Abstract
A long-term epidemiological study of Cryptosporidium molnari in aquacultured European sea bass (ESB) and gilthead sea bream (GSB) was performed in different types of facilities on the Atlantic, Cantabric, and Mediterranean coasts. Four types of studies were carried out. In study A, fish raised from juveniles to marketable size (ongrowing stage) were periodically sampled in three different types of cultures. Studies B and C focused on hatchery and nursery facilities. In study D, occasional samplings were performed during mortality or morbidity outbreaks. As a general trend, C. molnari was more prevalent in GSB than in ESB. Data on the distribution pattern of C. molnari in total sampled GSB (studies A, B, and D) had a variance higher than the mean (overdispersion). In GSB (study A), the type of ongrowing system (sea cages, earth ponds, or indoor tanks) was found to have no significant effect. There was a significant relationship between the presence of the parasite and both fish weight and season. The highest infection values were recorded in spring. Prevalence and intensity had convex weight profiles, with a peak in 30- to 100-g fish. In study D, the prevalence of infection was higher in fish recently introduced in sea cages and in preongrowing systems. In studies B and C, fish were almost never infected before entering the postlarval and nursery facilities. The parasite seems to enter the host mainly through the water in production steps with less stringent water treatment. Recirculation systems and fish cannibalism could contribute to oocyst concentration and dispersion in aquaculture facilities.
Parasite populations tend to show an aggregated distribution in host populations, in which different factors, such as host size, host density, and environment, are involved (4, 35). Host-parasite relationships at the population level are complex, and many factors related to the microhabitat (the host) and the macrohabitat (the host environment) have to be considered. In farmed hosts, culture conditions clearly affect this relationship, as they influence host density and other collateral factors. Gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.) are successfully cultured in the Mediterranean Sea and the Atlantic Ocean. Their production has reached almost 100,000 tons in the Mediterranean basin (17). With the increase of intensive farming, severe disease outbreaks have been reported, including those produced by emergent parasites (9).
A new protozoan parasite, Cryptosporidium molnari (Apicomplexa) was described from cultured sea bass and sea bream (3), and homologous transmission and cross-transmission have recently been demonstrated under experimental conditions (40). Cryptosporidia are small coccidian parasites recognized as significant pathogens for humans and many other vertebrates (12, 13, 18, 19, 46). The importance of piscine species remains to be determined, but C. molnari may produce pathological effects, mainly in small fish (3). Epidemiological data are very important for ascertaining the significance of this new parasite for aquaculture. In the present paper, a long-term epidemiological study of C. molnari in different culture facilities of sea bass and sea bream is presented. Fish were studied from the hatchery to the market size stage of production in several culture systems on the Atlantic, Cantabric, and Mediterranean coasts. The influence of host and environmental factors on parasite distribution and infection levels was analyzed.
MATERIALS AND METHODS
Farms and facilities surveyed.
Epidemiological surveys of gilthead sea bream and European sea bass were conducted from December 1997 to July 2001 in several aquaculture facilities, located primarily on the Spanish coasts. The surveyed farms or facilities, their geographical location, and the type of study are detailed in Table 1. Culture systems are identified according to the production cycle in which fish were sampled (H for the hatchery, postlarval, and nursery steps and F for the step that raises fish from juveniles to marketable size in aquaculture [ongrowing]; PFG is used for the preongrowing stage). A basic management model of marine fish farms including hatchery, postlarval, nursery, preongrowing, and ongrowing facilities is presented in Fig. 1. Some sampled facilities included only some culture phases, e.g., ongrowing. All fish from ongrowing systems were reared under natural temperature and photoperiodic conditions, following the standard procedures of each farm. The indoor experimental tanks of the Instituto de Acuicultura de Torre de la Sal (IATS) received a standard flowthrough seawater supply from a pump on shore (37% salinity; 30- to 40-μm-pore-size sand filter).
TABLE 1.
Farms and facilities surveyed in the epidemiological study
| Type of facility surveyed | Farm or facility designation | Geographical location | Study
|
|
|---|---|---|---|---|
| Sea bream | Sea bass | |||
| Hatchery and nursery | H-1 | South Atlantic (Spain) | C, D | |
| H-2 | Cantabric (Spain) | B, C | B, C | |
| H-3 | Atlantic (France) | A | ||
| Preongrowing indoor tanks | PGF-1 | South Mediterranean (Almería, Spain) | A, D | |
| PGF-2 | South Atlantic (Spain) | D | ||
| PGF-3 | Northwest Atlantic (Spain) | D | ||
| PGF-4 | Southwestern Mediterranean (Murcia, Spain) | D | ||
| Ongrowing sea cages | F-1 | Western Mediterranean (Castellón, Spain) | A, D | |
| F-2 | Western Mediterranean (Tarragona, Spain) | A, D | ||
| F-3 | Western Mediterranean (North Castellón, Spain) | D | A | |
| F-4 | Southwestern Mediterranean (Murcia, Spain) | D | ||
| F-5 | Atlantic (Canary Islands, Spain) | B | ||
| Ongrowing earth ponds | F-6 | River Ebro Delta region, (Tarragona, Spain) | A, D | |
| Indoor preongrowing and outdoor ongrowing concrete ponds | F-7 | River Ebro Delta region, (Tarragona, Spain) | D | |
| Experimental ongrowing indoor facilities | IATS | Western Mediterranean (Castellón, Spain) | A, B, D | D |
FIG. 1.
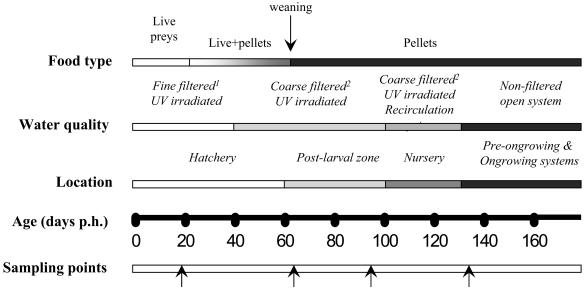
Diagrammatic representation of a typical production management model of gilthead sea bream and European sea bass from hatch to the end of the preongrowing stage, based on H-2 facilities. In study B, sampling points (arrows) were selected according to changes in food type, water quality, and location. Fine filtration, 30- to 40-μm-pore-size sand filter, plus 10- and 20-μm-pore-size sequential cartridge filters. Coarse filtration, 30- to 40-μm-pore-size sand filter only. UV irradiation was performed with 61.3 to 52.1 mJ/cm2 and a water flow of 25 to 30 m3/h (DeltaC30; Aquabona, S. L.).
Sampling schedules of four different types of studies. (i) Study A.
A total of 631 gilthead sea bream and 62 sea bass from different ongrowing systems were periodically surveyed, as detailed in Tables 2 and 3 for gilthead sea bream and sea bass, respectively. In all groups, the first sampling was done before fish entered the corresponding ongrowing facility (thus, it reflects the previous preongrowing situation) and then every 3 months until fish reached market size. For some groups, origins, including hatchery, nursery, and preongrowing facilities, were traced.
TABLE 2.
Prevalence and intensity of C. molnari in gilthead sea bream groups surveyed in ongrowing systems in study A
| System and farm | Previous origin
|
Sample no. | Sample date | nb | Fish weight (g) | Prevalence (%) | MIc | |
|---|---|---|---|---|---|---|---|---|
| Hatchery | Preongrowing | |||||||
| Sea cages | ||||||||
| F1 | H-3 | H-3 | 1a | Mar 1999 | 19 | 21.2 ± 4.7 | 0 | 0 |
| 2 | Jul 1999 | 20 | 92.3 ± 12.9 | 55 | 4 | |||
| 3 | Oct 1999 | 28 | 222.8 ± 35.0 | 3.6 | 1 | |||
| 4 | Feb 2000 | 20 | 313.5 ± 53.3 | 0 | 0 | |||
| 5 | Jul 2000 | 19 | 339.7 ± 50.1 | 0 | 0 | |||
| F2 | H-1 | PGF1 | 1a | Apr 1999 | 31 | 8.4 ± 7.2 | 3.2 | 2 |
| 2 | Jul 1999 | 16 | 41.0 ± 16.1 | 31.7 | 1.5 | |||
| 3 | Oct 1999 | 20 | 110.3 ± 19.5 | 0 | 0 | |||
| 4 | Jan 2000 | 20 | 142.3 ± 31.1 | 0 | 0 | |||
| 5 | Apr 2000 | 20 | 176.6 ± 38.6 | 0 | 0 | |||
| 6 | Jul 2000 | 17 | 281.3 ± 48.3 | 0 | 0 | |||
| Total | 230 | 6.5 | 3.3 | |||||
| Earth ponds, F6 | H-2 and H-3 | H-2 and H-3 | 1a | June 1999 | 22 | 2.1 ± 0.9 | 0 | 0 |
| 2 | Sept 1999 | 34 | 59.4 ± 12.9 | 0 | 0 | |||
| 3 | Dec 1999 | 39 | 119.2 ± 24.7 | 5 | 1.5 | |||
| 4 | Apr 2000 | 20 | 143.0 ± 31.9 | 10 | 1 | |||
| 5 | Jul 2000 | 20 | 201.8 ± 7.3 | 0 | 0 | |||
| 6 | Oct 2000 | 22 | 414.0 ± 76.5 | 0 | 0 | |||
| Total | 157 | 2.5 | 1.25 | |||||
| Indoor tanks | ||||||||
| IATS-1 | H-3 | H-3 | 1a | Mar 1999 | 19 | 21.2 ± 4.7 | 0 | 0 |
| 2 | June 1999 | 41 | 72.6 ± 16.3 | 0 | 0 | |||
| 3 | Sept 1999 | 35 | 266.1 ± 59.1 | 0 | 0 | |||
| IATS-2 | H-3 | H-3 | 1 | Jun 1999 | 20 | 9.1 ± 2.1 | 0 | 0 |
| 2 | Sept 1999 | 38 | 85.0 ± 16.3 | 0 | 0 | |||
| 3 | Dec 1999 | 34 | 157.3 ± 27.5 | 0 | 0 | |||
| 4 | Apr 2000 | 38 | 199.8 ± 44.1 | 42.1 | 4 | |||
| 5 | Jul 2000 | 30 | 349.3 ± 54.1 | 10 | 4 | |||
| Total | 244 | 7.8 | 4 | |||||
Sampling carried out before introduction in the ongrowing system.
n, number of fish examined.
MI, mean intensity of infection.
TABLE 3.
Prevalence and intensity of C. molnari in European sea bass groups surveyed in ongrowing systems in study Aa
| Sample no. | Sample date | nb | Fish weight (g) | Prevalence (%) | MI |
|---|---|---|---|---|---|
| 1c | Sept 1999 | 19 | 18.7 ± 5.8 | 57.9 | 1 |
| 2 | Jan 2000 | 5 | 42.2 ± 8.6 | 0 | 0 |
| 3 | Sept 2000 | 20 | 117.3 ± 49.6 | 0 | 0 |
| 4 | Dec 2000 | 18 | 328.8 ± 84.6 | 0 | 0 |
All groups sampled were from farm F-3, with hatchery H-1 and preongrowing facility PGF-1 as origins. For the total sample (62 fish), prevalence was 17.7% and mean intensity (MI) of infection was 1.
n, number of fish examined.
Sampling carried out before introduction in the ongrowing system.
(ii) Study B.
This study was carried out mainly at the hatchery, postlarval, and nursery facilities of H-2 throughout the year 2000, though some batches were also monitored after entering other ongrowing systems. Samplings at H-2 were usually carried out at significant points of the production cycle: before and after the weaning period and before and after 40 to 50 days of transfer to the recirculation system. Water quality and the food supply changed along with the different steps of production (Fig. 1).
Three different groups of gilthead sea bream were surveyed, as detailed in Table 4. Group B-1 was split into two subgroups with different filtration conditions on day 62 posthatching (p.h.). Group B-1a was reared with UV-irradiated and coarse-filtered water at facility H-2 (Fig. 1), and group B1-b was reared with UV-irradiated (80 to 70 mJ/cm2 with a flow of 3 m3/h) (Rex 1PE30, Sefiltra), and sequentially fine-filtered (10-, 5-, and 1-μm-pore-size filters) water at IATS facilities.
TABLE 4.
Prevalence and intensity of C. molnari in gilthead sea bream and European sea bass from the facilities surveyed in study B
| Fish species and group | Facility | Days p.h. | na | Prevalence (%) | MIb |
|---|---|---|---|---|---|
| Gilthead sea bream | |||||
| B1-a | H-2 | 19c | 9 | 0 | 0 |
| 62d | 19 | 0 | 0 | ||
| 85d | 20 | 0 | 0 | ||
| 120 | 20 | 94.7 | 5 | ||
| B1-b | H-2 | 19c | 9 | 0 | 0 |
| 62d | 19 | 0 | 0 | ||
| IATS | 132e | 20 | 0 | 0 | |
| 155e | 10 | 0 | 0 | ||
| 175e | 10 | 0 | 0 | ||
| 272e | 10 | 0 | 0 | ||
| B-2 | H-2 | 50d | 20 | 0 | 0 |
| 120 | 20 | 0 | 0 | ||
| 153g | 13 | 84.6 | 4 | ||
| IATS | 435f | 20 | 0 | 0 | |
| B-3 | H-2 | 80d | 20 | 0 | 0 |
| 117g | 20 | 0 | 0 | ||
| IATS | 152f | 24 | 100 | 5 | |
| 239f | 20 | 100 | 5 | ||
| European sea bass, B-4 | H-2 | 18c | 20 | 0 | 0 |
| 48c | 40 | 0 | 0 | ||
| 96d | 19 | 0 | 0 | ||
| 123d | 10 | 0 | 0 | ||
| F-5 | 344 | 10 | 0 | 0 |
n, number of fish examined.
MI, mean intensity of infection.
Fine-filtered water in H-2.
Coarse-filtered water in H-2.
IATS fine-filtered water.
IATS standard-filtered water.
Sampling 1 day before transfer to IATS facilities.
(iii) Study C.
Details on the sampling schedule can be found in Tables 5 and 6 for sea bream and sea bass, respectively. It included two samplings at facility H-1 (before and after weaning) and several samplings at H-2, according to the hatchery health control program.
TABLE 5.
Prevalence and intensity of C. molnari in gilthead sea bream from the facilities surveyed in study C
| Farm, facility type | Group | Sampling date | nb | Fish weight (g) or age (days p.h.) | Prevalence (%) | MIc |
|---|---|---|---|---|---|---|
| H1, hatchery | C-H1-1 | Dec 1998 | 5 | 30 days | 0 | 0 |
| C-H1-2 | Jan 1999 | 20 | 60 days | 0 | 0 | |
| Total | 25 | 0 | 0 | |||
| H2, hatchery | H2-1 | Dec 1997 | 30 | 0.1 g | 13 | 4 |
| H2-2 | Feb 1998 | 30 | 0.3 g | 40 | 4 | |
| H2-3 | May 1998 | 30 | 64 days | 0 | 0 | |
| Total | 90 | 17.7 | 4 | |||
| H2, postlarval plus nurserya | H2-1 | Dec 1997 | 90 | 1.5-3 g | 28.8 | 2 |
| H2-2 | Feb 1998 | 30 | 3 g | 30 | 2 | |
| H2-3 | May 1998 | 90 | 86-144 days | 28.9 | 2 | |
| H2-4 | Jul 1998 | 60 | 71-88 days | 11.6 | 4 | |
| H2-5 | Oct 1998 | 60 | 1-1.5 g | 28.3 | 2 | |
| H2-6 | Jan 2000 | 180 | 60-140 days | 25.5 | 4 | |
| Total | 510 | 25.7 | 2.4 | |||
| H1 plus H2, hatcherya | 115 | 13.9 | 4 |
Statistically significant difference between groups at P < 0.01.
n, number of fish examined.
MI, mean intensity of infection.
TABLE 6.
Prevalence and intensity of C. molnari in European sea bass in the facilities surveyed in study C
| Farm, facility type | Group | Sampling date | nb | Fish weight or agec | Prevalence (%) | MId |
|---|---|---|---|---|---|---|
| H1, hatchery | H1-1 | Mar 1999 | 12 | 37 days | 0 | 0 |
| H1-2 | Apr 1999 | 15 | 67 days | 0 | 0 | |
| Total | 27 | 0 | 0 | |||
| H2, hatchery | H2-1 | Feb 1998 | 30 | 0.3 g | 16.6 | 4 |
| H2-2 | May 1998 | 30 | 60 days | 26.6 | 2 | |
| H2-3 | Jan 2000 | 30 | 50 days | 0 | 0 | |
| Total | 90 | 14.4 | 3 | |||
| H2, postlarval plus nurserya | H2-1 | Feb 1998 | 90 | 0.7-1.5 g | 37.7 | 2 |
| H2-2 | May 1998 | 90 | 100-137 days | 47.7 | 3 | |
| H2-3 | Jan 2000 | 30 | 1-2 g | 0 | 0 | |
| H2-4 | May 2000 | 10 | 2.5 g | 50 | 2 | |
| Total | 220 | 37.3 | 2.8 | |||
| H1 plus H2, hatcherya | 117 | 11.1 | 3 |
Statistically significant difference between groups at P < 0.0001.
n, number of fish examined.
Weight in g; age in days p.h.
MI, mean intensity of infection.
(iv) Study D.
A total of 324 gilthead sea bream and 25 sea bass were occasionally sampled, mostly when mortality or morbidity outbreaks occurred, as detailed in Tables 7 and 8 for sea bream and sea bass, respectively.
TABLE 7.
Prevalence and intensity of C. molnari in gilthead sea bream surveyed in study D
| Type of facility | Groupa | Sampling date | nb | Fish weight (g) | Prevalence (%) | MIc |
|---|---|---|---|---|---|---|
| Preongrowing | D-PGF1-1 | Jul 2001 | 20 | 2.1 ± 0.5 | 25 | 5.7 |
| D-PGF2-1 | May 2001 | 20 | 12.8 ± 1.8 | 0 | 0 | |
| D-PGF3-1 | May 2000 | 20 | 67.9 ± 11.7 | 10 | 1 | |
| D-PGF3-2 | Sept 2000 | 18 | 38.7 ± 14.2 | 11.1 | 2 | |
| Sea cages | D-F1-1 | Apr 1999 | 10 | 123.4 ± 36.6 | 10 | 1 |
| D-F1-2 | Mar 2000 | 20 | 17.1 ± 5.8 | 10 | 2 | |
| D-F2-1 | Jul 1999 | 8 | 127.6 ± 49.6 | 0 | 0 | |
| D-F3-1 | Jul 1999 | 7 | 121.9 ± 31.7 | 0 | 0 | |
| Sept 1999 | 12 | 244.2 ± 34.9 | 0 | 0 | ||
| D-F3-2 | Jul 1999 | 8 | 81.9 ± 29.7 | |||
| D-F3-3 | Jul 1999 | 10 | 20.1 ± 6.4 | 8 | 3.8 | |
| D-F3-4 | Jul 1999 | 10 | 21 ± 5.3 | 8 | 2.1 | |
| Ongrowing earth ponds | D-F6-1 | Feb 2000 | 12 | 27.8 | 50 | 4.3 |
| Concrete ponds | D-F7-1 | May 2001 | 20 | 3.0 ± 1.1 | 0 | 0 |
| June 2001 | 20 | 5.4 ± 2.2 | 0 | 0 | ||
| Indoor ongrowing tanks and experimental facilities | D-IATS-1 | June 2000d | 20 | 3.9 ± 1.1 | 65 | 2.86 |
| Jul 2000 | 9 | 6.5 ± 2.3 | 0 | 0 | ||
| Aug 2000 | 20 | 16.9 ± 5.4 | 5 | 1 | ||
| Feb 2001 | 10 | 65.1 ± 7.8 | 0 | 0 | ||
| D-IATS-2 | Jul 2000e | 20 | 4.5 ± 1.3 | 10 | 3.5 | |
| Sept 2000 | 20 | 32.7 ± 8.3 | 5 | 1 | ||
| Feb 2001 | 10 | 68.7 ± 13.6 | 0 | 0 |
The corresponding farm or facility is included in the designation of each group.
n, number of fish examined.
MI, mean intensity of infection.
First sampling in the preongrowing facilities of F7.
First sampling in PGF-4.
TABLE 8.
Prevalence and intensity of C. molnari in European sea bass surveyed in study D
| Type of facility | Group | Sampling date | na | Fish weight (g) | Prevalence (%) | MIb |
|---|---|---|---|---|---|---|
| Hatchery | L-F4-1 | June 1999 | 5 | 250 | 0 | 0 |
| Indoor tanks | L-IATS-1 | May 2000 | 20 | 5.7 ± 1.09 | 10 | 3 |
n, number of fish examined.
MI, mean intensity of infection.
Parasitological study and histological processing.
Most fish were transported alive to IATS facilities and sampled at their arrival or within the next day. Occasionally, fish were sampled on-site, and formalin-fixed tissue portions were sent for histological processing. Fish were sacrificed, and tissue portions were processed for histology as previously described (3, 40). The presence of C. molnari stages was diagnosed mostly in histological sections, though in some groups only smears of mucosal scrapings stained with Giemsa were studied (3). The intensity of infection was semiquantitatively evaluated according to a scale (1+ to 6+) based on the number of parasitic stages per microscope field (at a magnification of ×625) as follows: 1+, 1 to 5; 2+, 5 to 10; 3+, 10 to 20; 4+, 20 to 30; 5+, 30 to 50; 6+, >50. Uninfected fish were scored 0.
Statistical analysis.
Prevalence and mean intensity were calculated for each sampled group. In studies A and C, the influence of the type of facilities and the production stage on the presence of C. molnari was statistically analyzed by using a chi-square test of independence (41), with a Yates correction for continuity when necessary. The Fisher exact test was run when the expected values of the contingency table were very low. This test was also used to analyze the relationship between prevalence of infection and fish weight and season in study A. In addition, a nonlinear regression was used to study the distribution of the prevalence of infection among fish weight classes and seasons. All the statistical analyses were performed with SigmaStat software (SPSS Inc., Chicago, Ill.).
In order to study the dispersion pattern and the age (weight)-intensity profile of C. molnari in gilthead sea bream cultures, data from studies A, B, and D were merged. The variance to mean ratio (VMR) was calculated for total sampled fish and for each weight class. In addition, the frequency distribution of the parasite within the sampled hosts was obtained.
RESULTS
Study A. (i) Gilthead sea bream (Table 2).
In both sea cage farms, prevalence and intensity of infection were maximum in the second sampling, though fish were introduced uninfected in ongrowing facility F-1 and infected in F-2. Later on, a decrease in infection levels occurred, and the parasite was not found in the last samplings. In earth ponds (F-6), fish were found infected only in samplings 3 and 4. In contrast to the remaining systems, C. molnari was only found in samplings 4 and 5 of the IATS-2 group (indoor facilities).
The overall results indicate slight differences according to the ongrowing system, with maximum infections in indoor facilities, followed by sea cages and ponds. It is remarkable that fish from the same origin introduced at the same time in facility F-1 and IATS were parasitized in F-1 but not in IATS (group IATS-1).
(ii) European sea bass (Table 3).
Fish were found infected only in the first sampling, before introduction in F-3 (sea cages). Subsequent samplings in this facility were negative.
Study B. (i) Gilthead sea bream (Table 4).
In group B-1, the parasite was not detected in the hatchery facilities (subgroup B-1a) until 120 days p.h., when prevalence was 94.7% In contrast, fish transferred to IATS (subgroup B-1b) and kept with fine-filtered water were never found parasitized, even at day 272 p.h.
In both groups B-2 and B-3, the two first samplings at H-2 were negative, whereas high prevalence and intensity were observed at the third sampling (in H-2 for group B-2 and in IATS for group B-3). However, in subsequent samplings after transfer to IATS (239 and 435 days p.h., respectively, for B-2 and B-3), the parasite was not found.
(ii) European sea bass (Table 4).
The parasite was not found in any of the samples carried out in group B-4.
Study C. (i) Gilthead sea bream (Table 5).
The two samplings carried out at facility H-1 (30 and 60 days p.h.) gave a negative result for C. molnari. In H-2, some fish groups were found in the hatchery already infected with medium intensity, and all groups examined from the postlarval-nursery tanks were infected. Thus, the total prevalence was somewhat higher in the postlarval-nursery facility than in the hatchery (25.7 versus 17.7%), though the mean intensity of infection was slightly lower. As there were no statistically significant differences between H-1 and H-2, data from both hatcheries were merged and compared to those of the postlarval-nursery facility. In such a comparison, a statistically significant relationship between the rearing step (hatchery and postlarval-nursery step) and the presence of the parasite was found (P < 0.01).
(ii) European sea bass (Table 6).
The two samplings carried out in H-1 (30 and 60 days) were also negative for C. molnari. In H-2, infection was found in both the hatchery and the postlarval-nursery periods, with a higher prevalence in the latter. As no statistically significant differences between H-1 and H-2 were found, merged data from both hatcheries were compared with those of the postlarval-nursery facility, and a statistically significant relationship between the type of facility and the presence of the parasite was also found for this host (P < 0.0001).
Study D. (i) Gilthead sea bream (Table 7).
Infection levels were highly variable among the different groups examined. High prevalence and intensity of infection were usually observed in fish sampled in preongrowing systems. A progressive decrease in infection levels was observed in IATS groups after their introduction into the facilities. In sea cages, the parasite was found occasionally, mainly in small fish. Notable were the high prevalence and intensity of infection in fish from earth ponds (F-6) with weights of 27.8 g.
(ii) European sea bass (Table 8).
Only small fish recently introduced in IATS facilities were found infected with low prevalence and medium intensity.
Analysis of the distribution of C. molnari throughout gilthead sea bream cultures (i) Dispersion pattern in total sampled fish (studies A, B, and D).
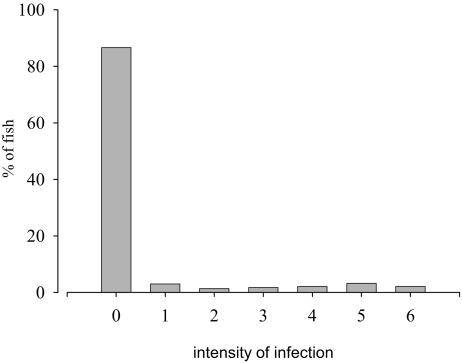
In Fig. 2, the frequency distribution of C. molnari for total sampled animals is shown. Pattern of distribution data had a variance clearly higher than the mean (overdispersed), with aggregation in the null intensity (absence of the parasite). The VMR was 2.46.
FIG. 2.
Frequency distribution of C. molnari for total sampled gilthead sea bream.
(ii) Culture system effect (study A).
There was no significant dependency between the type of ongrowing facilities (sea cages, earth ponds, or indoor tanks) and the presence of C. molnari. However, the type of cage facility was found to be significantly related to the prevalence of infection (P = 0.014), with infection levels higher in F-1 than in F-2 (Table 2).
(iii) Season effect (study A).
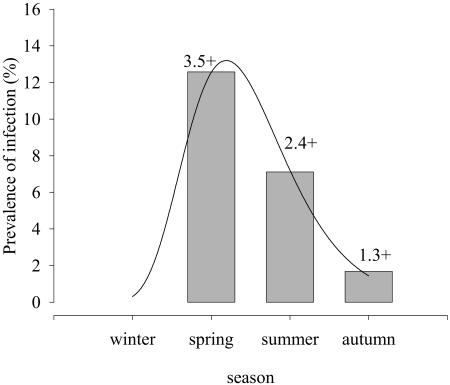
The relationship between the prevalence of infection and season adjusted well to a nonlinear regression curve (R 2 = 0.998, P = 0.041) (Fig. 3). A statistically significant dependency was found between prevalence of infection and the season (P < 0.001). No fish was found parasitized in winter, whereas prevalence and intensity reached peak values in spring, with a progressive decrease in summer and autumn.
FIG. 3.
Nonlinear regression adjustment of C. molnari prevalence of infection versus season in gilthead sea bream, study A (R2 = 0.998, P = 0.041). Values above each bar indicate the mean intensity of infection.
(iv) Fish weight effect.
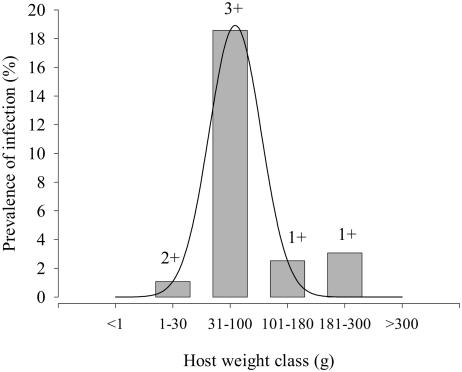
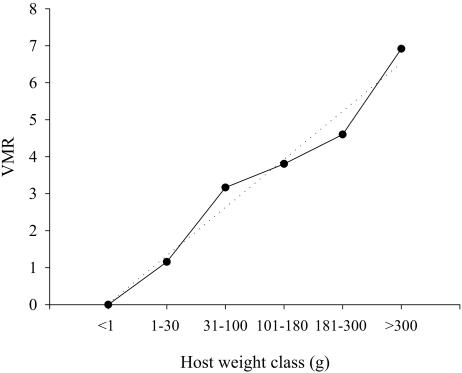
Figure 4 shows the distribution of C. molnari infection according to weight in fish from commercial farms of study A. Both the prevalence and the intensity of infection showed a convex profile, and the former followed a nonlinear regression curve with a good adjustment (R2 = 0.9631, P = 0.0071). The highest values were recorded in the 30- to 100-g fish class, with infection levels subsequently decreasing in larger animals and disappearing in fish weighing more than 300 g. The statistical analysis detected a significant dependency between the presence of the parasite and fish weight (P < 0.0001). Figure 5 shows the VMR for each weight class of all sampled gilthead sea bream in studies A, B, and D. The VMR increased steadily with weight class and adjusted well to a linear regression curve (R2 = 0.971, P < 0.001).
FIG. 4.
Nonlinear regression adjustment of C. molnari prevalence of infection versus weight class in gilthead sea bream, study A (R2 = 0.9631, P = 0.0071). Values above each bar indicate the mean intensity of infection.
FIG. 5.
Dispersion of C. molnari in total sampled gilthead sea bream, expressed as the VMR in relation to host weight. Dotted line represents the linear regression adjustment (R2 = 0.971, P < 0.001).
DISCUSSION
The information obtained from all four studies gives an overall picture of the situation of cryptosporidiosis in farmed fish in the different production steps, from the hatchery until fish reach market size. It is remarkable that C. molnari was highly prevalent in some of the surveyed cultured fish but that prevalence and intensity of infection were lower in European sea bass than in gilthead sea bream. This finding is in accordance with the results of oral experimental infections (40), thus suggesting probable differences in host susceptibility to the parasite.
In study A, only slight differences were detected among the different ongrowing culture systems. The statistically significant differences between F-1 and F-2 sea cages were probably influenced by the different previous origins of the fish, as fish from F-2 were introduced already parasitized. In contrast, ongrowing sea bass were never found infected in the studied sea cage farm (F-3), in spite of being parasitized before introduction.
Studies B and C provided some interesting results concerning infection in the hatchery, postlarval, and nursery facilities. As a general trend, gilthead sea bream were very rarely infected before weaning, as C. molnari was only found in two groups of 0.1 and 0.3 g sampled in Dec 97 and Feb 98 in H-2 (study C). On the remaining occasions, fish were always found infected after weaning. A similar situation was observed for sea bass in study C. For both fish species, such differences meant a significant relationship between the presence of the parasite and the production step, which could be related to different factors. Water quality might be involved, as treatment of the water supply changes substantially from the hatchery (fine filtration) to the postlarval and nursery zones (coarse filtration) of H-2. The results of group B-1 apparently support this hypothesis, as fish of the B-1a subgroup were positive for the disease 58 days after subgroup splitting and transfer to coarse-filtered water in H-2, whereas the sister B-1b subgroup fish, kept in IATS facilities with fine filtration, had not acquired the infection even 7 months after transfer. IATS water treatment (80 mJ/cm2; fine filtration) of group B-1b could have been more effective than the treatment applied in the sister subgroup in H-2, but we should also take into account that IATS water inflow can be considered free of C. molnari, according to our long-term records (IATS-positive fish came from other facilities).
However, the effect of water treatment must be carefully considered. The UV irradiation used in H-2 (applied in all the production steps) seems to be ineffective in preventing cryptosporidiosis. Modifications in the type of filtration used in the production steps seem to be responsible for differences in the prevalence and intensity of infection, as the finest filtration (10-μm-pore-size filters) corresponded to the lowest infection levels. UV irradiation (ranging between 20 and 120 mJ/cm2) and conventional filtration of tap water for human consumption have been shown to be unreliable for the removal of Cryptosporidium spp. (19). Furthermore, discrepancies in UV killing doses for Cryptosporidium spp. (8, 28, 39) could be explained by differences in the actual fluence (UV dose), which depends also on water turbidity and water flow rate (30). Thus, the efficacy of the UV irradiation used in H-2 (61.3 to 52.1 mJ/cm2) could have been lowered by turbidity, as coarse filtering was applied as early as 40 days p.h. Once parasites are inside the facilities, the recirculation system of postlarval and nursery steps could contribute to concentrate the oocysts.
Transmission of C. molnari seems to take place mostly through the water supply, as in other cryptosporidia (6). With fish parasites, transmission and dispersion by water are facilitated by the aquatic habitat of the host and the frequent releasing of fully sporulated oocysts with mucus casts or feces. Horizontal transmission of C. molnari to sea bass and gilthead sea bream was experimentally demonstrated (40). The ingestion of other infective stages besides oocysts, favored by frequent cannibalism among these fish, could contribute to the dispersion of the disease. However, the involvement of live food is not to be discounted. Although fish were found infected mostly after weaning, the infective stages could have been introduced during the hatchery period and remained undetectable until further samplings in the postweaning period. It is noticeable that different rotifers can ingest C. parvum oocysts when exposed to them (20). However, the presence of C. molnari in live fish food has not been ascertained, and it is difficult to demonstrate with the available diagnostic tools. The role of aquatic organisms as reservoirs and transmitters of cryptosporidiosis has been reported for other Cryptosporidium ssp. (22, 23, 44). Different aquatic organisms could also act as reservoirs of C. molnari and contribute to transmission, mainly in sea cages or earth ponds. In addition to changes in the water quality, weaning itself could be another risk factor as reported for humans (13, 46) and some farmed animals (24).
According to Arneberg et al. (4), transmission rates of macroparasites depend on host population density in natural parasite communities. Since there are no reports on the epidemiology of C. molnari in natural populations, the data obtained in the present study of cultured fish cannot be compared. However, host population densities are evidently higher in farming systems than in the wild, which probably facilitates transmission. Cryptosposidiosis has been frequently reported in farmed animals (12), but data on wild animals are scarce. Sturdee et al. (42) found the highest cumulative prevalence of Cryptosporidium with respect to pasture animals in home-bred calves. Wild animals can act as reservoirs of Cyrptosporidium spp., with subsequent significance for human welfare (29) or animal welfare (reviewed in reference 33).
The clear influence of the age and weight of fish can be deduced from the four studies presented here. Thus, the highest infection levels were observed in preongrowing and early ongrowing fish, with a tendency to decrease with fish weight. The results obtained confirm the age distribution of Cryptosporidum spp. in other hosts. In humans, all age groups are susceptible, but the majority of the cases in most Western studies are in children (45). In livestock, natural and experimental data have demonstrated that infections are usually high in neonates and the young and less prevalent in adults (5, 10, 12, 24, 32, 36). Although information on piscine cryptosporidiosis is very scarce, it mostly confirms the present results. In cultured turbot, epidemiological data obtained for Cryptosporidium scophthalmi (1) over a period of several years have shown a high prevalence and intensity of infection in small fish, with a sharp decrease in larger animals (P. Alvarez-Pellitero, M. J. Redondo, A. Sitjà-Bobadilla, A. Macías, A. Riaza and F. Padrós, Abstr. 5th Int. Symp. Fish Parasites, p. 4, 1999). The remaining data on piscine hosts are incomplete or based on few sampled animals (7, 25, 27, 31) but seem to indicate the same tendency.
Therefore, the distribution of cryptosporidiosis differs from the most generalized age- or size-dependent pattern for metazoan parasites, in which the prevalence and intensity of infection increase with the age or size of host, even in fish (14, 26, 35). The infection pattern with a peak at a particular age and size host and a subsequent decrease in older animals, a convex profile, has also been described for fish myxosporeans (2, 21, 34, 43) and some fish protozoans (37). Biological, ecological, and immunological aspects may affect the host age distribution and dispersion patterns. The age-dependent exposure and the density-dependent parasite establishment are among these factors (15). In several animal cryptosporidioses, the higher susceptibility of neonates has been attributed to the immunological immaturity of the host (32), and in humans the susceptibility to and the severity of cryptosporidiosis appears to be related to the immunocompetence of the host (10).
In fish, size (not age) has been determined to be critical for the maturity of the immune system and for reaching acceptable levels of protection after vaccination (16). The convex age and prevalence-intensity profile observed for C. molnari can be explained by a decrease in exposure to the parasite or a decrease in susceptibility to the parasite with age. Lower susceptibility due to acquired immunity has been reported for several parasites (reviewed in reference 15). Whether the immunocompetence of gilthead sea bream may play a role in the observed age distribution pattern of C. molnari needs further investigation. In addition, a lower exposure to the parasite in larger animals cannot be ruled out, since aquaculture procedures could favor parasite entrance, concentration, and dispersal in young fish. Consequently, different fish sizes in cultured populations imply changes in culture conditions that mirror those in feeding and migratory behaviors in some wild populations.
The dispersion pattern of C. molnari corresponds to that of an overdispersed parasite distribution, as the VMR of total sampled gilthead sea bream was >1, and the frequency distribution was clearly aggregated. In any host-parasite system, differences in the infectivity of parasites or the susceptibility of host will result in overdispersion of parasites throughout the host population. In fact, this is the model described for the great majority of parasites (38). Furthermore, VMR was higher than 1 in all gilthead sea bream size classes and increased in a linear manner with host weight, with no saturation in adult stages. Thus, the age-intensity profile was not convex in the dispersion pattern. Whether this profile would have become convex if larger fish had been sampled is unknown, but the combination of the different processes influencing parasite distribution leads usually to a convex profile in parasite distributions (5).
A seasonal distribution of C. molnari was found in gilthead sea bream, with maximum prevalence and intensity occurring in spring, followed by summer. Seasonality occurs in natural infections of different fish coccidia (reviewed in reference 11) and has also been found for Cryptosporidium spp. of some nonpiscine hosts (12). In a long-term study of livestock and small wild animals, seasonally combined data showed the highest Cryptosporidium prevalence in autumn (42). In humans, cryptosporidial infections are typically predominant during the warm and wet season in tropical developing areas (13). Temperature is probably one of the major factors involved in seasonal fluctuations of C. molnari infection, though other factors, such as the availability of infective stages and host density and, more probably, a combination of different processes could contribute to the observed situation.
In conclusion, C. molnari appears to be a ubiquitous parasite in gilthead sea bream cultures, reaching the highest infection levels in preongrowing and early ongrowing stages. The parasite seems to enter the host at a previous production stage (postlarval or nursery), either through the water or food. Aquaculture procedures that use recirculation systems and cannibalism among fingerlings may concentrate and facilitate oocyst dispersion. As a water filtration treatment that guarantees the total removal of oocysts is not feasible even in the first steps of the production cycle, early detection of the infection is advisable to avoid its dispersal in the facilities and its transfer to other systems through normal aquaculture trading.
Acknowledgments
Funding for this study was provided by the Spanish Ministerio de Educación, Cultura y Deporte MAR-98/1000 and FEDER Program 1FD97-0979-C02.
REFERENCES
- 1.Alvarez-Pellitero, P., M. I. Quiroga, A. Sitjà-Bobadilla, M. J. Redondo, O. Palenzuela, F. Padrós, S. Vázquez, and J. M. Nieto. 2004.. Cryptosporidium scophthalmi n. sp. (Apicomplexa: Cryptosporidiidae) from cultured turbot, Scophthalmus maximus L. Light and electron microscope description and histopathological study. Dis. Aquat. Organ. 62:133-145. [DOI] [PubMed]
- 2.Alvarez-Pellitero, P., and A. Sitjà-Bobadilla. 1993. Ceratomyxa spp. (Protozoa: Myxosporea) infections in wild and cultured sea bass, Dicentrarchus labrax, from the Spanish Mediterranean area. J. Fish Biol. 42:889-901. [Google Scholar]
- 3.Alvarez-Pellitero, P., and A. Sitjà-Bobadilla. 2002. Cryptosporidium molnari n. sp. (Apicomplexa: Cryptosporidiidae) infecting two marine fish species, Sparus aurata L. and Dicentrarchus labrax L. Int. J. Parasitol. 32:1007-1021. [DOI] [PubMed] [Google Scholar]
- 4.Arneberg, P., A. Skorping, and A. F. Read. 1998. Parasite abundance, body size, life histories, and the energetic equivalence rule. Am. Nat. 151:497-513. [DOI] [PubMed] [Google Scholar]
- 5.Atwill, E. R., E. Johnson, D. J. Klingborg, G. M. Veserat, G. Markegard, W. A. Jensen, D. W. Pratt, R. E. Delmas, H. A. George, L. C. Forero, R. L. Philips, S. J. Barry, N. K. McDougald, R. Gildersleeve, and W. E. Frost. 1999. Age, geographic and temporal distribution of fecal shedding of Cryptosporidium parvum oocysts in cow-calf herds. Am. J. Vet. Res. 60:420-425. [PubMed] [Google Scholar]
- 6.Brookes, J. D., J. Antenucci, M. Hipsey, M. D. Burch, N. J. Ashbolt, and C. Ferguson. 2004. Fate and transport of pathogens in lakes and reservoirs. Environ. Int. 30:741-759. [DOI] [PubMed] [Google Scholar]
- 7.Camus, A. C., and M. K. López. 1996. Gastric cryptosporidiosis in juvenile red drum. J. Aquat. Anim. Health. 8:167-172. [Google Scholar]
- 8.Clancy, J. L., M. M. Marshall, T. M. Hargy, and D. G. Korich. 2004. Susceptibility of five strains of Cryptosporidium parvum oocysts to UV light. J. Am. Water Works Assoc. 96:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colorni, A. 2004. Diseases of Mediterranean fish species: problems, research and prospects. Bull. Eur. Assoc. Fish Pathol. 24:22-32. [Google Scholar]
- 10.Current, W. L. 1989. Cryptosporidium spp., p. 281-341. In P. D. Walzer and R. M. Genta (ed.), Parasitic infections in the compromised host. Marcel Deker, New York, N.Y.
- 11.Davies, A. J., and S. J. Ball. 1993. The biology of fish coccidia. Adv. Parasit. 32:293-366. [DOI] [PubMed] [Google Scholar]
- 12.de Graaf, D. C., E. Vanopdenbosch, L. M. Ortega-Mora, H. Abbassi, and J. E. Peeters. 1999. A review of the importance of cryptosporidiosis in farm animals. Int. J. Parasitol. 29:1269-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillingham, R. A., A. A. Lima, and R. L. Guerrant. 2002. Cryptosporisiosis: epidemiology and impact. Microbes Infect. 4:1059-1066. [DOI] [PubMed] [Google Scholar]
- 14.Dogiel, V. A. 1961. Ecology of parasites of freshwater fishes, p. 1-47. In V. A. Dogiel, G. K. Petrushevski, and Y. I. Polyanski (ed.), Parasitology of fishes. Oliver and Boyd, Edinburgh, United Kingdom.
- 15.Duerr, H. P., K. Dietz, and M. Eichner. 2003. On the interpretation of age-intensity profiles and dispersion patterns in parasitological surveys. Parasitology 126:87-101. [DOI] [PubMed] [Google Scholar]
- 16.Ellis, A. E. 1988. Ontogeny of the immune system in teleost fish, p. 20-31. In A. E. Ellis (ed.), Fish vaccination. Academic Press, London, United Kingdom.
- 17.FAO. 2003. Yearbook of fishery statistics 2002. Aquaculture production, vol. 94. Food and Agriculture Organization of the United Nations, Rome, Italy.
- 18.Fayer, R., C. A. Speer, and J. P. Dubey. 1997. The general biology of Cryptosporidium, p. 1-41. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press Inc., Boca Raton, Fla.
- 19.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 20.Fayer, R., J. M. Trout, E. Walsh, and R. Cole. 2000. Rotifers ingest oocysts of Cryptosporidium parvum. J. Eukaryot. Microbiol. 47:161-163. [DOI] [PubMed] [Google Scholar]
- 21.Gbankoto, A., C. Pampoulie, A. Marques, G. N. Sakiti, and K. L. Dramane. 2003. Infection patterns of Myxobolus heterospora in two tilapia species (Teleostei: Cichlidae) and its potential effects. Dis. Aquat. Organ. 55:125-131. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Couso, H., F. Freire-Santos, J. Martínez-Urtaza, O. García-Martín, and M. E. Ares-Mazás. 2003. Contamination of bivalve molluscs by Cryptosporidium oocysts: the need for new quality control standards. Int. J. Food Microbiol. 2706:1-9. [DOI] [PubMed] [Google Scholar]
- 23.Gómez-Couso, H., F. Freire-Santos, M. R. Ortega-Iárrea, J. A. Castro-Hermida, and M. E. Ares-Mazás. 2003. Environmental dispersal of Cryptosporidium parvum oocysts and cross transmission in cultured bivalve molluscs. Parasitol. Res. 90:140-142. [DOI] [PubMed] [Google Scholar]
- 24.Guselle, N. J., A. J. Appelbee, and M. E. Olson. 2003. Biology of Cryptosporidium parvum in pigs: from weaning to market. Vet. Parasitol. 113:7-18. [DOI] [PubMed] [Google Scholar]
- 25.Hoover, D. M., F. J. Hoerr, W. W. Carlton, E. J. Hinsman, and H. W. Ferguson. 1981. Enteric cryptosporidiosis in a naso tang, Naso lituratus Bloch and Schneider. J. Fish Dis. 4:425-428. [Google Scholar]
- 26.Kennedy, C. R. 1975. Ecological animal parasitology. Blackwell, London, United Kingdom.
- 27.Landsberg, J. H., and I. Paperna. 1986. Ultrastructural study of the coccidian Cryptosporidium sp. from stomachs of juvenile cichlid fish. Dis. Aquat. Organ. 2:13-20. [Google Scholar]
- 28.Knott, M. 1996. Fatal dose for water parasite. New Sci. 150:23. [Google Scholar]
- 29.Legesse, M., and B. Erko. 2004. Zoonotic intestinal parasites of Papio anubis (baboon) and Cercopithecus aethipis (vervet) from four localities in Ethiopia. Acta Trop. 90:231-236. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo-Lorenzo, M. J., M. E. Ares-Mazás, I. Villacorta-Martínez de Maturana, and D. Durán-Oreiro. 1993. Effect of ultraviolet disinfection of drinking water on the viability of Cryptosporidium parvum oocysts. J. Parasitol. 79:67-70. [PubMed] [Google Scholar]
- 31.Muench, T. R., and M. R. White. 1997. Cryptosporidiosis in a tropical freshwater catfish (Plecostomus spp.). J. Vet. Diagn. Investig. 9:87-90. [DOI] [PubMed] [Google Scholar]
- 32.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 33.Olson, M. E., R. M. O'Handley, B. J. Ralston, T. A. Mcallister, and R. C. A. Thompson. 2004. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 20:185-191. [DOI] [PubMed] [Google Scholar]
- 34.Palenzuela, O., P. Alvarez-Pellitero, and A. Sitjà-Bobadilla. 1999. Glomerular disease associated to Polysporoplasma sparis (Myxosporea: Bivalvulida) infections in the gilthead sea bream, Sparus aurata (Pisces: Teleostei): aspects of the host-parasite relationship. Parasitology 118:245-256. [DOI] [PubMed] [Google Scholar]
- 35.Poulin, R. 2000. Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J. Fish Biol. 56:123-137. [Google Scholar]
- 36.Ralston, B. J., T. A. McAllister, and M. E. Olson. 2003. Prevalence and infection pattern of naturally acquired giardiasis and cryptosporidiosis in range beef calves and their dams. Vet. Parasitol. 114:113-122. [DOI] [PubMed] [Google Scholar]
- 37.Rintamäki-Kinnunen, P., and E. T. Valtonen. 1997. Epizootiology of protozoans in farmed salmonids at northern latitudes. Int. J. Parasitol. 27:89-99. [DOI] [PubMed] [Google Scholar]
- 38.Shaw, D. J., B. T. Grenfell, and A. P. Dobson. 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117:597-610. [DOI] [PubMed] [Google Scholar]
- 39.Shin, G. A., K. G. Linden, M. J. Arrowood, and M. D. Sobsey. 2001. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sitjà-Bobadilla, A., and P. Alvarez-Pellitero. 2003. Experimental transmission of Cryptosporidium molnari (Apicomplexa: Coccidia) to gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Parasitol. Res. 91:209-214. [DOI] [PubMed] [Google Scholar]
- 41.Sokal, R. R., and F. J. Rohlf. 1981. Biometry. W. H. Freeman and Co., New York, N.Y.
- 42.Sturdee, A. P., A. T. Bodley-Tickell, A. Archer, and R. M. Chalmers. 2003. Long-term study of Cryptosporidium prevalence on a lowland farm in the United Kingdom. Vet. Parasitol. 116:97-113. [DOI] [PubMed] [Google Scholar]
- 43.Su, X. Q., and R. W. G. White. 1996. Frequency distribution and host-parasite relationships of Zschokkella leptatherinae (Myxozoa: Myxiidae), a parasite of atherinid fishes. Aust. J. Zool. 44:97-106. [Google Scholar]
- 44.Tamburrini, A., and E. Pozio. 1999. Long-term survival of Cryptosporidium parvum oocysts in seawater and in experimentally infected mussels (Mytilus galloprovincialis). Int. J. Parasitol. 29:711-715. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, R. C. A., U. M. Morgan, R. M. Hopkins, and L. J. Pallant. 2000. Enteric protozoan infections, p. 194-209. In R. C. A. Thompson (ed.), Molecular epidemiology of infectious diseases. Arnold, London, United Kingdom.
- 46.Tzipori, S., and H. Ward. 2002. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 4:1047-1058. [DOI] [PubMed] [Google Scholar]