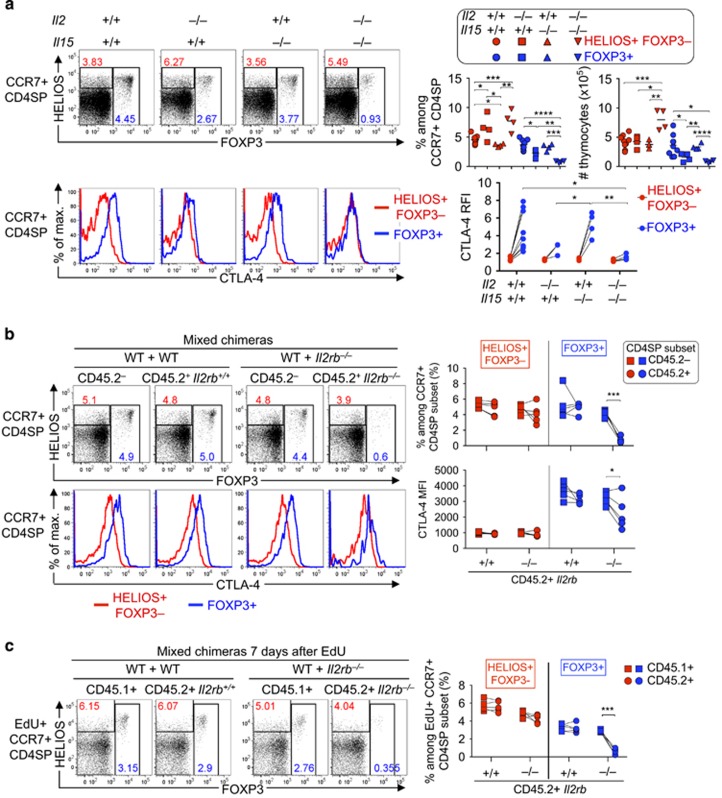
To test for effects of IL-15 on strongly TCR signalled CD4SP CCR7+ thymocytes, we examined Il15–/– mice. By most parameters Il15–/– mice were indistinguishable from wild-type littermate controls, except Il15–/– mice had a slightly lower frequency, but not number, of Helios+ Foxp3– thymocytes in the CCR7+ CD4SP population (Figure 4a). Il2–/– Il15–/– double-deficient mice had a significantly lower frequency of Foxp3+ cells than did Il2–/– mice (Figure 4a). However, Il2–/– Il15–/– mice had a significant increase in the number of Helios+ Foxp3– cells compared with all other groups (Figure 4a), indicating that the defect could not be attributed to loss of T-reg precursors. CTLA-4 expression was low to undetectable on thymic Foxp3+ T-reg cells from Il2–/– and Il2–/– Il15–/– mice, significantly lower than the Il15–/– group (Figure 4a, bottom row). These findings are consistent with the hypothesis that IL-15 contributes to thymic T-reg development when IL-2 is absent.20 They also indicate a requirement for IL-2, but not IL-15, in maintaining CTLA-4 expression on thymic T-reg cells.
Figure 4.
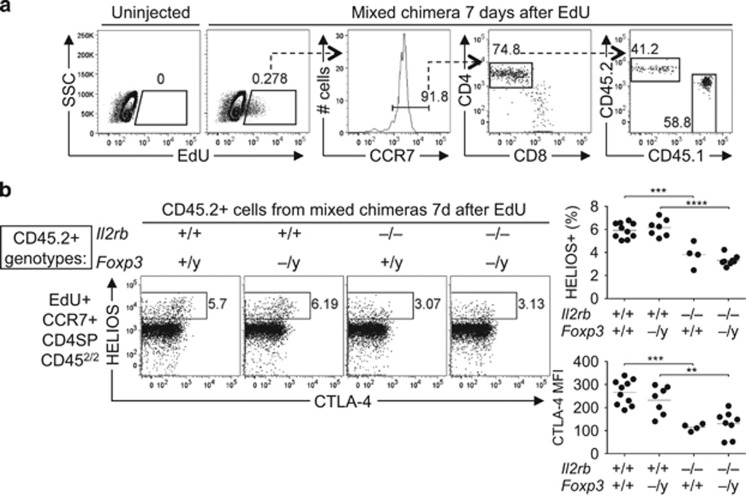
CD4SP CCR7+ Helios+ thymocytes that cannot respond to IL-2 or IL-15 are deleted. (a) Helios/Foxp3 phenotype of CCR7+ CD4SP thymocytes (top row) and CTLA-4 labelling on their Helios+ Foxp3– and Foxp3+ subsets (bottom) from wild type, Il2–/–, Il15–/– and Il2–/–Il15−/− mice on the B10.BR background examined at 28-47 days after birth. Graphs (right) show the frequency and absolute number of thymocyte subsets gated in plots (top row) or the CTLA-4 RFI (bottom row, normalised to the CCR7+ CD4SP Helios– Foxp3– subset in the same sample). (b) Helios/Foxp3 phenotype of CD45.2– and CD45.2+ subsets of CCR7+ CD4SP thymocytes (top row), and CTLA-4 labelling on their Helios+ Foxp3– and Foxp3+ subpopulations (bottom row), from B6.Rag1–/– mice that were irradiated and reconstituted 8 weeks earlier with BM mixtures from CD45.1+ wild-type mice plus CD45.2+ mice that were either Il2rb+/+ or Il2rb–/–. Graphs show the frequencies of populations gated in plots (top row) or the CTLA-4 MFI (bottom row). (c) Chimeric mice described in (b) were injected with EdU 7 days before analysis and EdU+ thymocytes were examined as in (b). Graph shows the frequencies of populations gated in plots. Lines on graphs join measurements from an individual mouse. Data in (a) were compiled from four experiments, (b and c) from one experiment. Two-tailed Student's t-tests (unpaired in a and paired in b and c) P-value symbols: * 0.01–0.05, ** 0.001–0.01, *** 0.0001–0.001; **** <0.0001
To examine the role of cell-autonomous IL-2 and IL-15 signalling in a context free of systemic immune dysregulation, mixed chimeras were generated in which some haemopoietic cells lacked expression of CD122 (encoded by Il2rb), a receptor for IL-2 and IL-15.21 At 8 weeks after reconstitution, no change was observed in the Helios+ Foxp3– cell frequency derived from Il2rb–/– BM, but there was a significant decrease in the Foxp3+ T-reg cell frequency derived from Il2rb–/– BM (Figure 4b). CTLA-4 expression on T-reg cells was also reduced in cells derived from Il2rb–/– BM (Figure 4b). Thus, a similar phenotype was observed in CD4SP CCR7+ thymocytes lacking either CD25 or CD122, with no detectable defect in the Helios+ Foxp3– population, but a sharp decrease in Foxp3+ T-reg cells.
The onset of IL-2 dependence coincides with Foxp3 upregulation
While the results above suggest that the onset of IL-2 dependence and Foxp3 upregulation coincide in CD4SP CCR7+ Helios+ thymocytes, some of the cells detected may have derived from mature cells that entered the thymus from the periphery.16, 22 To focus our analysis on a synchronised cohort of nascent thymocytes, we injected mice with 5-ethynyl-2'-deoxyuridine (EdU), which is incorporated into DNA-synthesising thymocytes predominantly at the stage immediately preceding αβ TCR-dependent selection.5 In a time-course experiment we demonstrated that nascent EdU+ T-reg cells peak at 6-8 days after EdU injection.5 Seven days after the mixed chimeras described in Figure 4b were injected with EdU, the EdU+ CD4SP CCR7+ population derived from Il2rb–/– BM cells showed a selective deficiency of Foxp3+ cells, but Helios+ Foxp3– cells were present at normal frequencies (Figure 4c). This indicates that IL-2 signalling is required for the transition from the Helios+ Foxp3– stage to the Foxp3+ T-reg stage of thymic T-reg differentiation.
Foxp3 is not required for thymocyte deletion
Since the CD25-deficient and CD122-deficient cells studied above had normal Foxp3 genes, it remained possible that Foxp3 was responsible for deleting the thymocytes that could not respond to IL-2 or IL-15, as recently hypothesised.8 To test this possibility we mixed BM from wild-type CD45.1+ mice and CD45.2+ donor mice with inactivating mutations in the Il2rb and/or Foxp3 genes and generated mixed chimeras as above. The chimeric mice were injected with EdU 7 days before analysis of EdU+ CD4SP CCR7+ CD45.2+ thymocytes. Foxp3-deficiency alone had no effect on the frequency of Helios+ cells, whereas CD122 deficiency significantly decreased the frequency of Helios+ cells (Figure 5b). If CD122 signalling were required to counteract Foxp3-dependent deletion, it would be hypothesised that the Helios+ cell frequency would increase in Foxp3/CD122 double-deficient cells in comparison with CD122 single-deficient cells. This was not the case, as similar frequencies of Helios+ cells were observed (Figure 5b), indicating that Foxp3 is not required for deletion of CD4SP CCR7+ Helios+ thymocytes that lack CD122 expression. CD122 deficiency significantly decreased CTLA-4 expression on EdU+ CD4SP CCR7+ Helios+ thymocytes, whereas Foxp3-deficiency had no effect on CTLA-4 expression (Figure 5b). These results show that CD122 prevents a deletion mechanism that is Foxp3 independent.
Figure 5.
Deletion of CD122-deficient CD4SP CCR7+ Helios+ cells is Foxp3 independent. CD45.1+ mice were irradiated and reconstituted with cell mixtures containing CD45.1+ wild-type BM plus CD45.2+ BM from wild-type, Foxp3null/y, Il2rb–/– or Il2rb–/– Foxp3null/y siblings. Chimeric mice were examined 6–7 weeks later, 7 days after a single EdU injection. (a) Gating strategy to analyse nascent cells derived from thymocytes that were proliferating 7 days prior to analysis. Left plots show side-scattered light (SSC) versus EdU on all thymocytes, with a gate for EdU+ events that was empty in the uninjected control. The CCR7+ subset of EdU+ cells was analysed for CD4/CD8, and the CD4SP subset was then analysed for expression of CD45.1 versus CD45.2. (b) Plots show Helios/CTLA-4 phenotype of EdU+ CCR7+ CD4SP CD45.2+ cells. Graphs show the frequencies of Helios+ events (top) or the CTLA-4 MFI of Helios+ events (bottom) as gated in the plots. We generated nine chimeras per group, except for the group bearing wild-type BM cells, which contained 10 mice. Possibly due to intraperitoneal injection failure, EdU+ cells were not detected in thymus samples from some EdU-injected mice; these samples were excluded from the analysis. Graphs show data compiled from two independent experiments with each symbol representing one mouse. Unpaired two-tailed Student's t-tests P-value symbols: ** 0.001–0.01, *** 0.0001–0.001; **** <0.0001
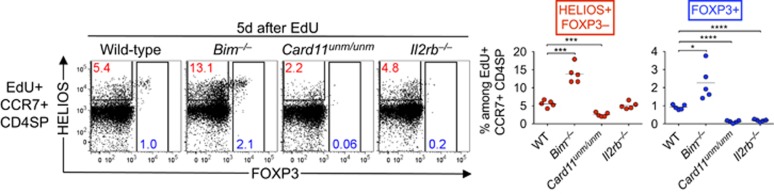
IL-2 signalling prevents deletion of developing T-reg cells at a later stage than Card11
In contrast to the results above, CD4SP CCR7+ Helios+ Foxp3– thymocytes are decreased by loss-of-function mutations in Card11 or Rel,6 suggesting that TCR-CD28 signalling through Card11 to activate NF-κB/c-Rel is required at an earlier stage of thymic T-reg differentiation than IL-2. We wished to test that hypothesis directly, and simultaneously verify that many thymocytes with the potential to become T-reg cells undergo deletion mediated by the proapoptotic protein, Bim (encoded by the Bcl2l11 gene). We irradiated B6.Rag1–/– mice and injected wild-type, Bcl2l11–/–, Card11unm/unm or Il2rb–/– BM to reconstitute haematopoiesis. These chimeras were examined 5 days after EdU injection, which is when EdU+ CCR7+ Helios+ Foxp3– thymocytes peak and EdU+ CCR7+ Foxp3+ thymocytes are first detectable in substantial numbers.5 Among EdU+ CD4SP CCR7+ cells, the frequency of Helios+ Foxp3– cells was increased in recipients of Bcl2l11–/– BM and decreased in recipients of Card11unm/unm BM (Figure 6), as previously observed in non-chimeric mice.6 In contrast, the Helios+ Foxp3– population in recipients of Il2rb–/– BM was not significantly different from wild-type controls (Figure 6). The frequency of Foxp3+ cells was increased in recipients of Bcl2l11–/– BM, and decreased in recipients of Card11unm/unm or Il2rb–/– BM, relative to wild-type controls (Figure 6). Thus, Card11 and IL-2 signals prevent Bim-dependent deletion at distinct stages of thymic T-reg cell differentiation, with Card11 required at the Helios+ Foxp3– stage, whereas IL-2 signalling is required later, approximately at the onset of Foxp3 expression.
Figure 6.
CD122 prevents Bim-dependent deletion at a later stage than Card11. Helios/Foxp3 phenotypes of EdU+ CCR7+ CD4SP thymocytes 5 days after EdU injection of B6.Rag1–/– mice that were irradiated and reconstituted 35 days before analysis with BM from wild-type, Bim–/–, Card11unm/unm or Il2rb–/– mice. Graphs (right) show the frequencies of populations gated in the plots with each symbol representing one mouse. Data were compiled from a single experiment. Two-tailed unpaired Student's t-tests were used to compare each gene-deficient group with the wild-type group; P-value symbols: * 0.01–0.05, *** 0.0001–0.001; **** <0.0001
Discussion
The expression of Helios at high levels marks T cells bound for deletion at any stage of thymocyte development and also marks CD4SP CCR7+ thymocytes poised to upregulate Foxp3. To elucidate mechanisms that distinguish deletion and T-reg differentiation, we concentrated on CD4SP CCR7+ Helios+ thymocytes that are susceptible to either fate. We show that Card11 and IL-2 signalling are essential to prevent deletion of CD4SP CCR7+ Helios+ thymocytes at distinct stages of development. Our results distinguish Card11-regulated and IL-2-regulated bottlenecks at which strongly self-reactive thymocytes undergo deletion or progress to the next stage of T-reg differentiation. The deletion prevented by IL-2 signalling is Foxp3 independent.
Although it was known that Card11 and IL-2 are necessary for thymic T-reg differentiation, the present study shows that these molecules are essential to prevent apoptotic deletion initiated by strong TCR signalling. Card11 is dispensable for a CD4SP CCR7+ thymocyte to register a strong TCR signal, but Card11 is essential to prevent apoptotic deletion in response to strong TCR signalling.6 This interpretation is consistent with several observations in the literature. First, in strongly TCR signalled CD4SP thymocytes, T-reg differentiation occurred when the thymocytes were induced to express CD25 (a Card11-dependent event6), whereas deletion occurred when the thymocytes did not upregulate CD25.23 Second, T-reg differentiation occurred when strongly TCR signalled CD4SP thymocytes could express the co-stimulatory receptor CD28, which promotes Card11-dependent CD25 upregulation in T cells,24 whereas deletion occurred when the thymocytes lacked CD28 expression.25 Third, 12 h after CD4SP thymocytes were injected into a thymus expressing a deleting self-antigen, almost all of the surviving cells were CD25+.26 We hypothesise that the outcome of the first strong TCR signalling event in a CD4SP CCR7+ thymocyte is determined by competition between a Bim-dependent pro-deletion programme and a Card11-dependent pro-survival programme. The latter programme is successful in a minority of cells, which attain a Helios+ Foxp3– phenotype. As the absence of IL-2 signalling did not decrease the CD4SP CCR7+ Helios+ Foxp3– thymocyte population, the Card11-regulated bottleneck in thymic T-reg differentiation is IL-2 independent.
The IL-2-regulated bottleneck occurs later in thymic T-reg differentiation, around the stage when CD4SP CCR7+ Helios+ Foxp3– cells upregulate Foxp3. While it was known that IL-2 is sufficient to upregulate Foxp3,11 we show here that CD4SP CCR7+ Helios+ thymocytes that cannot respond to IL-2 are deleted, whereas wild-type cells progress to the next stage of T-reg differentiation characterised by upregulation of Foxp3 and CTLA-4. It was hypothesised that IL-2 signalling is necessary for the proliferation of cells already expressing Foxp3.13 As most proliferating Foxp3+ cells in the thymus are mature CCR7– CD24– T-reg cells,5 preferential loss of this mature T-reg population in the thymus may account for the decreased proliferation observed in the absence of IL-2 signalling.13 Another hypothesis is that IL-2 signalling is required to counteract a proapoptotic protein signature induced by Foxp3.8 This hypothesis was based, in part, on the observation that transgene-driven Foxp3 expression in total thymocytes decreases thymic cellularity, and that inhibiting apoptosis offsets this effect.8 However, it cannot be assumed that Foxp3's proapoptotic effect in total thymocytes would also operate in cells that upregulate Foxp3 at the physiological stage of thymocyte development. The hypothesis was also based on the detection of a substantial thymic Foxp3+ population in apoptosis-defective mice that lack the common γ-chain cytokine receptor, CD132.8 This raised the possibility that cytokine signalling may be required for thymic T-reg development only after Foxp3 is upregulated. Our findings exclude this possibility because IL-2 is still required to prevent deletion of CD4SP CCR7+ Helios+ thymocytes that are incapable of expressing Foxp3. Our data are consistent with the hypothesis that IL-2 prevents deletion initiated by strong TCR signalling.15
It is accepted that thymic T-reg development can pass through either a Foxp3– CD25+ or a Foxp3+ CD25– stage. The standardisation of T-reg precursor analysis based on a Foxp3– CD25+ CD4SP phenotype advanced the field considerably.11 The identification of a thymic Foxp3+ CD25– T-reg precursor was also an advance,27 particularly when it was shown that apoptosis defects increase preferentially the Foxp3+ CD25– population over the Foxp3+ CD25+ population.7, 8 Because CD25 upregulation was thought to mark strongly TCR signalled CD4SP thymocytes,28 the detection of Foxp3+ CD25– thymocytes may lead to the interpretation that Foxp3 upregulation does not necessarily require a strong TCR signal. In this regard, it is notable that nascent Bcl2l11–/– Foxp3+ thymocytes are essentially all Helios+ (Figure 6). This suggests that Foxp3 upregulation in thymocytes does require TCR signalling above a certain threshold, including in the context of defective apoptosis, and that high Helios expression marks CD4SP CCR7+ thymocytes that have experienced TCR signalling above this threshold.
The alternative pathway for thymic T-reg development also attracted attention when it was shown that IL-15 was required for normal formation of Foxp3+ CD25– T-reg precursors, whereas this population remained intact when IL-2 was neutralised using anti-IL-2 antibodies.29 This conclusion was based on experiments that used Zap70-transgenic mice, which have a sevenfold lower frequency of Foxp3+ CD25+ cells than wild-type mice.29 Although not all reports have found an effect of IL-2 deficiency on thymic Foxp3+ cell frequency and number, a unifying finding is a marked decrease in Foxp3+ CD25+ cells.13, 14, 30, 31, 32 When all thymocytes are Zap70-transgenic, the amount of IL-2 in the thymus might be lower than in wild-type mice. This would explain the greater dependence of T-reg development on IL-1520 and the marked decrease in Foxp3+ CD25+ cells observed in the Zap70-transgenic model.29 We were unable to detect any role for IL-15 in T-reg development when IL-2 expression was intact. Our approach should be sensitive to effects on both pathways of thymic T-reg development. In recent studies using EdU pulse labelling to identify nascent thymocytes, ~50% of nascent T-reg cells were shown to be Foxp3+ CD25– cells.5 Therefore, the observed 80% decrease in nascent T-reg cells in thymocytes lacking CD122 (Figures 4 and 6) must involve decreases in both pathways of T-reg development. Our approach is informative in delineating steps required for CD4SP CCR7+ thymocytes to become T-reg cells. The Helios+ Foxp3– subset is susceptible to apoptotic deletion due to receipt of an above-threshold TCR signal, while Foxp3 upregulation marks the onset of IL-2 dependence in developing T-reg cells.
By distinguishing two bottlenecks regulated by Card11 and IL-2, our study draws attention to how the CD4SP thymocyte response to strong TCR signalling might mature over time. Imaging experiments have revealed that the first strong TCR signalling event is characterised by migratory arrest, but those cells that survive subsequently begin to migrate actively33 and interact repetitively with dendritic cells (DC) within ~30 μm of their original position.34 It was shown recently that thymic DC are an important source of IL-2,35 which has a focal distribution in the thymic medulla.36 Via repetitive interactions, T-reg precursors might induce local DC to produce the IL-2 that the T-reg precursors require to progress to the next stage of T-reg differentiation. If IL-2 synthesis increases with the avidity of T-reg precursor/DC interactions, it would explain why TCRs with higher avidity for self-antigen facilitate the development of larger thymic Foxp3+ T-reg cell populations.37 We speculate that apoptotic deletion at the CD4SP CCR7+ stage of thymocyte development impinges on T-reg differentiation not by eliminating cells with too high avidity for self-antigen, but instead by eliminating cells with too low avidity for self-antigen. This hypothesis is consistent with the extremely high avidity for self-antigen observed in T-reg TCRs,38 well above the threshold required for apoptotic deletion.39
Materials and methods
Mice
Foxp3GFP (Foxp3tm1.1Ayr), Foxp3null (Foxp3tm1Ayr), Il2–/– (Il2tm1Hor), Il15–/– (Il15tm1Imx), Rag1–/– (Rag1tm1Mom), Il2rb–/– (Il2rbtm1Mak), Bcl2l11–/– (Bcl2l11tm1Ast) and Card11unm mice have been described previously and were bred and housed at the Australian Phenomics Facility, Canberra or at Monash University, Melbourne. Foxp3null/nullRag1–/–females were bred with B6 males to produce Foxp3+/null and Foxp3null/y mice. All other experimental mice were the PCR-genotyped progeny of mice heterozygous for the variant allele(s). BM from Il2ra–/– (Il2ratm1Dw) and Il2rb–/– mice was kindly provided by Dr. Jaeho Cho and Prof. Jon Sprent, Garvan Institute, Sydney. To make chimeras, recipient mice were irradiated with X-rays (two doses of 4.5 Gy given 4 h apart for Rag1+/+ or one dose of 5 Gy for Rag1–/– recipients) and then injected intravenously with at least 2 × 106 bone marrow cells. The Animal Experimentation Ethics Committees of the Australian National University or Monash University approved all procedures.
Flow cytometry
For CCR7 staining, single-cell thymocyte suspensions were incubated for 60 min at 37 °C in pre-warmed FACS buffer (PBS containing 2% v/v heat-inactivated bovine serum and 0.01% m/v sodium azide) containing fluorochrome- or biotin-conjugated anti-CCR7 (BioLegend, San Diego, CA, USA; Cat #120104 or 120105). Cells were then pelleted by centrifugation and incubated for 30 min in FACS buffer at 4 °C containing assortments of fluorochrome-conjugated monoclonal antibodies against CD4 (BioLegend; Cat #100430), CD8α (BioLegend; Cat #100765), CD45.1 (BioLegend; Cat #110739), CD45.2 (BioLegend; Cat #109828). After washing in FACS buffer, cells were fixed and permeabilised using the Foxp3/Transcription Factor Staining Buffer Set (Affymetrix eBioscience, Santa Clara, CA, USA), and then incubated with antibodies specific for Helios (BioLegend; Cat #137220), CTLA-4 (eBioscience; Cat #11-5773-82) and Foxp3 (eBioscience; Cat # 11-5773-80). After washing in FACS buffer, data were acquired with LSR II flow cytometers (Becton Dickinson, Franklin Lakes, NJ, USA) and analysed using FlowJo software (FlowJo LLC, Ashland, OR, USA).
EdU labelling
0.25 mg EdU was injected i.p. (0.1 ml of a 2.5 mg/ml m/v in DMSO or PBS) per mouse. After intracellular staining as described above, cells were processed following the manufacturer's instructions (Click-iT EdU Flow Cytometry Assay Kit; Thermo Fisher Scientific, Waltham, MA, USA) except that Click-iT EdU buffer additive (Component G) was used at one-fifth of the concentration recommended. Samples were then washed in FACS buffer and incubated with streptavidin-PE (BioLegend; Cat# 405204) to detect biotin-conjugated anti-CCR7.
Cell culture
Thymocytes from Foxp3GFP mice were stained with antibodies specific for cell surface antigens as above with the addition of anti-CD24 (BioLegend; Cat # 101822) in the 4 °C incubation. GFP– CD4SP thymocyte subsets, sorted using a FACS Aria II instrument (Becton Dickinson), were cultured in complete medium for 20 h at 37 °C in the presence or absence of graded concentrations of recombinant human IL-2 (R&D Systems, Minneapolis, MN, USA) before intracellular staining for Helios, Foxp3 and CTLA-4 as described above.
Statistical analysis
GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA) was used to make summary graphs and perform statistical tests as described in figure legends.
Acknowledgments
We thank Anna Chan and Debbie Howard for expert technical assistance, Jon Sprent and Jaeho Cho for providing bone marrow from Il2ra–/– and Il2rb–/– mice, and the Australian Phenomics Facility staff for genotyping and animal husbandry. This research was supported by the National Health and Medical Research Council Program Grant 1016953 and Australia Fellowship 585490 to CCG, and by the Monash Biomedicine Discovery Institute.
Footnotes
Edited by P Bouillet
The authors declare no conflict of interest.
References
- Baldwin KK, Trenchak BP, Altman JD, Davis MM. Negative selection of T cells occurs throughout thymic development. J Immunol 1999; 163: 689–698. [PubMed] [Google Scholar]
- Douek DC, Corley KT, Zal T, Mellor A, Dyson PJ, Altmann DM. Negative selection by endogenous antigen and superantigen occurs at multiple thymic sites. Int Immunol 1996; 8: 1413–1420. [DOI] [PubMed] [Google Scholar]
- McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med 2008; 205: 2575–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med 2013; 210: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DY, Yap JY, Wirasinha RC, Howard DR, Goodnow CC, Daley SR. A timeline demarcating two waves of clonal deletion and Foxp3 upregulation during thymocyte development. Immunol Cell Biol 2016; 94: 357–366. [DOI] [PubMed] [Google Scholar]
- Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. J Exp Med 2013; 210: 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Zhang Y, Gray D, Carrington EM, Bouillet P, Ko HJ et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol 2011; 187: 1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 2013; 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J Immunol 2009; 182: 6736–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 2009; 206: 3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity 2008; 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol 2009; 9: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Yu A, Dee MJ, Malek TR. IL-2R signaling is essential for functional maturation of regulatory T cells during thymic development. J Immunol 2013; 190: 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005; 6: 1142–1151. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 2008; 28: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan JE, McCarthy NI, Anderson G. CCR7 controls thymus recirculation, but not production and emigration, of Foxp3(+) T cells. Cell Rep 2016; 14: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007; 445: 936–940. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330–336. [DOI] [PubMed] [Google Scholar]
- Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 1991; 352: 621–624. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol 2007; 178: 280–290. [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J 1994; 13: 2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C et al. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat Immunol 2015; 16: 628–634. [DOI] [PubMed] [Google Scholar]
- Cozzo Picca C, Simons DM, Oh S, Aitken M, Perng OA, Mergenthaler C et al. CD4(+)CD25(+)Foxp3(+) regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci USA 2011; 108: 14890–14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JE, Wilson LE, Vinuesa CG, Lesage S, Blery M, Miosge LA et al. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 2003; 18: 751–762. [DOI] [PubMed] [Google Scholar]
- Hinterberger M, Wirnsberger G, Klein L. B7/CD28 in central tolerance: costimulation promotes maturation of regulatory T cell precursors and prevents their clonal deletion. Front Immunol 2011; 2: 30.. [DOI] [PMC free article] [PubMed]
- Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc Natl Acad Sci USA 2009; 106: 10278–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med 2005; 202: 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol 2001; 2: 301–306. [DOI] [PubMed] [Google Scholar]
- Marshall D, Sinclair C, Tung S, Seddon B. Differential requirement for IL-2 and IL-15 during bifurcated development of thymic regulatory T cells. J Immunol 2014; 193: 5525–5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 2005; 6: 1152–1159. [DOI] [PubMed] [Google Scholar]
- Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol 2010; 185: 6426–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Siggs OM, Goodnow CC. Tracing the action of IL-2 in tolerance to islet-specific antigen. Immunol Cell Biol 2007; 85: 338–342. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Katagiri K, Tomiyama T, Yasuda K, Habiro K, Katakai T et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun 2012; 3: 1098. [DOI] [PubMed] [Google Scholar]
- Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol 2009; 10: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 2015; 16: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Snyder JA, Rothenberg EV. Spontaneous expression of interleukin-2 in vivo in specific tissues of young mice. Dev Immunol 1998; 5: 223–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity 2012; 37: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieback E, Hilgenberg E, Stervbo U, Lampropoulou V, Shen P, Bunse M et al. Thymus-derived regulatory T cells are positively selected on natural self-antigen through cognate interactions of high functional avidity. Immunity 2016; 44: 1114–1126. [DOI] [PubMed] [Google Scholar]
- Wyss L, Stadinski BD, King CG, Schallenberg S, McCarthy NI, Lee JY et al. Affinity for self antigen selects Treg cells with distinct functional properties. Nat Immunol 2016; 17: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]