ABSTRACT
Although launched in 2015, little is known about the accuracy of QuantiFERON-TB Gold-Plus (QFT-Plus) for diagnosis of latent M. tuberculosis infection (LTBI). Unlike its predecessor, QFT-Plus utilizes two antigen tubes to elicit an immune response from CD4+ and CD8+ T lymphocytes. We conducted a cross-sectional study in low-risk health care workers (HCWs) at a single U.S. center to compare QFT-Plus to QuantiFERON-TB Gold in-tube (QFT). A total of 989 HCWs were tested with both QFT and QFT-Plus. Risk factors for LTBI were obtained from a questionnaire. QFT-Plus was considered positive if either antigen tube 1 (TB1) or TB2 tested positive, per the manufacturer's recommendations, or if both TB1 and TB2 tested positive, using a conservative definition. Results were compared using Cohen's kappa and linear regression, respectively. Agreement of QFT with QFT-Plus was high, at 95.6% (95% confidence interval [CI], 94.3 to 96.9; kappa, 0.57). The majority of discordant results between QFT and QFT-Plus TB1 (84.8%) and QFT and QFT-Plus TB2 (88.6%) fell within the range of 0.2 to 0.7 IU/ml. The positivity rate in 626 HCWs with no identifiable risk factors and no self-reported history of positive LTBI tests was 2.1% (CI, 1.0 to 3.2) and 3.0% (CI, 1.7 to 4.3) with QFT and QFT-Plus, respectively. A conservative definition of a QFT-Plus-positive result yielded a positivity rate of 1.0% (CI, 0.2 to 1.7; P value of 0.0002 versus QFT-Plus and 0.07 versus QFT). On follow-up testing, of 11 HCWs with discordant QFT-Plus results, 90.9% (10/11) had a negative QFT result. The QFT-Plus assay showed a high degree of agreement with QFT in U.S. HCWs. A conservative interpretation of QFT-Plus eliminated nearly all nonreproducible positive results in low-risk HCWs. Larger studies are needed to validate the latter finding and to more clearly define conditions under which a conservative interpretation can be used to minimize nonreproducible positive results in low-risk populations.
KEYWORDS: QFT, QFT-Plus, healthcare worker, low incidence, tuberculosis, IGRA, Mycobacterium tuberculosis
INTRODUCTION
Periodic screening for latent Mycobacterium tuberculosis infection (LTBI) is a mandated component of occupation and student health programs in many high-income countries (1). It is intended to identify recently infected individuals and treat them with preventive therapy to avoid development of active disease (2).
In the past decade, many health care institutions in the U.S. have switched from tuberculin skin test (TST) to gamma interferon (IFN-γ) release assay (IGRA), in particular the QuantiFERON-TB Gold in-tube assay (QFT; Cellestis/Qiagen, Carnegie, Australia), for annual screening of health care workers (HCWs) (1, 3). Advantages of IGRA over the TST include improved specificity in individuals with bacillus Calmette-Guérin (BCG) vaccination and certain nontuberculous mycobacterial infections. Moreover, IGRA eliminates the need for a second nurse visit, thus offering operational and economic advantages over TST (2). However, studies conducted in HCWs and students in low-incidence settings have shown high conversion rates with IGRA which exceed the historical or contemporary TST rates (4–6). Also, high rates of reversions and issues with poor reproducibility have also been documented (4, 7). Since positive results can precipitate unnecessary follow up and preventive treatment in low-risk HCWs, the accuracy of IGRA has important implications for patient safety and overutilization of resources (8).
In 2015, the next generation of QFT, QuantiFERON-TB Gold-Plus (QFT-Plus) (Qiagen), was launched in Europe and is undergoing clinical trials in the United States. QFT-Plus employs two antigen tubes (TB1 and TB2) for diagnosis of M. tuberculosis infection. Per the manufacturer's recommendations, QFT-Plus is interpreted as positive when either antigen tube result is positive. Both antigen tubes include peptides from M. tuberculosis complex-specific antigens ESAT-6 and CFP-10. While peptides in TB1 and QFT antigen tubes are designed to elicit an IFN-γ response from CD4+ helper T lymphocytes, TB2 contains an additional set of peptides to also elicit a response from CD8+ cytotoxic T lymphocytes. CD8+ T lymphocytes are an important component of host immunity to M. tuberculosis and produce IFN-γ in vitro after stimulation with M. tuberculosis antigens (9–11). Moreover, ESAT-6- and CFP-10-responsive CD8+ T lymphocytes are more frequently detected in subjects with active tuberculosis (TB) than during latent infection (12–14). They are also detected at a higher frequency after recent infection compared with remote infection (14, 15). Therefore, detection of antigen-responsive CD4+ and CD8+ lymphocytes in QFT-Plus is designed for higher sensitivity in active TB cases and after recent exposure. This was recently suggested in a cohort of 119 patients with active TB (84.9% sensitivity with QFT-Plus TB2 versus 80.7% with TB1) (16). However, follow-up studies directly comparing QFT to QFT-Plus in active TB patients did not show a difference in sensitivity (15, 17, 18). Furthermore, the sensitivity and specificity of QFT-Plus for LTBI in low-risk individuals, such as low-risk North American HCWs, remains to be determined. Given the simultaneous availability of two antigen tube results, discordant TB1 and TB2 results might indicate false-positive results.
In a cross-sectional study, we prospectively compared the performance of QFT-Plus to QFT in low-risk HCWs undergoing TB screening at an academic institution in the United States. We also tested the hypothesis that a more conservative definition of QFT-Plus positivity based on double-positive antigen tube results would reduce positivity rates in low-risk HCWs.
RESULTS
Results with QFT and QFT-Plus.
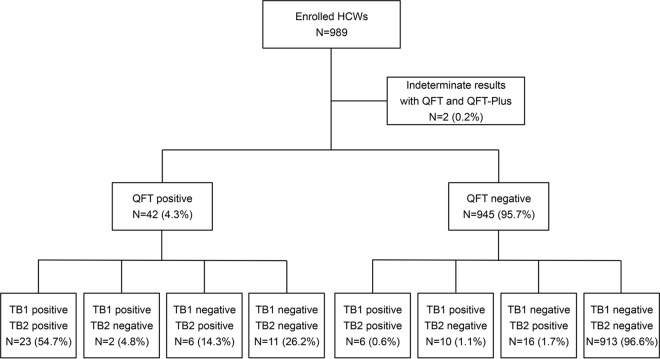
In total, 989 HCWs were tested with QFT and QFT-Plus. Demographic data and LTBI risk factors for all HCWs are summarized in Table 1. Two (0.2%) HCWs had indeterminate results with both QFT and QFT-Plus and therefore were excluded from further analysis. Figure 1 summarizes the QFT and QFT-Plus results for the remaining 987 HCWs. The positivity rate with QFT, QFT-Plus, QFT-Plus TB1, and QFT-Plus TB2 was 4.3% (95% confidence interval [CI], 3.0 to 5.6), 6.4% (CI, 4.9 to 7.9), 4.2% (CI, 3.0 to 5.5), and 5.2% (CI, 3.8 to 6.6), respectively (Table 2). Among 31 subjects that tested positive by both QFT and QFT-Plus, 61.3% had one or more risk factors for LTBI. Among 913 subjects that tested negative by both assays, 20.9% had one or more risk factors for LTBI (see Table S1 in the supplemental material). Among 82 subjects with a history of positive QFT or TST, the positivity rate was 20.7% (CI, 11.9 to 29.5) with QFT and 26.8% (CI, 17.2 to 36.4) with QFT-Plus. Among 68 subjects with a history of BCG vaccination, the positivity rate was 17.6% (CI, 8.6 to 26.7) with QFT and 22.1% (CI, 12.2 to 32.0) with QFT-Plus.
TABLE 1.
Demographic data and LTBI risk factors
| Parameter | Value (n = 989) |
|---|---|
| Age in yr, means ± SD | 38.0 ± 11.5 |
| Male gender [no. (%)] | 301 (30.4) |
| Prior positive QFT or TST [no. (%)] | 82 (8.3) |
| BCG vaccination [no. (%)] | 68 (6.9) |
| LTBI risk factora [no. (%)] | |
| 0 risk factors | 653 (66.0) |
| 1 risk factor | 107 (10.8) |
| ≥2 risk factors | 124 (12.6) |
| Unknown | 105 (10.6) |
LTBI risk factors per the questionnaire.
FIG 1.
Schematic overview of QFT and QFT-Plus results.
TABLE 2.
Qualitative comparison between QFT and QFT-Plus
| QFT result | Value [no. (%)] for: |
|||||||
|---|---|---|---|---|---|---|---|---|
| QFT-Plus |
QFT-Plus TB1 |
QFT-Plus TB2 |
QFT-Plus-C |
|||||
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Positive | 31 (3.1) | 11 (1.1) | 25 (2.5) | 17 (1.7) | 29 (2.9) | 13 (1.3) | 23 (2.3) | 19 (1.9) |
| Negative | 32 (3.2) | 913 (92.5) | 16 (1.6) | 929 (94.1) | 22 (2.2) | 923 (93.5) | 6 (0.6) | 939 (95.1) |
| Total | 63 (6.4) | 924 (93.6) | 41 (4.2) | 946 (95.8) | 51 (5.2) | 936 (94.8) | 29 (2.9) | 958 (97.1) |
Qualitative and quantitative comparisons between QFT and QFT-Plus.
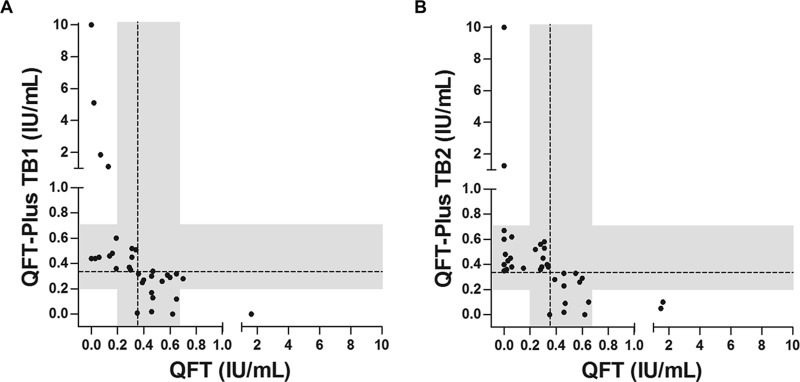
Binary agreement between QFT and QFT-Plus, QFT-Plus TB1, and QFT-Plus TB2 was >95% overall (Tables 2 and 3). Agreement in HCWs with one or more risk factors was 90.1% (CI, 86.3 to 94.0), 93.1% (CI, 89.8 to 96.4), and 93.5% (CI, 90.3 to 96.7), respectively (kappa, 0.60, 0.61, and 0.69, respectively). Among 42 (4.3%) HCWs with a positive QFT result, 11 (26.2%) were negative by QFT-Plus (Table 2 and Fig. 1). Among 945 (95.7%) HCWs with a negative QFT result, 32 (3.4%) were positive by QFT-Plus (Table 2 and Fig. 1). As shown in Fig. 2, 84.8% (28/33) and 88.6% (31/35) of HCWs with discordant results between QFT and QFT-Plus TB1 and QFT-Plus TB2, respectively, had a response within the range of 0.2 to 0.7 IU/ml for one or both assays. Similarly, among 34 HCWs with discrepant results between QFT-Plus TB1 and QFT-Plus TB2, 30 (88.2%) were within the same range. In 626 HCWs with no risk factors for LTBI, 76.9% (10/13) and 81.3% (13/16) of discrepancies between QFT and QFT-Plus TB1 and QFT-Plus TB2, respectively, were within the range of 0.2 to 0.7 IU/ml (Fig. S1).
TABLE 3.
Qualitative agreement between QFT and QFT-Plus
| Comparison | Agreement (%, 95% CI) | Kappa (95% CI) |
|---|---|---|
| QFT vs QFT-Plus | 944/987 (95.6, 94.3–96.9) | 0.57 (0.44–0.70) |
| QFT vs QFT-Plus TB1 | 954/987 (96.7, 95.6–97.8) | 0.59 (0.45–0.72) |
| QFT vs QFT-Plus TB2 | 952/987 (96.5, 95.4–97.7) | 0.61 (0.48–0.73) |
| QFT vs QFT-Plus-C | 962/987 (97.4, 96.4–98.4) | 0.64 (0.50–0.78) |
| QFT-Plus TB1 vs QFT-Plus TB2 | 953/987 (96.6, 95.5–97.7) | 0.61 (0.49–0.74) |
FIG 2.
Quantitative results in health care workers with discordant QFT and QFT-Plus results. Plots show quantitative results for QFT versus QFT-Plus TB1 (A) and QFT versus QFT-Plus TB2 (B) in health care workers with discordant results. The dashed reference lines at 0.35 IU/ml are the assay cutoffs, and the shaded areas mark the range of 0.2 to 0.7 IU/ml.
Quantitative IFN-γ results obtained with QFT showed a high degree of correlation with QFT-Plus TB1 and QFT-Plus TB2 (Pearson's correlation coefficient [R] of 0.74 and 0.75, respectively) (Fig. S2). QFT-Plus TB1 and QFT-Plus TB2 also showed very high correlation with each other (R = 0.90). The median TB response in HCWs with positive results was not significantly different between QFT and QFT-Plus TB1 (2.29 versus 1.77; n = 25; P = 0.21), QFT and QFT-Plus TB2 (1.58 versus 1.40; n = 29; P = 1.0), and QFT-Plus TB1 and TB2 (1.77 versus 1.89; n = 29; P = 0.29).
Positivity rates with QFT and QFT-Plus in no-risk HCWs.
Among 626 HCWs with no identifiable risk factors and no self-reported history of positive TST or IGRA, the positivity rate with QFT and QFT-Plus was 2.1% (CI, 1.0 to 3.2) and 3.0% (CI, 1.7 to 4.3), respectively (Table 4). A more conservative interpretation of QFT-Plus positivity (QFT-Plus-C), based on a double-positive TB1 and TB2 result, yielded a positivity rate of 1.0% (CI, 0.2 to 1.7), which is significantly lower than that for QFT-Plus (P < 0.001) and showed a reduced trend compared with QFT (P = 0.07) (Table 4). Among 310 HCWs with a documented history of negative QFT result and no risk factors for LTBI, the positivity rate was 2.6% (CI, 0.8 to 4.4), 2.6% (CI, 0.8 to 4.4), and 0.6% (CI, 0 to 1.5) with QFT, QFT-Plus, and QFT-Plus-C, respectively. In this group, the positivity rate with QFT-Plus-C was significantly lower than those for QFT-Plus (P = 0.03) and QFT (P = 0.03).
TABLE 4.
Positivity rates with QFT and QFT-Plus in 626 HCWs with no LTBI risk factors and no prior positive tests
| Assay | No. of positives | Positivity rate [% (95% CI)] | P valuea |
|---|---|---|---|
| QFT | 13 | 2.1 (1.0–3.2) | |
| QFT-Plus | 19 | 3.0 (1.7–4.3) | 0.24 |
| QFT-Plus TB1 | 10 | 1.6 (0.6–2.6) | 0.58 |
| QFT-Plus TB2 | 15 | 2.4 (1.2–3.6) | 0.80 |
| QFT-Plus-Cb | 6 | 1.0 (0.2–1.7) | 0.07 |
Compared with QFT using McNemar's test.
P value of 0.0002 compared with that of QFT-Plus.
Follow-up data were available for 11 of the 13 HCWs with discordant QFT-Plus results (Table 5). Zero HCWs developed active tuberculosis during the follow-up period. Ten HCWs had a negative QFT and 6 of 7 HCWs had a negative QFT-Plus (TB1 or TB2) at 9 to 13 months from enrollment. One HCW (study identity no. 6937), who was positive with QFT and QFT-Plus TB2 on enrollment, was subsequently positive with QFT after short-term retesting and with QFT and QFT-Plus (TB1 and TB2) 13 months later. Although this HCW did not have any known risk factors for LTBI, he was diagnosed with LTBI and was treated accordingly.
TABLE 5.
Follow-up results for 13 no-risk health care workers with discordant QFT-Plus results
| Study no. | Age (yr) | Sexa (M/F) | Result atb: |
Status since last screenc |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrollment |
Follow-up |
||||||||||||
| QFT |
QFT-Plus |
QFT |
QFT-Plus |
||||||||||
| Initial screen | Short-term retest | TB1 | TB2 | Annual screen | Short-term retest | TB1 | TB2 | Interval (mo) | TB exposure | Active TB | |||
| 6937 | 53 | M | 0.4 | 0.44 | 0.27 | 0.77 | 1.01 | ND | 0.91 | 1.12 | 13 | No | No |
| 823 | 30 | M | 0.47 | 0.16 | 0.34 | 0.36 | 0.16 | ND | ND | ND | 12 | No | No |
| 907 | 28 | F | 1.47 | 0.02 | 0.5 | 0.05 | 0.03 | ND | ND | ND | 13 | No | No |
| 1716 | 38 | F | 0.06 | ND | 0.45 | 0.25 | 0.16 | ND | 0.21 | 0.25 | 12 | No | No |
| 3958 | 28 | F | 0.07 | ND | 1.85 | 0.14 | 0 | ND | 0.03 | 0.01 | 13 | No | No |
| 6258 | 28 | F | 0.02 | ND | 5.11 | 0.02 | 0 | ND | ND | ND | 10 | No | No |
| 3720 | 26 | F | 0 | ND | 0 | 1.26 | 0 | ND | 0.13 | 0.15 | 13 | No | No |
| 4749 | 58 | F | 0 | ND | 0 | 0.67 | 0 | ND | 0.00 | 0.34 | 12 | No | No |
| 885 | 34 | F | 0.06 | ND | 0.23 | 0.62 | 0.03 | ND | 0.01 | 0.17 | 9 | No | No |
| 6156 | 23 | F | 0 | ND | 0.04 | 0.60 | ND | ND | ND | ND | NA | NA | NA |
| 2262 | 51 | M | 0.01 | ND | 0.06 | 0.48 | 0.01 | ND | 0.01 | 0.03 | 11 | No | No |
| 1588 | 55 | M | 0.28 | ND | 0.23 | 0.36 | 0.6 | 0.15 | ND | ND | 12 | No | No |
| 4698 | 43 | F | 0 | ND | 0.01 | 0.35 | ND | ND | ND | ND | NA | NA | NA |
M, male; F, female.
Results for TB Ag minus Nil (IU/ml) are shown, and positive results are shaded. ND, not done.
NA, not available.
DISCUSSION
The objective of this study was to compare the performance of QFT-Plus to QFT in low-risk HCWs at a U.S. institution and to test the hypothesis that a more conservative interpretation of QFT-Plus results will reduce positivity rate in HCWs with no known risk factors for LTBI. Overall, we found a high degree of agreement (>95%) between QFT-Plus and QFT. We also observed a high degree of correlation between quantitative QFT-Plus (TB1 and TB2) and QFT results. For the small number of patients with discordant results, the discordance was mostly due to quantitative results bordering the assay cutoff (0.2 to 0.7 IU/ml) (19–22). The latter finding is consistent with patterns of discordant results reported in prior IGRA reproducibility studies and suggests that sources of variability previously described for QFT also are acting on QFT-Plus (22).
Importantly, in this study we found that 2.1% and 3.0% of 626 HCWs with no identifiable risk factors for LTBI had positive QFT and QFT-Plus results, respectively. The higher positivity rate with QFT-Plus was more frequently due to positive results with TB2 than TB1 (2.4% versus 1.6%). Although IGRAs are more specific than TST in BCG-vaccinated populations (2), IGRAs have proven less specific in low-risk North American HCWs and college students (2). False-positive results likely are attributed to one of many sources of variability that cause IGRA results to cross the assay cutoff (22). Because of increasing awareness and recommendations (4, 23), practitioners commonly confirm positive IGRA results in low-risk patients with a short-term follow-up test, which results in added health care costs and overutilization of resources. The QFT-Plus assay, which employs two antigen tubes, was developed for increasing assay sensitivity for active TB by eliciting an IFN-γ response from both CD4+ and CD8+ T lymphocytes. The manufacturer interprets QFT-Plus results as positive when either TB1 or TB2 response reaches the assay cutoff. We showed that a more conservative definition of QFT-Plus positivity, based on double-positive antigen tube results (TB1 and TB2), significantly reduces the positivity rate to 0.6% in risk-free HCWs with a prior negative QFT, which is closer to historical TST conversion rates and somewhat better aligned with the TB epidemiology in the United States (4, 7). If we apply the conservative definition to a cohort of 106 low-risk controls from a recent study (16), the positivity rate would drop from 2.8% to 0%. In our study, follow-up investigation of 11 no-risk HCWs with discordant QFT-Plus TB1 and TB2 results showed that in all but one, follow-up testing with QFT and QFT-Plus (TB1 or TB2) remained negative. This finding suggests the conservative interpretation is a useful strategy for increasing QFT-Plus specificity in low-risk settings. However, larger studies are needed to validate the conservative definition and to better define conditions (i.e., quantitative cutoffs) under which a conservative interpretation can be used to accurately identify nonreproducible positive results in low-risk populations.
Reproducibility studies have identified different causes of IGRA variability (22). The sources of variability can be broadly classified as preanalytical, analytical, postanalytical, manufacturing, and immunological (22). The use of standardized IGRA testing protocols may minimize variable results after serial testing (22). In this study, QFT and QFT-Plus assays were simultaneously performed using the same preanalytical test processes. In this setting, apart from differences in antigen makeup in TB2, analytical variability would have had to arise from enzyme-linked immunosorbent assay (ELISA). Metcalfe and colleagues estimated that variability of QFT derived from ELISA is ±0.6 IU/ml for all individuals and ±0.24 IU/ml for subjects with initial response in the borderline range of 0.25 to 0.8 IU/ml (20). This is consistent with our study, in which most discordant results (84.8% for QFT versus QFT-Plus TB1 and 88.6% for QFT versus QFT-Plus TB2) lie in a range of 0.2 to 0.7 IU/ml. The fact that we saw a similar finding in HCWs with no risk factors for LTBI argues for a borderline zone which accounts for variability due to random sources.
This study had several strengths and weaknesses. The strengths of our study include the availability of LTBI risk factors for participants. This allowed us to identify low-risk HCW and calculate the positivity rate in this population. In addition, we had prior QFT results for a subset of patients with no risk factors, which allowed us to also assess the positivity rate in this group. Further, the number of study participants was large and sufficient for comparison of two assays in low-risk HCWs. Lastly, with standardization of preanalytical processes (22) and simultaneous testing design, we minimized the preanalytical sources of variability. The fact that all testing was performed under routine clinical practice should render our findings applicable to other health care institutions in nonendemic settings. A limitation of this study is the low number of latently infected HCWs, which limited our ability to assess agreement between assays in HCWs with LTBI. It also limited our ability to assess the performance of QFT-Plus in recently exposed versus remotely infected HCWs (14). However, this distribution reflects the low-incidence setting we are operating in and therefore our interest in improving assay specificity. Future observational studies in high-incidence settings with long-term follow ups are needed to assess the sensitivity of QFT-Plus for LTBI after recent exposure.
In conclusion, the QFT-Plus assay showed high agreement with the QFT assay in low-risk HCW. A conservative interpretation of QFT-Plus identified nearly all positive results in HCW with no known risk factors for LTBI. Larger studies are needed to validate our findings and to better characterize the conservative interpretation in low-risk populations.
MATERIALS AND METHODS
Ethics.
Per the Stanford University Institutional Review Board (IRB), this study was exempt from written informed consent because it constituted a quality improvement project for premarket validation of QFT-Plus.
Study design.
A cross-sectional study was conducted in HCWs at Stanford Health Care to compare the performance of QFT-Plus to that of QFT and to test the hypothesis that a more conservative interpretation of QFT-Plus results would reduce positivity rates in low-risk HCWs. HCWs with no risk factors and discordant QFT-Plus results were evaluated for LTBI and active tuberculosis (TB) on their follow-up visits.
Subjects.
Between 7 August and 19 November 2015, HCWs presenting to the Stanford Health Care Occupational Health Clinic for annual and new employee LTBI screening were randomly enrolled in this study. The occupational health program performs QFT on all HCWs with a negative or undocumented history of LTBI. Risk factors for TB exposure are routinely collected using a questionnaire. HCWs are also evaluated for active TB. LTBI risk factors assessed in a questionnaire include history of close contact with a TB patient, country of birth outside U.S., long-term stay outside the U.S., travel to countries where TB is endemic, and employment or volunteer work at high-risk facilities (correctional facility or homeless shelter). Previous history of TST and IGRA positivity and BCG vaccination were also assessed. Positivity rates of QFT and QFT-Plus were assessed in HCWs with no identifiable risk factors and no self-reported history of positive TST or IGRA and also in HCWs with a documented history of negative QFT result in the previous year. No-risk HCWs with discordant QFT-Plus results (n = 13) were assessed for active TB and retested with QFT and QFT-Plus on their follow-up visits.
QFT and QFT-Plus testing.
Blood was drawn, in a single venipuncture, for QFT and QFT-Plus in the following tube order: purge, Nil (negative control without any additive), QFT-Plus TB1, QFT-Plus TB2, QFT TB Antigen, and QFT TB Mitogen. Both assays were performed according to the manufacturer's instructions as outlined in the package insert. Briefly, blood was drawn into Vacutainer tubes up to the 1-ml mark and mixed gently. The samples were incubated immediately at 37°C for 16 to 24 h and then transported to the clinical microbiology laboratory for ELISA. The plasma was separated by centrifugation and stored at ambient temperature for same-day ELISA or stored at 4°C for ELISA within 72 h. ELISA was performed within 24 h on an automated robotic ELISA system (DSX; Dynex Technologies, Chantilly, VA). Plasma samples derived from each subject were tested on the same ELISA plate. For QFT, the results are considered positive when the TB Antigen minus Nil IFN-γ concentration was ≥0.35 IU/ml and ≥25% of the Nil value. For QFT-Plus, two different interpretative criteria were applied. First, per the manufacturer's instructions, the QFT-Plus assay was interpreted as positive when either TB antigen tube (TB1 or TB2) minus Nil IFN-γ concentration was ≥0.35 IU/ml and ≥25% of the Nil value. Second, using a conservative interpretative criteria (QFT-Plus-C), QFT-Plus was interpreted as positive when both TB antigen tubes (TB1 and TB2) minus Nil IFN-γ concentration were ≥0.35 IU/ml and ≥25% of the Nil value. TB1 and TB2 results were also analyzed separately using the QFT interpretive criteria.
Statistical analysis.
Concordance between binary results was measured using Cohen's kappa (24, 25). Linear regression was used to evaluate quantitative relations between continuous variables. The confidence intervals for proportions were calculated from the binomial distribution. McNemar's test was used to compare proportions. Sample size was calculated as previously described (26). All reported P values were two tailed and calculated with statistical significance set at a P value of less than 0.05. Statistical analysis was performed using MedCalc statistical software (version 12.3.0; MedCalc Software, Mariakerke, Belgium) and IBM SPSS Statistics 22.0 (IBM Corporation, Armonk, NY, USA).
Supplementary Material
ACKNOWLEDGMENTS
We thank Qiagen for providing QFT-Plus TB1 and TB2 tubes. Qiagen did not provide any financial support or have any influence on the design and analysis of results in this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02498-16.
REFERENCES
- 1.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. 2012. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 67:62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 2.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringshausen FC, Schablon A, Nienhaus A. 2012. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol 7:6. doi: 10.1186/1745-6673-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. 2013. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 188:1005–1010. doi: 10.1164/rccm.201305-0831OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMullen SE, Pegues DA, Shofer FS, Sheller AC, Wiener EB. 2014. Performance of QuantiFERON-TB Gold and tuberculin skin test relative to subjects' risk of exposure to tuberculosis. Clin Infect Dis 58:1260–1266. doi: 10.1093/cid/ciu119. [DOI] [PubMed] [Google Scholar]
- 6.Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, Wang Y, Cronin W, Hirsch-Moverman Y, Teeter LD, Parker M, Garrett DO, Daley CL. 2014. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 189:77–87. [DOI] [PubMed] [Google Scholar]
- 7.Zwerling A, Benedetti A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, Menzies D, Pai M. 2013. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PLoS One 8:e54748. doi: 10.1371/journal.pone.0054748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menzies D, Joshi R, Pai M. 2007. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 11:593–605. [PubMed] [Google Scholar]
- 9.Turner J, Dockrell HM. 1996. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology 87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busch M, Herzmann C, Kallert S, Zimmermann A, Hofer C, Mayer D, Zenk SF, Muche R, Lange C, Bloom BR, Modlin RL, Stenger S. 2016. Lipoarabinomannan-responsive polycytotoxic T cells are associated with protection in human tuberculosis. Am J Respir Crit Care Med 194:345–355. doi: 10.1164/rccm.201509-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brookes RH, Pathan AA, McShane H, Hensmann M, Price DA, Hill AV. 2003. CD8+ T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages. Eur J Immunol 33:3293–3302. doi: 10.1002/eji.200324109. [DOI] [PubMed] [Google Scholar]
- 12.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, Harari A. 2013. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 43:1568–1577. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Ohmiti K, Goletti D, Bart PA, Hanekom W, Scriba TJ, Nicod L, Pantaleo G, Harari A. 2015. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis 60:432–437. doi: 10.1093/cid/ciu795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M. 2013. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. 2016. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 22:701–703. doi: 10.1016/j.cmi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, Saito T, Fukushima K, Igarashi Y, Aono A, Chikamatsu K, Yamada H, Takaki A, Mori T, Mitarai S. 2016. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6:30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitworth WC, Hamilton LR, Goodwin DJ, Barrera C, West KB, Racster L, Daniels LJ, Chuke SO, Campbell BH, Bohanon J, Jaffar AT, Drane W, Maserang D, Mazurek GH. 2012. Within-subject interlaboratory variability of QuantiFERON-TB gold in-tube tests. PLoS One 7:e43790. doi: 10.1371/journal.pone.0043790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. 2013. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 187:206–211. doi: 10.1164/rccm.201203-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detjen AK, Loebenberg L, Grewal HM, Stanley K, Gutschmidt A, Kruger C, Du Plessis N, Kidd M, Beyers N, Walzl G, Hesseling AC. 2009. Short-term reproducibility of a commercial interferon gamma release assay. Clin Vaccine Immunol 16:1170–1175. doi: 10.1128/CVI.00168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banaei N, Gaur RL, Pai M. 2016. Interferon-gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol 54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera V, Perry S, Parsonnet J, Banaei N. 2011. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin Infect Dis 52:1031–1037. doi: 10.1093/cid/cir068. [DOI] [PubMed] [Google Scholar]
- 24.McHugh ML. 2012. Interrater reliability: the kappa statistic. Biochem Med 22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 25.Fleiss JI. 1981. Statistical methods for rates and proportions, 2nd ed John Wiley & Sons Inc., New York, NY. [Google Scholar]
- 26.Banoo S, Bell D, Bossuyt P, Herring A, Mabey D, Poole F, Smith PG, Sriram N, Wongsrichanalai C, Linke R, O'Brien R, Perkins M, Cunningham J, Matsoso P, Nathanson CM, Olliaro P, Peeling RW, Ramsay A. 2010. Evaluation of diagnostic tests for infectious diseases: general principles. Nat Rev Microbiol 8:S17–S29. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.