Abstract
We describe two Malawian adults on successful antiretroviral therapy who experienced frequent malaria episodes after stopping cotrimoxazole prophylaxis. We argue that, in addition to stopping cotrimoxazole, diminished malaria immunity and drug interactions between efavirenz and artemether-lumefantrine may have played a causative role in the recurrent malaria our patients experienced.
Introduction
Recurrent malaria illness is uncommon in non-pregnant adults living in endemic areas, owing to protection conferred by naturally acquired immunity.1,2 HIV-associated immune suppression contributes to increased frequency and severity of clinical malaria illness in malaria-experienced adults,3,4 but the combination of antiretroviral therapy (ART), cotrimoxazole preventative therapy (CPT) and insecticide-treated bed net use is highly effective in preventing malaria in persons living with HIV.5,6 CPT provides a survival benefit to patients with HIV infection by preventing severe bacterial and parasitic infections, although its exact role for patients on successful ART in sub-Saharan Africa remains uncertain.7 We report 2 case histories of Malawian adults on ART and CPT who experienced repeated episodes of malaria illness soon after stopping CPT and discuss potential contributing factors.
Case 1
A 49-year-old woman was on the standard first-line ART regimen of tenofovir, lamivudine, and efavirenz, along with CPT for 7 years before enrolment in “A randomized, open-label controlled trial of daily trimethoprim-sulfamethoxazole or weekly chloroquine among adults on antiretroviral therapy in Malawi”. This study examines whether there is a benefit of lifelong CPT, as recommended by current WHO and Malawi guidelines, for patients with excellent clinical, immunological, and virological responses to ART.7 She had a CD4 count of 461 cells/µL and undetectable HIV-1 RNA at baseline. She was randomised to the arm without prophylaxis and CPT was discontinued. Four weeks later, she developed intermittent fevers, headache, and malaise. On examination, tachycardia (113 beats per minute) and tachypnoea (24 breaths per minute) were noted, while other vital signs and the rest of the physical examination were normal. A blood film showed Plasmodium falciparum (Figure 1A) with a parasite density of 2480/mm3. A full blood count showed no abnormalities and blood cell morphology was normal on a peripheral blood smear. She was treated with artemetherlumefantrine (80/480 mg tablets), 4 tablets twice a day for 3 days; her response was evaluated using the standardised World Health Organization (WHO) methods for surveillance of antimalarial drug efficacy, with follow-up visits on days 1, 2, 3, 7, 14, 21 and 28.8 She completed malaria treatment with good self-reported adherence, was parasite-free from day 2 through day 28, and symptoms had resolved by day 3. One month after day 28 she presented with new-onset fever, and malaria smear showed P. falciparum with a density of 7720/mm3. She was treated with artemether-lumefantrine again. On day 2 she was afebrile and no parasites were seen on blood films from day 2 through day 28. Six weeks later, she returned with fever, headache, and malaise. The blood film showed Plasmodium ovale with a parasite density of 120/mm3 (Figure 1B), and she was treated with artemether-lumefantrine for a third time. Symptoms resolved quickly and blood smears from day 2 through day 14 were negative for parasites. A glucose 6-phosphate dehydrogenase (G6PD) test revealed that she was deficient and treatment with primaquine was not given. On day 21 she was asymptomatic, but a blood film showed P. falciparum with parasite density of 320/mm3. A fourth treatment with artemether-lumefantrine was started; parasitaemia resolved by day 7 and remained negative through day 28. She reported being fully adherent to all antimalarial treatments and denied vomiting any doses. Blood cultures taken during the first 3 presentations yielded no growth. Real-time polymerase chain reaction (PCR) analysis of dried blood spot specimens collected at all visits confirmed the species identified by microscopy and excluded mixed infections.
Figure 1.
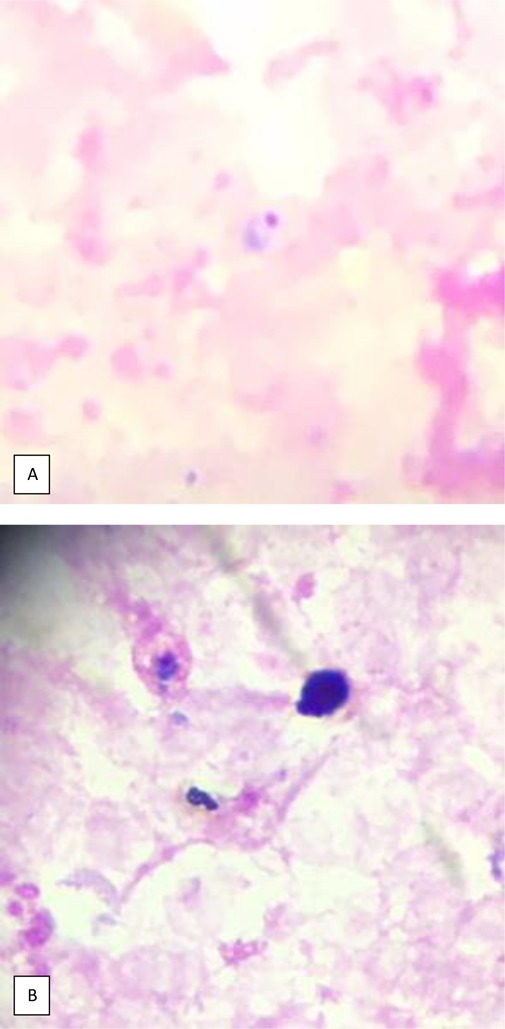
A. Plasmodium falciparum trophozoite
B. Plasmodium ovale trophozoite
Case 2
A 37-year-old woman was enrolled into the same trial as the first patient, after having been on tenofovir, lamivudine, and efavirenz, with CPT, for 3 years. Her CD4 count at enrolment was 883 cells/µL, and HIV-1 RNA was undetectable. She was also randomised to no prophylaxis arm, and CPT was discontinued. Starting 2 weeks after CPT discontinuation and over a period of 7 months, she experienced 6 malaria epiodes and was found to have asympomatic parasitaemia on one occasion during routine follow-up, as summarised in Table 1. Findings from physical examination during each episode were unremarkable. This patient also reported complete adherence to antimalarial treatments and denied vomiting during any malaria episode. Only P. falciparum species were observed in microscopy, but molecular analysis was not performed for this patient. Due to the frequent recurrences of malaria illness, the study team decided to resume CPT in this patient.
Table 1.
Summary of malaria episodes for Case 2
| Malaria episode sequence |
Date of presentation |
Parasite count (per mm3) |
Symptom onset to resolution (days) |
Interval to parasite clearance by microscopy (days) |
Events during 28 day follow up |
| 1 | 2nd May 2016 | 120 | 2 | 2 | Symptomatic parasitaemia day 23 |
| 2 | 26th May 2016 | 6640 | 3 | 3 | Symptomatic parasitaemia day 28 |
| 3 | 23rd June 2016 | 400 | 3 | 3 | None |
| 4 | 23rd Aug 2016 | 3880 | 3 | 3 | None |
| 5 | 10th Oct 2016 | 17,600 | 3 | 2 | Asymptomatic parasitaemia day 28 |
| 6 | 7th Nov 2016 | 14,160 | Asymptomatic on day 0 |
2 | Symptomatic parasitaemia day 25 (parasite count 280/mm3) |
| 7 | 2nd Dec 2016 | 280 | 7 | 1 | None |
Discussion
Both adult patients experienced frequent malaria episodes within the months following CPT discontinuation. Recurrent malaria infection after treatment can be the result of 3 different circumstances: malaria treatment failure (recrudescence), activation of hepatic hypnozoites of ovale or vivax malaria (relapse), or new infection (reinfection). Because cotrimoxazole provides malaria prophylaxis,5,6,9 discontinuing CPT increases individual susceptibility to malaria illness. We consider additional reasons why our patients had frequently recurring malaria episodes.
In HIV-infected adults living in regions with high malaria burden, ART, CPT, and insecticide-treated bed net use effectively reduce incident malaria infection and illness.5,6,9,11
Such longstanding protection against blood stage infection may lead to waning of naturally acquired immunity to malaria and increased vulnerability to malaria infection and illness when CPT is discontinued. The observation that long-term CPT in HIV-exposed, uninfected children led to reduced development of IgG anti-malarial antibodies10 and the experience that adults who move from highly endemic-to non-transmission areas become susceptible to severe malaria upon return to their original residence12 support the hypothesis that CPT discontinuation after its prolonged and consistent use can lead to frequent malaria attacks as a rebound effect due to decreased naturally acquired immunity.
Malaria treatment failure is defined as failure to clear malarial parasitaemia or to resolve clinical symptoms after treatment with antimalarial drugs.13 This can be due to subtherapeutic exposure to antimalarial drugs or due to drug-resistant parasites. In all episodes described here, malaria parasitaemia was rapidly and completely cleared after artemether-lumefantrine treatment. The patients were in good general health, had close to normal CD4 counts, and no gastrointestinal symptoms suggesting HIV enteropathy. They reported consistent bednet use, full adherence to antimalarial treatment, and did not vomit at any point. These arguments make it likely that treatments were taken as prescribed and were well-absorbed.
In Malawi, standard first-line ART regimens include efavirenz and nevirapine, and artemether-lumefantrine is the first-choice antimalarial drug. These 4 drugs are all metabolised by enzymes in the cytochrome P450 system (CYP450) in the liver and concomitant use of these antiretroviral and antimalarial drugs may affect malaria treatment. Lumefantrine has a long half-life (3 to 6 days) and clears residual parasitaemia with the aim to prevent recrudescence when used in combination with artemether.14 Lumefantrine is metabolised by CYP450 enzyme 3A4 (CYP3A4). Efavirenz is also metabolised by CYP3A4, as well as by CYP2B6 and CYP2A6, and efavirenz induces the activity of these 3 enzymes in a dose-dependent manner. Some individuals are genetically determined slow metabolisers of efavirenz, mainly due to single-nucleoside polymorphisms in CYP2B6, and they maintain high efavirenz levels and high-level induction of CYP3A4 as a consequence.14–18 CYP3A4 induction by efavirenz enhances lumefantrine metabolism, which may lead to plasma concentrations that are subtherapeutic14,15 and which may then decrease the clinical efficacy of artemether-lumefantrine. Late treatment failures have been previously reported in patients with high baseline parasitaemia and low lumefantrine concentrations.19 There are also drug interactions between efavirenz and artemether, and between nevirapine and artemether-lumefantrine, but these appear to be of less clinical relevance.15 Further insight into the role of these mechanisms in our patients could have been gained by determining plasma lumefantrine and efavirenz levels and by pharmacogenetic analysis looking for CYP2B6 polymorphisms—tests that are not available in our setting. Despite adequate adherence to the artemether-lumefantrine dosing, our patients may have had insufficient exposure to lumefantrine, and this would have contributed to treatment failure.
Drug resistance is another cause of treatment failure, but clinical or parasitological failures of artemisinin drug combinations have not been reported from Malawi and none of the relevant resistance-conveying mutations (in particular, in the kelch propellor gene domain) have been found in P. falciparum on the African continent.20
Case 2 had recurrent malaria late within the 28-day treatment follow-up period (2nd, 3rd, 6th and 7th episodes), but we did not perform P. falciparum genotyping to distinguish reinfection from recrudescence. We can therefore not definitively determine if malaria treatment failure occurred in these episodes.
P. ovale infection occasionally occurs in Malawi and can cause relapses due to the persistence of hypnozoites in the liver. Case 1 had such an episode, and while artemether-lumefantrine is adequate treatment for P. ovale blood stages, primaquine is required to treat the hypnozoites. We identified G6PD deficiency in Case 2, and thus did not prescribe primaquine, which is associated with haemolysis and increased risk of cerebrovascular accident in persons with G6PD deficiency. P. ovale relapse did not appear to play a role in our second case.
Conclusions
Several potential factors can explain why HIV-infected patients experience recurrent malaria illness episodes after stopping CPT: poor adherence to antimalarial drugs; vomiting tablets; malabsorption due to HIV enteropathy; antimalarial drug resistance; P. ovale hypnozoite relapse; waning of acquired malaria immunity under long-term use of bed nets and effective ART and CPT; and drug-drug interactions between lumefantrine and efavirenz. Only the last 2 are likely to have contributed in our cases. Previous clinical trials have demonstrated that CPT protects against uncomplicated malaria in African adults on ART, without providing evidence of benefit in terms of survival, other morbidity, and HIV-related outcomes.9,11 The study in which both our patients are enrolled will provide conclusive insight into the role of CPT in clinically stable adults on effective ART.
Ethical considerations
The clinical trial was reviewed and approved by the College of Medicine Research and Ethics Committee (COMREC ref. number: P.02/02/490) and the University of Maryland, Baltimore Institutional Review Board (ref. number H-25835, HP-00043360).
Acknowledgements
We would like to acknowledge the participants and staff from the Tisungane Research Clinic at Zomba Central Hospital and the Blantyre Malaria Project Research Clinic at the Ndirande Health Centre in Blantyre.
Competing interests
The authors declare that they have no conflicts of interest.
References
- 1.Doolan DL, Dobaño C, Baird JK. Acquired Immunity to Malaria. Clinical Microbiology Reviews. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(9):1208–1213. doi: 10.1086/522181. [DOI] [PubMed] [Google Scholar]
- 3.Idemyor V. Human immunodeficiency virus (HIV) and malaria interaction in sub-Saharan Africa: the collision of two Titans. HIV clinical trials. 2007;8(4):246–253. doi: 10.1310/hct0804-246. [DOI] [PubMed] [Google Scholar]
- 4.Berg A, Patel S, Aukrust P, David C, Gonca M, Berg ES, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: a prospective cross-sectional study. PLoS One. 2014;9(2):e88257. doi: 10.1371/journal.pone.0088257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. Aids. 2007;21(15):2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 6.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet (London, England) 2006;367(9518):1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 7.Laurens MB, Mungwira RG, Nyirenda OM, Divala TH, Kanjala M, Muwalo F, et al. TSCQ study: a randomized, controlled, open-label trial of daily trimethoprim-sulfamethoxazole or weekly chloroquine among adults on antiretroviral therapy in Malawi: study protocol for a randomized controlled trial. Trials. 2016;17(1):322. doi: 10.1186/s13063-016-1392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OrganizationWH, author. Methods for surveillance of antimalarial drug efficacy. 2009. [Google Scholar]
- 9.Walker AS, Ford D, Gilks CF, Munderi P, Ssali F, Reid A, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet (London, England) 2010;375(9722):1278–1286. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longwe H, Jambo KC, Phiri KS, Mbeye N, Gondwe T, Hall T, et al. The Effect of Daily Co-Trimoxazole Prophylaxis on Natural Development of Antibody-Mediated Immunity against P. falciparum Malaria Infection in HIV-Exposed Uninfected Malawian Children. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0121643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell JD, Moore D, Degerman R, Kaharuza F, Were W, Muramuzi E, et al. HIV-infected ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/muL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(8):1204–1211. doi: 10.1093/cid/cis013. [DOI] [PubMed] [Google Scholar]
- 12.Farnert A, Wyss K, Dashti S, Naucler P. Duration of residency in a non-endemic area and risk of severe malaria in African immigrants. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2015;21(5):494–501. doi: 10.1016/j.cmi.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 13.White NJ. The assessment of antimalarial drug efficacy. Trends in parasitology. 2002;18(10):458–464. doi: 10.1016/s1471-4922(02)02373-5. [DOI] [PubMed] [Google Scholar]
- 14.Hoglund RM, Byakika-Kibwika P, Lamorde M, Merry C, Ashton M, Hanpithakpong W. Artemether-lumefantrine coadministration with antiretrovirals; population pharmacokinetics and dosing implications. British journal of clinical pharmacology. 2015:79. doi: 10.1111/bcp.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maganda BA, Ngaimisi E, Kamuhabwa AAR, Aklillu E, Minzi OMS. The influence of nevirapine and efavirenz-based anti-retroviral therapy on the pharmacokinetics of lumefantrine and anti-malarial dose recommendation in HIV-malaria co-treatment. Malaria Journal. 2015;14(1):1–11. doi: 10.1186/s12936-015-0695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colic A, Alessandrini M, Pepper MS. Pharmacogenetics of CYP2B6, CYP2A6 and UGT2B7 in HIV treatment in African populations: focus on efavirenz and nevirapine. Drug metabolism reviews. 2015;47(2):111–123. doi: 10.3109/03602532.2014.982864. [DOI] [PubMed] [Google Scholar]
- 17.Haas DW, Kwara A, Richardson DM, Baker P, Papageorgiou I, Acosta EP, et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. J Antimicrob Chemother. 2014;69(8):2175–2182. doi: 10.1093/jac/dku110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross R, Aplenc R, Tenhave T, Foulkes AS, Thakur R, Mosepele M, et al. Slow efavirenz metabolism genotype is common in Botswana. J Acquir Immune Defic Syndr. 2008;49(3):336–337. doi: 10.1097/QAI.0b013e31817c1ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maganda BA, Minzi OM, Kamuhabwa AA, Ngasala B, Sasi PG. Outcome of artemether-lumefantrine treatment for uncomplicated malaria in HIV-infected adult patients on anti-retroviral therapy. Malar J. 2014;13:205. doi: 10.1186/1475-2875-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A Worldwide Map of Plasmodium falciparum K13-Propeller Polymorphisms. The New England journal of medicine. 2016;374(25):2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]


