Abstract
Farm management practices that reduce the prevalence of food-borne pathogens in live animals are predicted to enhance food safety. To ascertain the potential role of livestock bedding in the ecology and epidemiology of Escherichia coli O157:H7 on farms, the survival of this pathogen in used-sand and used-sawdust dairy cow bedding was determined. Additionally, a longitudinal study of mature dairy cattle housed on 20 commercial dairy farms was conducted to compare the prevalence of E. coli O157:H7 in cattle bedded on sand to that in cattle bedded on sawdust. E. coli O157:H7 persisted at higher concentrations in used-sawdust bedding than in used-sand bedding. The overall average herd level prevalence (3.1 versus 1.4%) and the number of sample days yielding any tests of feces positive for E. coli O157:H7 (22 of 60 days versus 13 of 60 days) were higher in sawdust-bedded herds. The choice of bedding material used to house mature dairy cows may impact the prevalence of E. coli O157:H7 on dairy farms.
Cattle manure is considered the primary source of Escherichia coli O157:H7 contamination of foods and the environment. Reduction of the prevalence and the magnitude of fecal E. coli O157:H7 excretion by cattle is predicted to enhance food and environmental safety (17). Unfortunately, despite many years of research focused specifically on E. coli O157:H7, the understanding of the epidemiology and ecology of this organism on farms and in livestock populations is still insufficient to provide scientifically based recommendations for preharvest intervention strategies for this important food-borne pathogen.
In contrast, research focused on the broader group of mastitis-causing coliform bacteria in dairy cattle has yielded some valuable information concerning the epidemiology and ecology of generic E. coli in the farm environment. As observed with E. coli O157:H7 colonization, coliform mastitis is more frequently observed during the warm months of the year (8, 27). This summertime increase in clinical mastitis is correlated with the seasonal peak of gram-negative-bacterium contamination in dairy cow bedding (15). On a gram-per-gram basis, bacterial counts in organic bedding are typically higher than those in inorganic bedding materials, such as sand (15). The purpose of this study was to determine the associations between bedding material and prevalence values for E. coli O157:H7 in cattle. The survival of E. coli O157:H7 in sand and sawdust bedding was assessed. The hypothesis that cows bedded with sand would have a lower fecal prevalence of E. coli O157:H7 than cows bedded on sawdust was tested in a prospective longitudinal study.
MATERIALS AND METHODS
Survival of E. coli O157:H7 in laboratory microcosms.
Three composite samples of used-sand bedding material and three composite samples of used-sawdust bedding material were collected from the floor in the lactating-cow barns of six commercial dairy farms. A green fluorescent protein-expressing, ampicillin-resistant strain of E. coli O157 (strain B6914) was grown overnight at 37°C in brain heart infusion broth (ca. 108 CFU/ml) (7). Bacterial cells were concentrated by centrifugation and resuspended in 1/10 volume of phosphate-buffered saline (PBS). Ten milliliters of the concentrated cells was evenly distributed in 500 ml of each bedding sample. Bedding samples were maintained at laboratory ambient temperature (approximately 22°C).
Ten-milliliter volumes of each bedding material were removed periodically (days 0, 1, 2, 3, 6, 8, 9, 13, 20, 27, 34, 41, 49, 55, 76, and 112). Each 10-ml sample was added to 90 ml of buffered peptone water (BPW) and homogenized manually for approximately 30 s, and serial 10-fold dilutions of the supernatant were immediately made in BPW. Five hundred microliters of multiple dilutions was spread plated onto 150-mm-diameter Luria-Bertani agar plates containing 100 μg of cycloheximide/ml and 100 μg of ampicillin/ml (LBCycAmp). LBCycAmp plates were incubated overnight at 37°C, and fluorescent green colonies on plates with isolated colonies were enumerated with the aid of UV illumination.
Longitudinal study.
Twenty commercial dairy farms located in northeast Ohio were enrolled in the study based on herd size (>120 lactating cows), type of bedding used for lactating cows (10 used sand, and 10 used sawdust), location (ease of sampling), and producer willingness to participate in the study. Pairs of farms (one with a sand-bedded herd and one with sawdust-bedded herd of similar size) were enrolled in the study between 9 June and 22 July 2003. Farms were visited at 2-week intervals for sample collection. Each farm was visited six times. The last sample collection was on 15 September 2003. At the time of the first sample visit an extensive questionnaire regarding farm management practices was completed. Subjects covered included biosecurity, animal health, feed, water, and waste management practices.
Sample collection.
Sixty-milliliter samples of used bedding material were collected from the back one-third of 10% of the stalls (evenly spaced throughout the free-stall barn). All bedding samples collected on a single farm were pooled in sterile plastic bags. Pooled samples were mixed thoroughly by rolling and shaking sample bags.
One hundred milliliters of drinking water was collected from the surface of one designated water trough in the lactating-cow barn on each farm. In addition, up to 50 ml of sediment (if available) from the bottom of the water trough was collected.
On each sampling occasion feces were collected by inserting sterile cotton-tipped swabs into 30 fresh fecal pats from lactating cows. Swabs coated with feces were immediately placed in 3 ml of BPW and transported chilled immediately to the laboratory. Ambient temperature and the temperature of the free-stall bedding material were measured on the last four sample collection dates on each farm. Relative humidity data were obtained from a local meteorological monitoring station.
Microbiological analyses.
By displacement, bedding was added to 225 ml of PBS to bring total volume of sample and buffer to 250 ml. Bedding samples diluted in PBS were shaken on ice for 15 min to suspend bacteria into solution. Additional serial 10-fold dilutions of the bedding wash solution were made in PBS. The most probable numbers (MPN) of coliforms and generic E. coli cells present in the bedding wash solution were determined with a commercially available assay (Quanti-Tray/2000; IDEXX Labs, Westbrook, Maine).
One hundred milliliters of the 1:9 dilution of bedding in PBS was added to 100 ml of 2× BPW, and the mixture was incubated at 42°C for 24 h for detection of E. coli O157:H7 (21, 22). Briefly, E. coli O157 present in any 1-ml aliquot of the overnight enrichment was concentrated with anti-O157-specific immunomagnetic beads (Dynal, Oslo, Norway). Beads were plated on sorbitol-MacConkey agar plates (Difco Laboratories, Detroit, Mich.) containing cefixime (50 ng/ml) and potassium tellurite (2.5 mg/ml; Sigma Chemical Company; St. Louis, Mo.) (SMACCT). Up to five sorbitol-negative (white) colonies were picked from each plate. Suspect colonies picked from the SMACCT plates were further identified as E. coli O157:H7 based on lactose fermentation and the inability to cleave 4-methylumbelliferyl-β-d-glucuronide to a fluorescent product (2, 32). The presence of the O157 antigen was determined by a particle agglutination test (Oxoid, Basingstoke, Hampshire, United Kingdom). Colonies phenotypically typical of E. coli O157:H7 were tested by a multiplex PCR assay for rfbO157, stx1, stx2, fliCH7, and eaeA (14).
Ten grams of the original bedding sample was weighed for dry-matter calculation. Samples were allowed to dry in the 42°C incubator until they achieved a constant weight as determined by weighing on two consecutive days.
MPN of coliform and generic E. coli cells present in the undisturbed livestock drinking water were determined by using the IDEXX Quanti-Tray/2000 procedure on 10-fold dilutions of the sampled water. Twenty-five grams of sediment from each trough was enriched overnight at 42°C in 225 ml of BPW. E. coli O157:H7 present in the sediment enrichments was detected as described above.
Within 6 h of collection, feces on each swab were dislodged by vortex agitation and dissolved in the BPW enrichment broth. Samples were incubated overnight at 42°C. E. coli O157:H7 presence was determined by immunomagnetic separation as outlined above for each enrichment.
Statistical analyses.
The difference in the in the rates of survival of E. coli O157:H7 inoculated into sand and sawdust bedding was determined by repeated-measures analysis of variance (ANOVA). Differences in bedding coliform and E. coli counts between treatment groups were also determined by a repeated-measures ANOVA. The effect of bedding type on the incidence of E. coli O157:H7 in live animals was determined by several methods. The overall herd E. coli O157:H7 prevalence values (over all sample dates) between groups were compared by a Wilcoxon matched-pair signed-rank test (25). Furthermore, differences in herd level fecal prevalence between sand- and sawdust-bedded herds were assessed by a repeated-measures analysis for nonparametric data (24). Finally, to compare the numbers of sample days positive (days on which one or more fecal samples tested positive for E. coli O157:H7), results were dichotomized into positive and negative on each sample date and the total numbers of sample days positive for the two groups (sand versus sawdust) were compared by a chi-squared goodness-of-fit test (25).
Pearson product-moment correlation coefficients for bedding coliform and E. coli counts, bedding temperature at the time of sampling, ambient temperatures at the time of sampling and the average daily maximum temperature in the 7 days prior to sampling, maximum daily relative humidity at the time of sampling and in the 7 days prior to sampling, the dry matter content of the bedding and microbial water quality parameters, and bedding dry matter content were determined (25). Correlations between the aforementioned parameters and the fecal E. coli O157 prevalence were assessed by using the Spearman rank correlation coefficient. All tests were conducted using SAS System for Windows, version 8.02 (Statistical Analysis Systems, Cary, N.C.) with unidirectional alternative hypotheses (sawdust > sand) and an alpha (type I error rate) of 0.05.
RESULTS
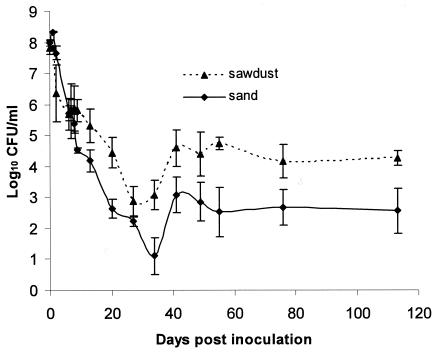
In the experimental microcosms, E. coli O157:H7 declined following initial inoculation in both sand and sawdust bedding but survived at higher concentrations in used-sawdust bedding than in used-sand bedding (P = 0.03; Fig. 1).
FIG. 1.
Survival of E. coli O157 in cattle bedding. Bars, standard errors.
The farms represented in each group were of similar sizes (farms using sand, 267 ± 139 milking cows; farms using sawdust, 311 ± 158 milking cows [means ± standard deviations]; P = 0.55). For 8 of the 10 sawdust-bedded herds, fresh bedding material was added to stalls two or more times a week, whereas bedding material for only 2 of 10 sand-bedded herds was replenished at this frequency.
The total coliform and E. coli concentrations and presence of E. coli O157:H7 in 120 bedding and 120 water trough samples were assessed. Coliforms counts the two groups were not different (log10 MPN per 10 ml, 8.36 versus 8.24; P = 0.67). The E. coli counts in sand and sawdust bedding were identical (log10 MPN per 10 ml for both, 7.55; P = 0.98). Using enrichment methods, E. coli O157:H7 was detected at similar frequencies in sand bedding (2 of 60 samples, 3.3%) and sawdust bedding samples (3 of 60 samples, 5%; P = 1.0). E. coli O157:H7 was detected at equal frequencies in water troughs from sand-bedded and sawdust-bedded herds, once (1 of 60 samples, 1.7%; P = 1.0) in each group. Water coliform counts (log10 MPN/100 ml, 4.3 versus 3.7; P = 0.05), but not water E. coli counts (log10 MPN/100 ml, 3.4 versus 3.0; P = 0.07), were significantly higher in sawdust-bedded herds than in sand-bedded herds.
A total of 3,600 bovine fecal samples were tested for E. coli O157:H7; 1,800 from sand-bedded animals and 1,800 from sawdust-bedded animals. The prevalence of fecal-material-positive samples identified on each sample date on each farm is outlined in Table 1. A total of 81 of 3,600 (2.3%) fecal samples tested positive for E. coli O157:H7. There were more than twice as many positive samples from sawdust-bedded (56 of 1,800) animals as from sand-bedded animals (25 of 1,800). On a herd level basis, the average E. coli O157:H7 prevalence was significantly higher among the sawdust-bedded herds (3.1%) than among the sand-bedded herds (1.4%), regardless of whether the Wilcoxon rank-sum test (sum of positive samples on each farm over all sample dates; P = 0.03) or the more appropriate repeated-measures analysis was used (P = 0.05). Furthermore, the total number of days on which herds were positive for E. coli O157:H7 was higher for sawdust-bedded herds than for sand-bedded herds (22 versus 14; P = 0.05).
TABLE 1.
Fecal prevalence of E. coli O157:H7 in Ohio dairy herdsa
| Bedding | Farm | Fecal prevalence (%) during the sampling period (mo/day):
|
Overall prevalence (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6/9-6/14 | 6/15-6/29 | 6/30-7/13 | 7/14-7/27 | 7/28-8/10 | 8/10-8/24 | 8/25-9/7 | 9/8-9/15 | |||
| Sand | A | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| B | 0.0 | 0.0 | 3.3 | 3.3 | 0.0 | 10.0 | 2.8 | |||
| C | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| D | 13.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 | |||
| E | 0.0 | 0.0 | 6.7 | 0.0 | 0.0 | 0.0 | 1.1 | |||
| F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| G | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.6 | |||
| H | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| I | 6.7 | 13.3 | 3.3 | 0.0 | 6.7 | 0.0 | 5.0 | |||
| J | 0.0 | 3.3 | 0.0 | 3.3 | 6.7 | 0.0 | 2.2 | |||
| Avg | 0.0 | 2.2 | 1.0 | 2.7 | 0.3 | 1.3 | 1.9 | 0.8 | 1.4 | |
| Sawdust | K | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 3.3 | 1.1 | ||
| L | 3.3 | 0.0 | 0.0 | 0.0 | 3.3 | 20.0 | 4.4 | |||
| M | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.0 | 1.7 | |||
| N | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 0.0 | 3.3 | |||
| O | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | 6.7 | 1.7 | |||
| P | 0.0 | 3.3 | 0.0 | 3.3 | 0.0 | 0.0 | 1.1 | |||
| Q | 6.7 | 0.0 | 6.7 | 0.0 | 0.0 | 0.0 | 2.2 | |||
| R | 0.0 | 3.3 | 0.0 | 0.0 | 3.3 | 13.3 | 3.3 | |||
| S | 6.7 | 0.0 | 0.0 | 3.3 | 6.7 | 3.3 | 3.3 | |||
| T | 0.0 | 0.0 | 0.0 | 6.7 | 0.0 | 46.7 | 8.9 | |||
| Avg | 1.1 | 0.0 | 1.7 | 0.3 | 4.0 | 4.3 | 2.4 | 15.8 | 3.1 | |
| Overall avg | 0.6 | 1.1 | 1.3 | 1.5 | 2.2 | 2.8 | 2.1 | 8.3 | 2.3 | |
Thirty samples were tested per herd during each sampling period. Average values for farms using sand and sawdust are in boldface.
Other than the expected strong correlation between bedding E. coli counts and bedding coliform counts, no other significant associations between bedding coliform counts were identified. However, several weak, but significant correlations between several other parameters measured in this study were identified (Table 2). Notably, bedding generic E. coli counts were correlated with the fecal prevalence of E. coli O157:H7.
TABLE 2.
Significant correlations among variables observed on Ohio dairy farmsa
| Parameter 1 | Parameter 2 | n | r2 | P |
|---|---|---|---|---|
| Bovine E. coli O157 prevalence | E. coli count in bedding | 118 | 0.19 | 0.04 |
| % of E. coli among bedding coliforms | 119 | 0.22 | 0.02 | |
| Dry matter content of bedding | 119 | −0.18 | 0.05 | |
| % of E. coli among water coliforms | 120 | 0.24 | 0.01 | |
| Relative humidity at time of sample collection | 120 | 0.18 | 0.04 | |
| Relative humidity at sampling | Count of E. coli in water | 120 | 0.20 | 0.03 |
| % of E. coli among bedding coliforms | 119 | 0.21 | 0.02 | |
| Count of coliforms in bedding | Count of E. coli in bedding | 118 | 0.63 | <0.001 |
| Count of coliforms in water | Count of E. coli in water | 120 | 0.74 | <0.001 |
| Dry-matter content of bedding | Count of coliforms in water | 119 | −0.38 | <0.001 |
| Count of E. coli in water | 119 | −0.21 | 0.03 | |
| % of E. coli among water coliforms | 119 | 0.20 | 0.03 |
Correlations with fecal prevalence are calculated with Spearman's rank-order correlation coefficient; all others are determined with Pearson's product-moment correlation coefficient.
DISCUSSION
In this study, the fecal prevalence of E. coli O157:H7 among mature dairy cattle was associated with the choice of bedding material used on the farm. The use of sawdust for bedding material for lactating dairy cows, as opposed to sand, was associated with a significantly higher fecal prevalence of E. coli O157:H7. Specifically, the prevalence of E. coli O157 was correlated with the magnitude of bedding contamination with generic E. coli. Increased magnitude of bedding contamination with generic E. coli may be predictive of the increased likelihood of environmental contamination with E. coli O157. These results are in accord with the increased incidence of coliform mastitis among sawdust-bedded cattle (27). In experimental microcosms, E. coli O157:H7 persisted at higher concentrations in sawdust bedding than in sand bedding. Differences in water content, nutrient availability, and competitive microbial flora may account for some the within-group variability observed. Collectively, these results suggest that the choice of bedding material impacts the magnitude of E. coli O157:H7 in the farm environment and highlights the role of the dairy farm environment as a potential source of E. coli O157:H7 for cattle.
Factors that govern the persistence and growth of bacteria in various bedding materials are complex. This study was conducted during the summer and early fall in order to capture the highest expected bovine prevalence of E. coli O157:H7. Coincidently, the levels of total coliform and generic E. coli contamination in livestock bedding are greatest during the warm months of the year (15, 19). Other factors that can influence the magnitude of bacterial contamination of the environment include the nutrient content of the bedding material and the presence of substances toxic to bacteria (29). It is generally accepted that sand bedding has less available organic matter and nutrients than sawdust, although this parameter was not analyzed in this study. Lower water content in sand than in sawdust, as observed in this study and described by others, is also believed to inhibit bacterial growth in fresh bedding (4, 29). Smith et al. have previously reported that the prevalence of E. coli O157 among feedlot cattle housed in muddy or wet pens was higher than that among cattle housed in pens subjectively assessed as being drier (26).
Our methodology of measuring bedding material by volume differs from that for many previous studies where the unit of measure was weight (13, 14, 16). Since bedding material is placed in barns based on a volume basis, this method of comparison provides a more appropriate measure of exposure to environmental bacteria. Sand bedding may be five to eight times denser than sawdust (6). It is highly probable that significant differences in the bacterial counts between the two bedding materials in this study would have been identified if they had been measured on a per-gram basis. Gabler et al. found that comparing the numbers of Streptococcus sp. cells in bedding samples on a per-weight basis was highly skewed in favor of the denser material and that, in fact, when the same samples were compared on a per-volume basis, the significant differences in bacterial counts were actually completely (and significantly) inverted (6). One author has suggested that bacterial counts higher than 106 cells per gram of bedding may be associated with increased health risk for cattle (3). Assuming that the density of sawdust is at least 0.32 g/ml (6), 116 of 120 (97%) bedding samples exceeded this threshold for coliform counts alone.
Mature cattle do not routinely consume large volumes of soil or bedding material (5, 11); however, bacterial contaminants present in bedding material can be aerosolized when handled, distributed, or removed from the barn. It is estimated that up to 5.7 × 106 microorganisms per dairy cow may be liberated into the air during the spreading of the bedding. Epidemiological evidence collected from a human outbreak of E. coli O157:H7 associated with a dance held in a multipurpose agricultural building with a sawdust-covered floor suggests that this organism is readily aerosolized when contaminated sawdust is disturbed (28). Aerosolized particles of dust are usually trapped in the upper airway and then swallowed. Alternatively, E. coli O157:H7 carried on dust particles may contaminate feed, water sources, or the hide of animals (18). As with humans, many cattle may become colonized with E. coli O157:H7 following low-exposure doses (2).
The experimental inoculation of E. coli O157:H7 demonstrated that, following an initial decline in numbers, as observed for generic E. coli, this pathogen can survive for extended periods in ambient temperatures. Humidity and temperature fluctuations in barns may be more extreme than those observed under these controlled laboratory conditions. Nevertheless, these results are consistent with field data that suggest the extended persistence of E. coli O157 in sawdust (28). This has implications not only for human health (as mentioned above in regard to visits to contaminated sites) but also for cattle. Calves and heifers are frequently housed on manure pack or other organic materials and have the highest prevalence of E. coli O157:H7 (20).
Because of the sporadic and transient nature of E. coli O157 carriage by cattle, the detection of differences in bovine E. coli O157 prevalence among different groups of animals is challenging (1, 10). Although we have presented the overall fecal prevalence values in this report for completeness, the true unit of observation is the farm. Each farm was sampled on multiple occasions; thus, the measurements cannot be considered independent. Moreover, parametric comparisons of prevalence values can be easily skewed if a single farm has an extremely high prevalence on a single sample date, which would not be uncommon given the epidemiology of E. coli O157 in cattle populations. Furthermore, when samples are collected at intermittent intervals, it is not possible to determine if the prevalence was increasing, decreasing, or at its peak. To circumvent this problem, we also report in this study the days on which herds were positive for E. coli O157 as a measure of food and environmental safety. We posit that animals (cull cattle), milk, and manure leaving farms with a greater number of days on which herds were positive (sawdust-bedded herds) are more likely to be contaminated with this pathogen than those from farms with fewer days on which herds were positive. Thus, products leaving farms more frequently contaminated with E. coli O157 may pose a greater risk to food and environmental safety.
The specific means by which the type of bedding material affects E. coli O157 prevalence in bovine feces was not determined in this study. We have proposed that reduced survival of E. coli O157 in sand bedding lowers the environmental load of this food-borne pathogen. However, other plausible explanations, which may have worked in concert with the proposed reduction in aerosolized bacteria, may also exist. For example, drinking water contamination with organic bedding material on farms using sawdust bedding may contribute to increased E. coli O157 survival in water. This alternative hypothesis is partially supported by the fact that water coliform counts (but not E. coli counts) from sawdust-bedded herds also were higher than those from sand-bedded herds. A second indirect mechanism by which bedding type may influence E. coli O157 prevalence is in insect control. Houseflies and stable flies on farms have been frequently identified as E. coli O157-positive (9, 12). The use of sand bedding on farms has been shown to greatly suppress fly larva densities compared to the use of organic materials (23). If flies play an important role in the transmission of E. coli O157 among cattle, it may be that farms with smaller fly populations will prove to have lower E. coli O157 counts. The choice of bedding material used on farms is dependent on many factors, including economics, animal health, manure management, and animal well-being. Additional studies are required to elucidate the precise mechanisms by which the beneficial results of using sand bedding were attained. Regardless of the outcome of these more detailed experiments, the present data provide evidence that specific (modifiable) farm management practices can influence the prevalence of E. coli O157:H7 on the farm.
Acknowledgments
This work was supported in part by federal and state funds provided to the Ohio Agricultural Research and Development Center.
We acknowledge Ohio State University Dairy Extension Agents Tom Noyes, Dean Slates, Dave Marrison, and Ernie Oelker for their support and participation as well as the cooperation of the 20 participating dairy farms.
REFERENCES
- 1.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 2.Besser, T. E., B. L. Richards, D. H. Rice, and D. D. Hancock. 2001. Escherichia coli O157:H7 infection of calves: infectious dose and direct contact transmission. Epidemiol. Infect. 127:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bramley, A. J., and F. K. Neave. 1975. Studies on the control of coliform mastitis in dairy cows. Br. Vet. J. 131:160-169. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, E. J., and D. E. Jasper. 1978. Distribution of enterobacteriaceae in recycled manure bedding on California dairies. J. Dairy Sci. 61:1498-1508. [DOI] [PubMed] [Google Scholar]
- 5.Fries, G. F., G. S. Marrow, and P. A. Snow. 1982. Soil ingestion by dairy cattle. J. Dairy Sci. 65:611-618. [DOI] [PubMed] [Google Scholar]
- 6.Gabler, M. T., J. K. Reneau, and R. J. Farnsworth. 2001. Comparison of number of Streptococcus uberis calculated on a volume or weight basis in sand and sawdust bedding. Am. J. Vet. Res. 62:171-173. [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi, J. V., and J. S. Karns. 2000. Leaching of Escherichia coli O157:H7 in diverse soils under various agricultural management practices. Appl. Environ. Microbiol. 66:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock, D., T. Besser, J. Lejeune, M. Davis, and D. Rice. 2001. The control of VTEC in the animal reservoir. Int. J. Food Microbiol. 66:71-78. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, D. D., T. E. Besser, D. H. Rice, E. D. Ebel, D. E. Herriott, and L. V. Carpenter. 1998. Multiple sources of Escherichia coli O157 in feedlots and dairy farms in the northwestern USA. Prev. Vet. Med. 35:11-19. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, D. D., T. E. Besser, D. H. Rice, D. E. Herriott, and P. I. Tarr. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol. Infect. 118:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herlin, A., and I. Andersson. 1996. Soil ingestion in farm animals. Report 105 from the Swedish University of Agricultural Sciences, Department of Biosystems and Technology. Swedish University of Agricultural Sciences, Lund, Sweden.
- 12.Heuvelink, A. E., F. L. A. M. van den Biggelaar, J. T. M. Zwartkruis-Nahuis, R. G. Herbes, R. Huyben, N. Nagelkerke, W. J. G. Melchers, L. A. H. Monnens, and E. de Boer. 1998. Occurrence of verocytotoxin-producing Escherichia coli O157 on Dutch dairy farms. J. Clin. Microbiol. 36:3480-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan, J. S., V. L. Bogacz, L. M. Thompson, S. Romig, P. S. Schoenberger, W. P. Weiss, and K. L. Smith. 1999. Bacterial counts associated with sawdust and recycled manure bedding treated with commercial conditioners. J. Dairy Sci. 82:1690-1695. [DOI] [PubMed] [Google Scholar]
- 14.Hogan, J. S., and K. L. Smith. 1997. Bacteria counts in sawdust bedding. J. Dairy Sci. 80:1600-1605. [DOI] [PubMed] [Google Scholar]
- 15.Hogan, J. S., K. L. Smith, K. H. Hoblet, D. A. Todhunter, P. S. Schoenberger, W. D. Hueston, D. E. Pritchard, G. L. Bowman, L. E. Heider, B. L. Brockett, et al. 1989. Bacterial counts in bedding materials used on nine commercial dairies. J. Dairy Sci. 72:250-258. [DOI] [PubMed] [Google Scholar]
- 16.Hogan, J. S., K. L. Smith, D. A. Todhunter, and P. S. Schoenberger. 1990. Bacterial counts associated with recycled newspaper bedding. J. Dairy Sci. 73:1756-1761. [DOI] [PubMed] [Google Scholar]
- 17.Jordan, D., S. A. McEwen, A. M. Lammerding, W. B. McNab, and J. B. Wilson. 1999. Pre-slaughter control of Escherichia coli O157 in beef cattle: a simulation study. Prev. Vet. Med. 41:55-74. [DOI] [PubMed] [Google Scholar]
- 18.Keen, J. E., and R. O. Elder. 2002. Isolation of shiga-toxigenic Escherichia coli O157 from hide surfaces and the oral cavity of finished beef feedlot cattle. J. Am. Vet. Med. Assoc. 220:756-763. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. J., B. W. Beasley, L. J. Yanke, F. J. Larney, T. A. McAllister, B. M. Olson, L. B. Selinger, D. S. Chanasyk, and P. Hasselback. 2003. Bedding and seasonal effects on chemical and bacterial properties of feedlot cattle manure. J. Environ. Qual. 32:1887-1894. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen, E. M., C. Tegtmeier, H. J. Andersen, C. Gronbaek, and J. S. Andersen. 2002. Influence of age, sex and herd characteristics on the occurrence of Verocytotoxin-producing Escherichia coli O157 in Danish dairy farms. Vet. Microbiol. 88:245-257. [DOI] [PubMed] [Google Scholar]
- 21.Ogden, I. D., N. F. Hepburn, M. MacRae, N. J. Strachan, D. R. Fenlon, S. M. Rusbridge, and T. H. Pennington. 2002. Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett. Appl. Microbiol. 34:100-104. [DOI] [PubMed] [Google Scholar]
- 22.Ogden, I. D., M. MacRae, and N. J. Strachan. 2004. Is the prevalence and shedding concentrations of E. coli O157 in beef cattle in Scotland seasonal? FEMS Microbiol. Lett. 233:297-300. [DOI] [PubMed] [Google Scholar]
- 23.Schmidtmann, E. T. 1991. Suppressing immature house and stable flies in outdoor calf hutches with sand, gravel, and sawdust bedding. J. Dairy Sci. 74:3956-3960. [DOI] [PubMed] [Google Scholar]
- 24.Shah, D., and L. Madden. 2004. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology 94:33-43. [DOI] [PubMed] [Google Scholar]
- 25.Sheskin, D. 2000. Handbook of parametric and nonparametric statistical procedures, 2nd ed. CRC Press, Boca Raton, Fla.
- 26.Smith, D., M. Blackford, S. Younts, R. Moxley, J. Gray, L. Hungerford, T. Milton, and T. Klopfenstein. 2001. Ecological relationships between the prevalence of cattle shedding Escherichia coli O157:H7 and characteristics of the cattle or conditions of the feedlot pen. J. Food Prot. 64:1899-1903. [DOI] [PubMed] [Google Scholar]
- 27.Smith, K. L., D. A. Todhunter, and P. S. Schoenberger. 1985. Environmental mastitis: cause, prevalence, prevention. J. Dairy Sci. 68:1531-1553. [DOI] [PubMed] [Google Scholar]
- 28.Varma, J. K., K. D. Greene, M. E. Reller, S. M. DeLong, J. Trottier, S. F. Nowicki, M. DiOrio, E. M. Koch, T. L. Bannerman, S. T. York, M. A. Lambert-Fair, J. G. Wells, and P. S. Mead. 2003. An outbreak of Escherichia coli O157 infection following exposure to a contaminated building. JAMA 290:2709-2712. [DOI] [PubMed] [Google Scholar]
- 29.Zehner, M. M., R. J. Farnsworth, R. D. Appleman, K. Larntz, and J. A. Springer. 1986. Growth of environmental mastitis pathogens in various bedding materials. J. Dairy Sci. 69:1932-1941. [DOI] [PubMed] [Google Scholar]