Abstract
Mycobacterium goodii X7B, which had been primarily isolated as a bacterial strain capable of desulfurizing dibenzothiophene to produce 2-hydroxybiphenyl via the 4S pathway, was also found to desulfurize benzothiophene. The desulfurization product was identified as o-hydroxystyrene by gas chromatography (GC)-mass spectrometry analysis. This strain appeared to have the ability to remove organic sulfur from a broad range of sulfur species in gasoline. When Dushanzi straight-run gasoline (DSRG227) containing various organic sulfur compounds was treated with immobilized cells of strain X7B for 24 h, the total sulfur content significantly decreased, from 227 to 71 ppm at 40°C. GC flame ionization detection and GC atomic emission detection analysis were used to qualitatively evaluate the effects of M. goodii X7B treatment on the contents of gasoline. In addition, when immobilized cells were incubated at 40°C with DSRG275, the sulfur content decreased from 275 to 54 ppm in two consecutive reactions. With this excellent efficiency, strain X7B is considered a good potential candidate for industrial applications for the biodesulfurization of gasoline.
Sulfur oxides released from fossil fuel combustion contribute to acid rain and air pollution (11, 24). With the increasing demands for energy and more stringent environmental policies, deep desulfurization of petroleum is becoming more and more desired. In terms of available technologies, the sulfur content in gasoline can be reduced to <30 ppm by current hydrotreatment processes. The major problem with deep desulfurization of gasoline is that conventional hydrodesulfurization (HDS) technology results in a significant reduction in the octane number due to the saturation of olefins in naphtha from fluid catalytic cracking, which also causes more hydrogen consumption (21). However, thiophenic compounds such as benzothiophene (BTH) and thiophene (T) and their derivates, as well as thiol, are major sulfur compounds in gasoline (23). If biodesulfurization (BDS) can be applied effectively to gasoline, the refiner will be offered a less expensive alternative to HDS that also avoids the drawback of octane degradation (20). Several studies on BTH- and dibenzothiophene (DBT)-desulfurizing bacteria have been reported (8, 13, 14, 22, 30, 31).
The desulfurization pathway of Rhodococcus erythropolis IGTS8 (9) has been characterized. The dszA, -B, and -C genes, which are responsible for DBT desulfurization, have been cloned and sequenced, and their products have been characterized (5, 6, 16, 17, 19, 27). In addition, the BTH desulfurization pathway has been demonstrated for six bacteria: Gordonia sp. strain 213E (8), Rhodococcus sp. strain T09 (22), Paenibacillus sp. strain A11-2 (14), Sinorhizobium sp. strain KT55 (31), Rhodococcus sp. strain KT 462 (30), and Rhodococcus sp. strain WU-K2R (13). Furthermore, the desulfurization of both DBT and BTH by a single bacterium has only been reported for Paenibacillus sp. strain A11-2 (14) and Rhodococcus sp. strain KT462 (30). On the other hand, since distillate fractions are often treated at high temperatures, there may be some cost savings through the use of moderate thermophiles if BDS is integrated with HDS during refinery without cooling the stock to 30°C (15). Moreover, the desulfurization activity will also be enhanced due to the higher mass transfer rate at high temperatures (1). For practical BDS, it is useful to obtain microorganisms that exhibit much higher DBT and BTH desulfurization activities at high temperatures.
In this paper, we describe the microbial desulfurization pathway of BTH by the previously reported Mycobacterium goodii strain X7B (17, 18). We examined the biodesulfurization of various organic sulfur compounds by strain X7B. The ability of M. goodii X7B to desulfurize gasoline in an immobilized-cell system was also evaluated. M. goodii X7B metabolized a broad range of organic sulfur compounds, suggesting its potential application for the desulfurization of fossil fuels.
MATERIALS AND METHODS
Chemicals.
DBT, methylated DBTs, DBT sulfone, thiophene, thiophene acetic acid, thiophene carboxylic acid, 3,3′-thiodipropionic acid, 2-hydroxybiphenyl, and Tween 80 of the highest quality available were purchased from Sigma-Aldrich Chemical Co., Inc. BTH was purchased from ACROS Organic Co., Inc. 5-Methyl-BTH was purchased from Lancaster Synthesis (Morecambe, United Kingdom). Propylmercaptan (with a purity exceeding 97%) was purchased from Fluka Chemika (Buchs, Switzerland). All other commercially available chemicals were of analytical grade.
Bacterial strains and medium.
Mycobacterium sp. strain X7B was primarily isolated as a facultative thermophilic bacterial strain capable of degrading DBT to 2-hydroxybiphenyl at 45°C (17). The sulfur-free medium used for the growth of thermophilic bacteria was modification of A medium (MAM) as described previously (17).
Estimation of organic sulfur compounds.
M. goodii X7B was shaken at 45°C in MAM with BTH or other organic sulfur compounds dissolved in ethanol or N,N′-dimethylformamide as the sole sulfur source for bacterial growth. During the time course of bacterial growth, aliquots of the culture were removed and acidified to pH 2.0 by the addition of 6 N HCl, followed by extraction with ethyl acetate. A portion of the ethyl acetate layer was measured by use of a gas chromatograph (GC) (CP3380; Varian Associates, Inc.) fitted with an SPB-5 column (0.32-mm internal diameter by 30-m length; Supelco). When cultivated with BTH as the sole sulfur source, cells were inoculated in 50 ml of MAM containing BTH in a 300-ml screw-cap Erlenmeyer flask. Cultures containing BTH were cooled on ice for 20 min to prevent the volatilization of BTH before sampling according to a previously reported method (30).
DBT, methylated DBTs, and other thiophenic compounds and their metabolites were also prepared in 50% ethanol and detected by high-performance liquid chromatography (Agilent 1100 series; Hewlett-Packard) with a reverse-phase C18 column (4.6 by 150 mm; Hewlett-Packard). The column was eluted with 80% methanol at a flow rate of 1.0 ml/min. DBT, 2-hydroxybiphenyl, BTH, and 5-methyl-BTH were monitored by measuring the A254. The molecular structures of metabolites of BTH and its derivates were analyzed by GC-mass spectrometry (GC-MS) (GCD 1800C; Hewlett-Packard) with a 50-m DB-5MS column (J & W Scientific, Folsom, Calif.). Before injection, the samples were concentrated under nitrogen gas.
Analyses of gasoline were performed by GC-flame ionization detection (FID) and GC-atomic emission detection (AED). Hydrocarbon-containing compounds were detected by use of a FID instrument. The distribution of organic sulfur-containing compounds was determined by use of an AED instrument (Agilent G2350A; Hewlett-Packard). Sulfur removal from gasoline was determined by comparing the difference in the sulfur content in control oil and that in oil treated with immobilized X7B cells. The total sulfur content was determined in triplicate for each sample by measuring the combustion of oil and the amount of released sulfur dioxide by use of a sulfur analyzer (model 7000 SN; ANTEK, Houston, Tex.).
Desulfurization reactions.
Cells were immobilized by entrapment with calcium alginate, carrageenan, agar, polyvinyl alcohol, polyacrylamide, and gelatin-glutaraldehyde. We found that calcium alginate-immobilized cells had the highest DBT desulfurization activity, and therefore calcium alginate was selected as the biosupport material for this study (3, 26). Bacteria were cultured in MAM containing 0.5 mM DBT or 1 mM dimethyl sulfoxide as the sole sulfur source at 45°C. Cells were harvested in the mid-exponential phase of growth by centrifugation at 2,500 × g for 10 min at 4°C, washed twice with a sodium chloride solution (0.85%), and resuspended in the same solution containing 0.3% Tween 80 and 2% sodium alginate at a concentration of 12.4 mg of dry cells/ml. The mixture was then dropped into a 5% calcium chloride solution containing 0.3% Tween 80 to obtain beads of immobilized cells (about 1.0 mm in diameter). The beads were kept in the solution for 4 h at room temperature to ensure that they were rigid.
Fifty grams of beads was added to 90 ml of a 0.85% sodium chloride solution containing 0.3% Tween 80 supplemented with 2% glucose as an energy source (7). Ten milliliters of gasoline was added for desulfurization, and thus the volumetric phase ratio of the aqueous phase to oil was 9. Dushanzi straight-run gasoline 227 (DSRG227) and DSRG275 were kindly provided by Petrochina Karamay Petrochemical Company. The numbers 227 and 275 refer to the concentrations of sulfur in the oil, in parts per million. BDS was performed for 24 h at 40°C in a 4-liter seal-capped bottle to ensure that there was enough oxygen. The oil was separated by centrifugation at 5,000 × g for 5 min (7).
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain X7B has been submitted to GenBank and assigned accession number AF513815.
RESULTS
Identification of the DBT-desulfurizing bacterium M. goodii X7B.
Strain X7B was previously identified as a Mycobacterium species by our laboratory (17, 18). Further identification of strain X7B was performed by the Deutsche Sammlung von Mikrooganismen und Zellkulturen GmbH.
Strain X7B is a nonmotile, non-spore-forming rod and has a 2-μm length and a 1-μm diameter. Its colonies are golden yellow, whereas colonies of the type strain of M. goodii, DSM 44492, are light ivory. The utilization pattern for 35 carbon sources was investigated. Because M. goodii was not included in the physiological database, strain X7B was assigned to Rhodococcus fascians, but with a similarity which was not sufficient for identification. High-performance liquid chromatography elution profiles of mycolic acids of X7B and M. goodii DSM 44492T were quite similar, indicating that the two strains belong to the same species. The GC chromatograms of X7B showed identical mycolic acid pyrolysis products, which indicated that both strains were the same. Strain X7B synthesized fatty acid patterns which are diagnostic for mycobacteria. A comparison of the fatty acid patterns synthesized by strain X7B and those synthesized by M. goodii showed that they were identical. Secondary alcohols (18:0 alcohol, 20:0 alcohol), which are diagnostic for some mycobacteria, could not be detected in either X7B or M. goodii. A comparison of the partial sequences of X7B revealed 99.5% sequence similarity to the type strain of M. goodii, DSM 44492 (EMBL accession no. Y12872). The RiboPrint pattern of strain X7B was different from that of M. goodii DSM 44492T. The phenomenon that mycobacterial strains of the same species are separated into two RiboPrint clusters has also been observed for some other Mycobacterium species (2). Based on comparative 16S rRNA gene sequencing, chemotaxonomy, and morphological and physiological data, we can conclude that strain X7B belongs to the species M. goodii.
Biodesulfurization of BTH by growing cells of M. goodii X7B.
M. goodii X7B was able to grow in MAM with BTH as the sole source of sulfur. The strain showed maximum growth after 24 h of cultivation; thereafter, the biomass decreased a little, and the turbidity at 620 nm at this time point was 2.46.
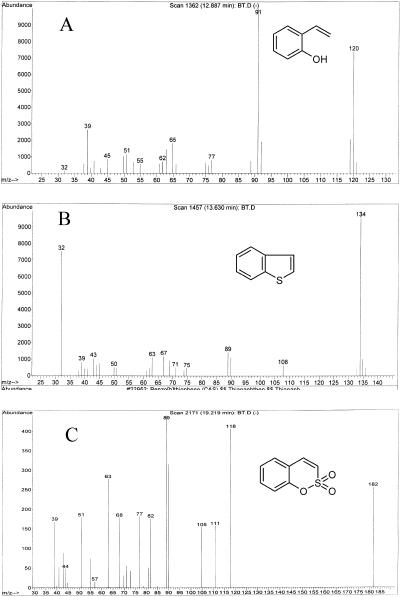
Broth that was sampled after 12 h of culturing with BTH was positive by the Gibbs assay (15), indicating the production of phenolic compounds. In order to elucidate the metabolic pathway of BTH by strain X7B, we extracted the metabolites of BTH with ethyl acetate and then analyzed them. Strain X7B was grown in MAM containing 0.5 mM BTH as the sole sulfur source to the end of the exponential growth phase, and the culture broth was extracted with ethyl acetate. GC-MS analysis revealed several main peaks. Besides the main solvent peaks detected after about 3 min, the other three peaks were further analyzed by use of their mass spectra to deduce the structures which were not detected in the extracts prepared from cells grown with sulfate instead of BTH. Mass spectral data are shown in Fig. 1. These mass spectral data agreed with those of o-hydroxystyrene, BTH, and benzo(c)(1, 2)oxathiin S,S-dioxide (31).
FIG. 1.
MS spectra of BTH metabolites. (A) o-Hydroxystyrene (molecular weight, 120); (B) BTH (molecular weight, 134); (C) benzo(c)(1,2)oxathiin S,S-dioxide (molecular weight, 182). These spectra corresponded to peaks detected at retention times of 12.89, 13.63, and 19.22 min, respectively.
Degradation of various organic sulfur compounds by M. goodii X7B.
Petroleum-based fuel oils, such as gasoline and diesel oil, contain enormous numbers of organic sulfur compounds, including thiophenes, BTHs, and DBTs. The capability of strain X7B to use organic sulfur compounds other than DBT as a sole sulfur source was investigated. M. goodii X7B was cultivated in MAM containing various sulfur sources at a concentration of 0.5 mM at 45°C. As shown in Table 1, this strain grew well on dimethyl sulfoxide, 4,6-dimethyl-DBT, DBT sulfone, DBT, BTH, 5-methyl-BTH, 2-thiopheneacetic acid, and 2-thiophene carboxylic acid for 24 h. 4-Methyl-DBT propylmercaptan also supported growth, but only after incubation for 48 h; 3,3′-thiodipropionic acid was also used as a sole sulfur source for the growth of X7B, and it was shown that X7B could grow well after 3 days, which may have been caused by the toxic effect of the sulfur compounds (12) used in the medium. The reduction of these sulfur compounds was also calculated, and most of them were degraded by the growing cells of strain X7B. Therefore, M. goodii X7B possesses a broad substrate range, suggesting its potential use for fuel desulfurization. Without glucose in the medium, strain X7B could not grow on any of the compounds listed in Table 1 as a sole sulfur source.
TABLE 1.
Growth of M. goodii X7B in the presence of various sulfur compounds at 45°C
| Sulfur compounda | Growth (OD620) | Degradation (%)e |
|---|---|---|
| Control | 0.75b | NT |
| Dimethyl sulfoxide | 11.50b | NT |
| 4,6-Dimethyl-DBT | 6.73b | 45.8 |
| DBT sulfone | 7.75b | 56.6 |
| DBT | 2.25b | 74.4 |
| 4-Methyl-DBT | 2.38c | 61 |
| BTH | 2.46b | NT |
| 5-Methyl-BTH | 4.63b | NT |
| 3-Methyl-BTH | 6.13b | NT |
| 2-Thiophene acetic acid | 8.85b | NT |
| 2-Thiophene carboxylic acid | 4.83b | NT |
| Thiophene | 1.04b | NT |
| 3,3′-Thiodipropionic acid | 2.9d | NT |
| Propylmercaptan | 3.6c | NT |
All sulfur compounds were added to MAM at 0.5 mM.
Cultures were incubated for 24 h.
Cultures were grown for 48 h.
Cultures were grown for 72 h.
NT, not tested.
Biodesulfurization of gasoline in M. goodii X7B immobilized-cell system.
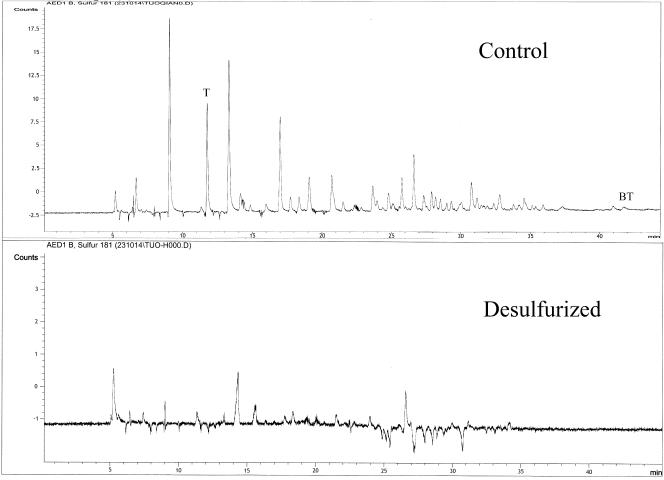
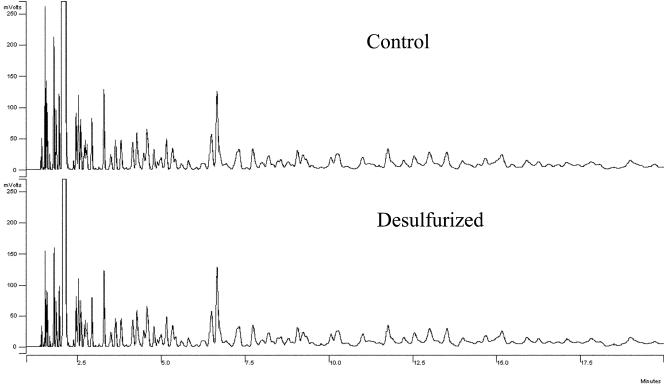
The feasibility of gasoline desulfurization was investigated by the use of DSRG227. GC-FID and GC-AED analyses were used to qualitatively evaluate the effects of M. goodii X7B treatment on the hydrocarbon and sulfur contents, respectively, of the gasoline. The GC-AED chromatograms of gasoline samples from the immobilized-cell reaction system revealed an extensive depletion of sulfur compounds across the entire boiling range of the oil (Fig. 2). The GC-FID chromatograms of gasoline samples (Fig. 3) from the reaction system and of untreated gasoline showed that hydrocarbon components with lower boiling points decreased slightly, while no visible changes in other hydrocarbon components were detected. The total sulfur content of the gasoline after treatment with immobilized cells of M. goodii X7B was 71 ppm, corresponding to a reduction of 69%. When beads of immobilized cells prepared from cell suspension cultures grown in the presence of Na2SO4 and noncell beads were used for the desulfurization reaction, no decrease in the sulfur content of the gasoline was detected (Table 2). After the desulfurization reaction, immobilized cells were collected and washed once with 0.85% NaCl. Ten milliliters of gasoline was then brought into contact with an immobilized-cell suspension, as described in Materials and Methods, for 24 h at 40°C. The longevity of the immobilized cells was evaluated from the residual desulfurization activity as a fraction of the initial activity. The residual activity was 82% for strain X7B.
FIG. 2.
GC-AED chromatograms of control and M. goodii X7B-desulfurized gasoline. Thiophene (T) and benzothiophene (BT) peaks are indicated in the figure. The control was an untreated gasoline sample.
FIG. 3.
GC-FID chromatograms of control and M. goodii X7B-desulfurized gasoline. The control was an untreated gasoline sample. Ethyl acetate, n-heptane, and n-octane were detected at 2.1, 3.0, and 5.5 min, respectively.
TABLE 2.
Biodesulfurization of gasoline by M. goodii X7B immobilized-cell systems
| Type of beads | Avg sulfur content (ppm) | Reduction rate (%) |
|---|---|---|
| Grown in DMSO | 71 | 69 |
| Grown in Na2SO4 | 218 | 4 |
| No cells | 222 | 2 |
When immobilized M. goodii X7B cells were incubated at 40°C with DSRG275 in a reaction mixture containing 10% (vol/vol) oil for 24 h, the sulfur content of the gasoline decreased from 275 to 121 ppm. After a 24-h reaction, the desulfurization reaction was repeated by exchanging the used immobilized cells for fresh ones. The sulfur content of DSRG275 decreased from 275 to 54 ppm through two consecutive reactions, corresponding to a reduction of 81%.
DISCUSSION
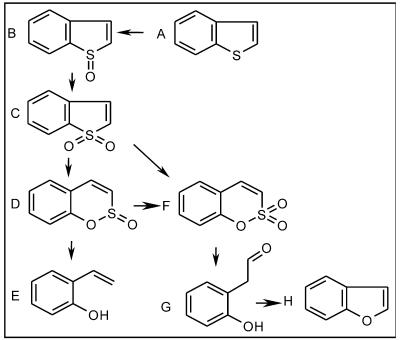
The BTH desulfurization pathways of Gordonia sp. strain 213E (8), Rhodococcus sp. strain T09 (22), Paenibacillus sp. strain A11-2 (14), Sinorhizobium sp. strain KT55 (31), Rhodococcus sp. strain WU-K2R (13), and Rhodococcus sp. strain KT462 (30) have been elucidated. However, the desulfurization of both BTH and DBT by a single bacterial strain has only been reported for the thermophilic bacterium Paenibacillus sp. strain A11-2 and the mesophilic bacterium Rhodococcus sp. strain KT462. As shown in this paper, M. goodii X7B, a facultative thermophilic bacterium, can also grow with either BTH or DBT as the sole sulfur source. The desulfurization pathway of Sinorhizobium sp. strain KT55, Paenibacillus sp. strain A11-2, and Rhodococcus sp. strain KT462 is as follows: BTH→BTH sulfoxide→BTH sulfone→benzo(e)(1,2)oxathiin S-oxide→o-hydroxystyrene. The BTH degradation routes in Gordonia sp. strain 213E and Rhodococcus sp. strain T09 were found to be the same, and the deduced pathway of BTH was similar to that of the three bacteria mentioned above except for the last step, that is, the end product of BTH desulfurization was not o-hydroxystyrene but, rather, 2-(2′-hydroxyphenyl)ethan-1-al. In an analysis of BTH metabolites of strain X7B, benzo(e)(1,2)oxathiin S,S-dioxide and o-hydroxystyrene were both observed. Therefore, we postulated that M. goodii X7B shares the same BTH degradation pathway as Sinorhizobium sp. strain KT55, Paenibacillus sp. strain A11-2, and Rhodococcus sp. strain KT462 (14, 30, 31). It was interesting that the three BTH- and DBT-desulfurizing strains had the same BTH degradation steps (Fig. 4). This clearly indicates that there are some common characteristics among them. However, it has been reported that IGTS8 cannot utilize BTH as a sole sulfur source (10), suggesting that X7B may carry a novel system for the desulfurization of heterocyclic sulfur-containing xenobiotics. More detailed analyses of the purified enzymes and genes involved in BTH degradation by strain X7B would provide a further corroborative explanation for the biodesulfurization of both BTH and DBT by a single bacterial strain.
FIG. 4.
Proposed pathway of biodesulfurization of BTH. (A) BTH; (B) BTH sulfoxide; (C) BTH sulfone; (D) benzo(e)(1,2)oxathiin S-oxide; (E) o-hydroxystyrene; (F) benzo(e)(1,2)oxathiin S,S-dioxide; (G) 2-(2′-hydroxyphenyl)ethan-1-al; (H) benzofuran (29).
Most of the sulfur species in gasoline are a result of fluid catalytic cracking of gas oils or residuum materials that are relatively rich in sulfur. There are three main classes of organic molecules, namely, thiophenes, BTHs, and mercaptans. Therefore, refineries using fluid catalytic cracking to produce gasoline components are concerned with sulfur reduction in gasoline (28). We found that the range of substrates utilized as sulfur sources by M. goodii X7B is quite broad. Besides BTH and DBT, strain X7B could also grow by using alkylated BTHs, alkylated DBTs, thiophene carboxylic acid, thiophene acetic acid, 3,3′-thiodipropionic acid, and propylmercaptan as sole sulfur sources (Table 1).
The major problem with the deep desulfurization of gasoline is that conventional hydrodesulfurization technology results in a significant reduction in the octane number due to the saturation of olefins in naphtha from fluid catalytic cracking (21, 28). Also, thiophenic compounds such as BTH, thiophene, and their derivates, as well as thiol, are major sulfur compounds in gasoline (23). If BDS can be applied effectively to gasoline, the refiner will be offered a less expensive alternative to HDS that also avoids the drawback of octane degradation. Furthermore, BDS will significantly reduce energy requirements and, consequently, the environmental impact brought about by gasoline combustion (20).
The process challenges to developing gasoline BDS lie primarily in reactor design, especially that for oil separations (4, 28). The process must also minimize the problems associated with the toxicity of gasoline to the biocatalyst. Most BDS processes are triphasic systems containing cells, water, and oil (7, 15, 28). It is very difficult to separate oil and water from the emulsified oil. In this study, the immobilized-cell system adopted for BDS made it easier to separate oil from the reaction system and greatly minimized the toxicity of gasoline to the biocatalyst. Chang et al. immobilized Gordona sp. strain CYKS1 and Nocardia sp. strain CYKS2 in Celite for the desulfurization of light gas oil (3). Naito et al. entrapped R. erythropolis KA2-5-1 cells in a photo-cross-linkable prepolymer (ENT-4000) for model oil (n-tetradecane) desulfurization (26). Pseudomonas delafieldii R-8 was immobilized on magnetic polyvinyl alcohol beads, and a diesel oil desulfurization reaction was performed. After desulfurization, immobilized cells could be easily separated magnetically from the BDS reactor (29). Encapsulation provided the bacterial cells with a protective solid barrier, which may have somewhat limited the diffusion of gasoline hydrocarbons, reducing the bioavailable concentration in the inner space of the beads with respect to that in the bulk liquid (25). However, according to our knowledge, no studies using immobilized cells for gasoline biodesulfurization have ever been reported. In this study, calcium alginate was selected for cell immobilization, and the biocatalyst prepared from X7B possesses a broad substrate specificity toward organic sulfur compounds in gasoline. We are currently investigating the enzymatic and genetic characteristics of this moderately thermophilic bacterium.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 30270045), by the Tenth Five-Year National Key Technologies R&D Programme (grant no. 2001BA707B01), and by a doctoral research grant from the Ministry of Education, People's Republic of China (grant no. 20020422050).
REFERENCES
- 1.Adams, M. W. W., and R. M. Kelly. 1998. Finding and using hyperthermophilic enzymes. Trends Biotechnol. 16:329-332. [DOI] [PubMed] [Google Scholar]
- 2.Brown, B. A., B. Springer, V. A. Steingrube, R. W. Wilson, G. E. Pfyffer, M. J. Garcia, M. C. Menendez, B. Rodriguez-Salgado, K. C. Jost, Jr., S. Chiu, G. O. Onyi, E. C. Bŏttger, and R. J. Wallace, Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Bacteriol. 49:1493-1511. [DOI] [PubMed] [Google Scholar]
- 3.Chang, J. H., Y. K. Chang, H. W. Ryu, and H. N. Chang. 2000. Desulfurization of light gas oil in immobilized-cell systems of Gordona sp. CYKS1 and Nocardia sp. CYKS2. FEMS Microbiol. Lett. 182:309-312. [DOI] [PubMed] [Google Scholar]
- 4.Coco, W. M., W. E. Levinson, M. J. Crist, H. J. Hektor, A. Darzins, P. T. Pienkos, C. H. Squires, and D. J. Monticello. 2001. DNA shuffling method for generating highly recombined genes and evolved enzymes. Nat. Biotechnol. 19:354-359. [DOI] [PubMed] [Google Scholar]
- 5.Denome, S. A., C. Oldfield, and L. J. Nash. 1994. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J. Bacteriol. 176:6707-6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denome, S. A., E. S. Olson, and K. D. Young. 1993. Identification and cloning of genes involved in specific desulfurization by Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 59:2837-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folsom, B. R., D. R. Schieche, and P. M. Digrazia. 1999. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl. Environ. Microbiol. 65:4967-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert, S. C., J. Morton, S. Buchanan, C. Oldfield, and A. McRoberts. 1998. Isolation of a unique benzothiophene-desulphurizing bacterium, Gordonia sp. strain 213E (NCIMB 40816), and characterization of the desulphurization pathway. Microbiology 144:2545-2553. [DOI] [PubMed] [Google Scholar]
- 9.Gray, K. A., O. S. Pogrebinsky, and G. T. Mrachko. 1996. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat. Biotechnol. 14:1705-1709. [DOI] [PubMed] [Google Scholar]
- 10.Kayser, K. J., B. A. Bielaga-Jones, K. Jackowski, O. Odusan, and J. J. Kilbane II. 1993. Utilization of organosulphur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. J. Gen. Microbiol. 139:3123-3129. [Google Scholar]
- 11.Kilbane, J. J. 1989. Desulfurization of coal: the microbial solution. Trends Biotechnol. 7:97-101. [Google Scholar]
- 12.King, R. W. 1988. Petroleum: its composition, analysis and processing. Occup. Med. 3:409-430. [PubMed] [Google Scholar]
- 13.Kirimura, K., T. Furuya, R. Sato, Y. Ishii, K. Kino, and S. Usami. 2002. Biodesulfurization of naphthothiophene and benzothiophene through selective cleavage of carbon-sulfur bonds by Rhodococcus sp. strain WU-K2R. Appl. Environ. Microbiol. 68:3867-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi, J., T. Onaka, Y. Ishii, and M. Suzuki. 2000. Demonstration of the carbon-sulfur bond targeted desulfurization of benzothiophene by thermophilic Paenibacillus sp. strain A11-2 capable of desulfurization of dibenzothiophene. FEMS Microbiol. Lett. 187:151-215. [DOI] [PubMed] [Google Scholar]
- 15.Konishi, J., Y. Ishii, and T. Onaka. 1997. Thermophilic carbon-sulfur-bond-targeted biodesulfurization. Appl. Environ. Microbiol. 63:3164-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei, B., and S. C. Tu. 1996. Gene overexpression, purification, and identification of a desulfurization enzyme from Rhodococcus sp. strain IGTS8 as a sulfide/sulfoxide monooxygenase. J. Bacteriol. 178:5699-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, F. L., P. Xu, C. Q. Ma, L. L. Luo, and X. S. Wang. 2003. Deep desulfurization of hydrodesulfurization-treated diesel oil by a facultative thermophilic bacterium Mycobacterium sp. X7B. FEMS Microbiol. Lett. 223:301-307. [DOI] [PubMed] [Google Scholar]
- 18.Li, F. L., P. Xu, C. Q. Ma, Y. Zheng, and Y. B. Qu. 2003. Biodesulfurization of dibenzothiophene by a newly isolated bacterium Mycobacterium sp. X7B. J. Chem. Eng. Jpn. 36:1174-1177. [Google Scholar]
- 19.Li, M. Z., C. H. Squires, and D. J. Monticello. 1996. Genetic analysis of the dsz promoter and associated regulatory regions of Rhodococcus erythropolis IGTS8. J. Bacteriol. 178:6409-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linguist, L., and M. Pacheco. 1999. Enzyme-based diesel desulfurization offers energy, CO2 advantages. Oil Gas J. 97:45-48. [Google Scholar]
- 21.Ma, X. L., L. Sun, and C. S. Song. 2002. A new approach to deep desulfurization of gasoline, diesel fuel and jet fuel by selective adsorption for ultra-clean fuels and for fuel cell applications. Catal. Today 77:107-116. [Google Scholar]
- 22.Matsui, T., O. Toshimitsu, T. Yasuhiro, T. Toshiyuki, S. Masanori, and K. Ryuichiro. 2000. Alkylated benzothiophene desulfurization by Rhodococcus sp. strain T09. Biosci. Biotechnol. Biochem. 64:596-599. [DOI] [PubMed] [Google Scholar]
- 23.McFarland, B. L., D. J. Boron, W. Deever, J. A. Meyer, A. R. Johnson, and R. M. Atlas. 1998. Biocatalytic sulfur removal from fuels: applicability for producing low sulfur gasoline. Crit. Rev. Microbiol. 24:99-147. [DOI] [PubMed] [Google Scholar]
- 24.Monticello, D. J. 2000. Biodesulfurization and the upgrading of petroleum distillates. Curr. Opin. Biotechnol. 11:540-546. [DOI] [PubMed] [Google Scholar]
- 25.Moslemy, P., R. J. Neufeld, and S. R. Guiot. 2002. Biodegradation of gasoline by gellan gum-encapsulated bacterial cells. Biotechnol. Bioeng. 80:175-184. [DOI] [PubMed] [Google Scholar]
- 26.Naito, M., T. Kawamoto, K. Fujino, M. Kobayashi, K. Maruhashi, and A. Tanaka. 2001. Long-term repeated biodesulfurization by immobilized Rhodococcus erythropolis KA2-5-1 cells. Appl. Microbiol. Biotechnol. 55:374-378. [DOI] [PubMed] [Google Scholar]
- 27.Piddington, C. S., B. R. Kovacevich, and J. Rambosek. 1995. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 61:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pienkos, P. T. 2002. Gasoline biodesulfurization DE-FC07-97ID13570 final report. Enchira Biotechnology Corp. (www.osti.gov/dublincore/gpo/servlets/purl/791501-cPnHAz/native/).
- 29.Shan, G. B., J. M. Xing, M. F. Luo, H. Z. Liu, and J. Y. Chen. 2003. Immobilization of Pseudomonas delafieldii with magnetic polyvinyl alcohol beads and its application in biodesulfurization. Biotechnol. Lett. 25:1977-1983. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka, Y., T. Matsui, J. Konishi, and K. Maruhashi. 2002. Biodesulfurization of benzothiophene and dibenzothiophene by a newly isolated Rhodococcus strain. Appl. Microbiol. Biotechnol. 59:325-328. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka, Y., T. Onaka, T. Matsui, K. Maruhashi, and R. Kurane. 2001. Desulfurization of benzothiophene by the gram-negative bacterium, Sinorhizobium sp. KT55. Curr. Microbiol. 43:187-191. [DOI] [PubMed] [Google Scholar]