ABSTRACT
Sensititre YeastOne (YO) panels were assessed for in vitro susceptibility testing of echinocandins against 39 isolates of Aspergillus fumigatus, A. flavus, and A. terreus, including two echinocandin-resistant A. fumigatus strains, using different inocula (103, 104, and 105 CFU/ml), incubation times (16 to 48 h), and endpoints (first blue or purple well) and compared to CLSI M38-A2. The best agreement was found with an inoculum of 104 CFU/ml, incubation times of 20 h for A. flavus and of 30 h for A. fumigatus and A. terreus, and reading the first purple well. The reproducibility within ±1 2-fold dilutions was 100% for all three echinocandins. YO color endpoints were 2 to 3 2-fold dilutions lower than CLSI minimum effective concentrations (MECs) of caspofungin and 1 to 2 2-fold dilutions higher than CLSI MECs of micafungin. For anidulafungin, off-scale YO color endpoints were observed. Nevertheless, A. fumigatus echinocandin-resistant isolates were detected after 24 h of incubation.
KEYWORDS: echinocandins, Aspergillus spp., Sensititre, antifungal susceptibility testing, MEC
INTRODUCTION
Aspergillus isolates with reduced susceptibility to current antifungal agents have been increasingly reported, highlighting the importance of antifungal susceptibility testing in clinical microbiology laboratory routine (1). In vitro reduced susceptibility of Aspergillus fumigatus isolates to echinocandins can be developed via FKS-dependent and FKS-independent mechanisms (2). Although clinical resistance of Aspergillus to echinocandins is limited (3), breakthrough infections in patients treated with echinocandins has been reported (4), and non-wild-type clinical isolates with minimal effective concentrations (MECs) above the epidemiological cutoff value have been found (5). Sensititre YeastOne (YO) is an adapted susceptibility system of the microbroth CLSI method based on the M27-A3 standard for yeasts, which uses alamarBlue as a colorimetric indicator. Sensititre YeastOne has been extensively evaluated for yeasts. There are fewer studies for molds, demonstrating high levels of agreement with the reference methodology for amphotericin B and azoles with Aspergillus spp. (6–12), while limited data exist for echinocandins. Since Sensititre YeastOne is widely used in routine microbiology laboratories, the aim of the present study was to assess its performance for in vitro susceptibility testing of echinocandins against the three most commonly isolated Aspergillus spp.—A. fumigatus, A. flavus, and A. terreus—compared to the reference Clinical and Laboratory Standards Institute (CLSI) broth microdilution (BMD) method.
(This study was presented in part at the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 2013, abstr P982.)
RESULTS
Optimal conditions.
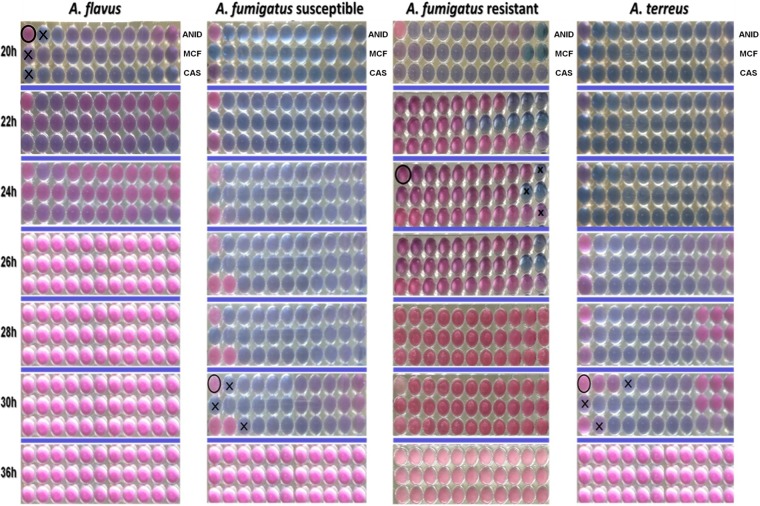
Inocula of 103 and 105 CFU/ml resulted in very slow (>40-h incubation) and fast (<20-h incubation) red development, respectively, whereas for the inoculum of 104 CFU/ml color changes (from blue to red) were observed between 20 and 36 h of incubation for most isolates. The time of plate reading significantly affected the colorimetric endpoints and was found to be species-dependent (Fig. 1). Color change (from blue to purple) was observed in all wells after >20 h of incubation for most A. flavus isolates and after >24 h for most A. fumigatus strains. No color change was observed in the growth control after incubation for <20h, <20h, and <24h for the majority of A. fumigatus, A. flavus, and A. terreus isolates, respectively. In any case, the incubation time was critical and should not be ≥26 h for A. flavus and ≥36 h for A. fumigatus and A. terreus, since exceeding the aforementioned periods resulted in red color being seen in all wells, so the YO color endpoints could not be interpreted (Fig. 1). The best agreement between CLSI and YO was found with an inoculum of 104 CFU/ml after 20 h for A. flavus (mainly for micafungin) and after 30 h for A. fumigatus and A. terreus (mainly for caspofungin) using the first purple well (YO-P). YO color endpoints for A. fumigatus did not change significantly between 24 h and 30 h.
FIG 1.
Different time points of reading of Sensititre YeastOne susceptibility testing of echinocandins against Aspergillus spp. and detection of echinocandin-resistant A. fumigatus isolate (CLSI echinocandin MECs 8 to 16 mg/liter). The first, second, and third rows of each microtitration plate correspond to anidulafungin (ANID), micafungin (MCF), and caspofungin (CAS), respectively. The upper left well corresponds to the growth control, while drug concentrations increase from left (0.008 mg/liter for caspofungin and micafungin, 0.015 mg/liter for anidulafungin) to right (8 mg/liter) well. The symbol “X” represents the visually determined YO color endpoints (first purple well), and the circled well corresponds to the drug-free control for the optimal incubation time. Red color was observed at high concentrations (>0.12 mg/liter), particularly that of anidulafungin with A. flavus, corresponding to the paradoxical phenomenon.
Reproducibility.
The absolute interexperimental agreements between replicates of YO-P color endpoint using the settings described above were 100% for anidulafungin, 78% for micafungin, and 89% for caspofungin. The essential agreement was 100% for all three echinocandins.
Agreement.
The YO-P color endpoints obtained by the colorimetric technique and MECs determined with the reference BMD method, as well as the essential agreement between them are presented in Table 1. Overall, the levels of agreement within ±1 log2 dilution were poor, with the exception of micafungin against A. fumigatus. Greater agreement was found within ±2 log2 dilutions. For anidulafungin, off-scale YO color endpoints were found for all isolates. For micafungin, the agreements were 100, 89, and 77%, with a median (range) CLSI-YO differences of 0 (range, −1 to 2), −1 (range, −4 to 1), and −2 (range, −3 to −2) 2-fold dilutions, respectively, although off-scale YO color endpoints were found for 5/9 A. flavus, 9/17 A. fumigatus, and 9/13 A. terreus isolates. On the other hand, for caspofungin the agreements were poor, with 41, 0, and 54% as a result of 3 (range, 1 to 5), 3 (range, −4 to 4), and 2 (range, 1 to 4) 2-fold CLSI-YO differences for A. fumigatus, A. flavus, and A. terreus, respectively (off-scale YO color endpoints were found for 6/9 A. flavus, 1/17 A. fumigatus, and 4/13 A. terreus isolates). Similar results were found also when off-scale YO color endpoints were excluded.
TABLE 1.
Agreement between Sensititre YeastOne and CLSI BMD method for anidulafungin, micafungin, and caspofungin susceptibility testing of Aspergillus spp.a
| Species (no. of isolates) | Antifungal | CLSI MEC median (range)b | YO color endpoints median (range) | Median (range) 2-fold difference | Agreement (%) |
|
|---|---|---|---|---|---|---|
| ±1 2-fold dilution | ±2 2-fold dilutions | |||||
| A. flavus (9) | ANID | 0.008 (≤0.002 to 0.015) | ≤0.015 (≤0.015 to ≤0.015) | NDc | ND | ND |
| MCF | 0.008 (≤0.002 to 0.015) | ≤0.008 (≤0.008 to 0.06) | −1 (−4 to 1) | 67 | 89 | |
| CAS | 0.06 (0.06 to 0.125) | ≤0.008 (≤0.008 to 1) | 3 (−4 to 4) | 0 | 0 | |
| A. fumigatus (17) | ANID | 0.008 (0.008 to 0.015) [8, 16] | ≤0.015 (≤0.015 to ≤0.015) [8, 8] | ND | ND | ND |
| MCF | 0.008 (0.004 to 0.015) [8, 16] | ≤0.008 (≤0.008 to 0.03) [2, 4] | 0 (−1 to 2) | 88 | 100 | |
| CAS | 0.25 (0.125 to 0.25) [16, 16] | 0.03 (≤0.008 to 0.125) [8, 8] | 3 (1 to 5) | 24 | 41 | |
| A. terreus (13) | ANID | 0.004 (0.004 to 0.008) | ≤0.015 (≤0.015 to ≤0.015) | ND | ND | ND |
| MCF | ≤0.002 (≤0.002 to 0.004) | ≤0.008 (≤0.008 to 0.015) | −2 (−3 to −2) | 0 | 77 | |
| CAS | 0.125 (0.06 to 0.125) | 0.015 (≤0.008 to 0.06) | 2 (1 to 4) | 23 | 54 | |
The agreement between Sensititre YeastOne (first purple well after 20 h for A. flavus and 30 h for A. fumigatus and A. terreus with 104 CFU/ml initial inoculum) and the CLSI BMD method for anidulafungin (ANID), micafungin (MCF), and caspofungin (CAS) susceptibility testing of Aspergillus spp. was evaluated.
Where applicable, the CLSI MECs and YO color endpoints of the two resistant isolates are indicated within brackets.
ND, not determined because of off-scale endpoints.
Interestingly, for the two resistant A. fumigatus isolates, both YO color endpoints were high (2 to 8 mg/liter) for all three echinocandins even after 24 h of incubation (Fig. 1). Furthermore, YO MECs were 1 (range, −1 to 3) 2-fold dilutions higher for anidulafungin and micafungin and 2 (range, 0 to 3) 2-fold dilutions lower for caspofungin than the corresponding CLSI MECs (data not shown).
DISCUSSION
Using the optimal conditions for the YO method (inoculum of 104 CFU/ml, incubation for 20 h for A. flavus and 30 h for A. fumigatus and A. terreus, reading the first purple well), the agreement with the CLSI M38-A2 reference method was poor for caspofungin (0 to 54%) but good for micafungin (77 to 100%) and inconclusive for anidulafungin because of off-scale YO color endpoints. In order to achieve those incubation times in an average clinical laboratory, A. fumigatus and A. terreus isolates could be inoculated in the morning and read the next day afternoon, and A. flavus isolates could be inoculated in the afternoon and read the next day morning. Overall, YO color endpoints were 2 to 3 2-fold dilutions lower than CLSI MECs of caspofungin and 1 to 2 2-fold dilutions higher than CLSI MECs of micafungin. Nevertheless, detection of echinocandin-resistant Aspergillus isolates may be feasible with YO method since both resistant isolates converted alamarBlue within 24 h at high concentrations.
Sensititre YeastOne is a commercially prepared, broth-based MIC panel that requires only the addition of a medium containing the fungal inoculum, while the identification of the endpoints is facilitated by the inclusion of a metabolic dye, alamarBlue. Based on the current literature, no published studies have yet evaluated the performance of the colorimetric assay versus the reference CLSI BMD method for the in vitro susceptibility testing of Aspergillus spp. to echinocandins. Comparisons of both methods with other antifungal agents against the most commonly isolated Aspergillus spp., i.e., A. fumigatus, A. flavus, and A. terreus, have yielded agreement rates of 32 to 100% for amphotericin B (7, 8, 11, 12), 78 to 98% for itraconazole (7, 11, 12), 96 to 100% for posaconazole (6, 8), and 62 to 99% for voriconazole (7, 9, 10). Taking these findings into account, the manufacturer recommends that Sensititre YeastOne be used for susceptibility testing Aspergillus spp. against the four aforementioned antifungals after 48 h of incubation (Sensititre YeastOne for In Vitro Diagnostic Use [http://www.mcsdiagnostics.com/site/upload/file/pdf/yo_8_yo10_v1.4_e.pdf]).
Echinocandin MECs obtained in the present study using the reference CLSI BMD method were comparable to values recorded in previous surveys (13, 14). To date, the only available public accessible data concerning the use of Sensititre panel in susceptibility testing of echinocandins against Aspergillus spp. derive from a conference abstract (15). Particularly, Trinidad et al. compared the results obtained by the YO method with the corresponding CLSI MECs demonstrating excellent agreement for anidulafungin and micafungin and lower for caspofungin, although no levels of agreement were stated in the abstract. In addition, the optimal inoculum size and incubation period were not explored as in our study, in which we found that time of plate reading was species dependent. Regarding the testing conditions, for the CLSI BMD method, Trinidad et al. used drug concentrations of ≥0.03 mg/liter and concordance to low concentrations could not be concluded, since these researchers reported that all isolates showed YO color endpoints of ≤0.015 and ≤0.008 mg/liter for anidulafungin and micafungin, respectively. Although off-scale YO color endpoints were also found in the present study particularly with anidulafungin, the conclusions were the same when only on-scale YO color endpoints were analyzed for micafungin and caspofungin. Furthermore, for the colorimetric technique, Trinidad et al. used 80% of growth inhibition as a reading endpoint and 48 h of incubation. Nevertheless, YO color endpoints are determined visually as the color of alamarBlue transitions from blue (0% growth) to red (100% growth), and only an intermediate purple color can be indicative of 50% growth. Moreover, in our study, we investigated the full time of reading profile, and we found that the incubation period should not exceed 26 h for A. flavus and 36 h for A. fumigatus and A. terreus because red color was seen in all wells (Fig. 1). Hence, 48 h of incubation should not be considered for Sensititre and echinocandins as proposed for antifungals belonging to other classes by various publications (6–12) and the manufacturer (Sensititre YeastOne for In Vitro Diagnostic Use). Finally, no resistant isolates were included in that study in order to better assess the ability of the commercial system to detect resistant strains.
Based on our observations, the level of agreement between the Sensititre and CLSI methods was drug and species dependent. For caspofungin, YO color endpoints were lower than CLSI endpoints, whereas the opposite was observed for micafungin. YO color endpoints depend on the conversion of the oxidation-reduction indicator alamarBlue by living cells, whose decrease may signify an impairment of cellular metabolism or decrease of fungal growth due to antifungal action. Depending on the alamarBlue concentration, this conversion can take place at low or high fungal growth/metabolic levels, increasing or decreasing, respectively, the YO color endpoints compared to the CLSI visual growth endpoints depending on species growth/metabolic rate and drug action. Thus, caspofungin YO color endpoints were lower than CLSI growth endpoints, probably due either to an insufficient alamarBlue concentration to detect fungal growth/metabolism or to sub-MEC effects influencing metabolism but not visual growth at concentrations 2 to 3 dilutions lower than the CLSI MEC. On the other hand, the higher YO color endpoints than CLSI growth endpoints found for micafungin and isolates with on-scale endpoints although it needs verification with more isolates may be due to high alamarBlue concentration that detect even small amount of fungal growth/metabolism, particularly for micafungin which have shallower concentration-effect curves compared to caspofungin (16). However, the fact that these differences were verified with the microscopically determined YO MECs may indicate an interaction between the dye and echinocandins that decrease caspofungin MECs and increase micafungin. This hypothesis needs further investigation.
Species-dependent differences were found particularly for caspofungin for which the agreement between YO and CLSI was 0, 41, and 54% for A. flavus, A. fumigatus, and A. terreus, respectively. Using a different colorimetric marker (XTT), Antachopoulos et al. also reported a proportionate species-dependent reduction in metabolic activity at the MEC for all Aspergillus isolates tested, which was more pronounced for A. flavus (median metabolic activity 25% of control) compared to A. fumigatus and A. terreus (median metabolisms of 42 and 53%, respectively) (17). Thus, the current alamarBlue concentration may be sufficient for the slower-growing A. fumigatus and A. terreus but not for the fast-growing A. flavus. Overall, these findings indicate that optimal concentration of alamarBlue should be explored and validated for each echinocandin and Aspergillus species.
In summary, a head-to-head comparison of Sensititre YeastOne versus the CLSI M38-A2 method for the susceptibility testing of echinocandins against Aspergillus spp. was performed, and for the first time the optimal conditions for the colorimetric assay, including the inoculum size, incubation time, and endpoint reading, were determined. In anticipation of susceptibility breakpoint assignments, further optimization of the commercial system, as well as examination of additional non-wild-type strains, is required in a multicenter study in order to improve its concordance with the reference method and to facilitate the routine susceptibility testing of Aspergillus spp. to echinocandins.
MATERIALS AND METHODS
Isolates.
A total of 39 clinical isolates of Aspergillus spp. were tested, including 9 A. flavus, 13 A. terreus, and 17 A. fumigatus isolates (two with known reduced susceptibility to anidulafungin, caspofungin, and micafungin with a CLSI MEC ≥ 8 mg/liter kindly provided by D. Perlin) (18). The reference strains Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, and A. fumigatus ATCC MYA-3626 were used as quality controls. Isolates were stored in normal saline with 10% glycerol at −70°C and revived by subculturing the samples twice on Sabouraud dextrose agar plates with gentamicin and chloramphenicol (SGC2; bioMérieux) at 30°C for 5 to 7 days to ensure purity, viability, and adequate sporulation.
CLSI method.
For the reference BMD testing, conidial suspensions were prepared as outlined in the CLSI M38-A2 document (19) using laboratory-grade standard powders of anidulafungin (Pfizer, Inc., Groton, CT), caspofungin acetate (Merck & Co., Inc., Whitehouse, NJ), and micafungin (Astellas Pharma, Inc., Tokyo, Japan) dissolved in sterile dimethyl sulfoxide (Chem-Lab NV, Zedelgem, Belgium), and 2-fold serial concentrations ranging from 0.002 to 0.25 mg/liter anidulafungin and micafungin and 0.008 to 1 mg/liter caspofungin (for the two resistant A. fumigatus isolates, 0.12 to 16 mg/liter of all three echinocandins) were used. The plates were incubated at 35°C, and MECs were defined as the lowest drug concentration at which short, stubby, and highly branched hyphal clusters were observed compared to the growth control well of the panel after 24 and 48 h using an inverted microscope.
Sensititre YeastOne.
A colorimetric microdilution method was performed using commercially available Sensititre YeastOne (YO) panels (Thermo Fisher Scientific, Cleveland, OH) according to the manufacturer's recommendations. CFU counts were affirmed each time by spread plate counts on SGC2 plates. The actual size of 0.5 McFarland standard ranged from 0.4 × 106 to 3 × 106 CFU/ml.
Optimizing in vitro conditions.
The impact of inoculum size, incubation time, and color change endpoints on YO results were assessed in preliminary experiments. Increasing inocula of 103, 104, and 105 CFU/ml of three isolates/species were tested in the YO method, and the results were compared to the CLSI MECs using the standard inoculum of 104 CFU/ml. Visual readings were made after 16, 20, 22, 24, 26, 28, 30, 36, 40, and 48 h of incubation using an inverted magnifying reading mirror. YO color endpoints were determined as the lowest drug concentration corresponding to the first blue (ΥΟ-Β) or purple well (ΥΟ-P). Furthermore, the bottom of the YO plates were inspected with an inverted microscope, and an YO MEC was determined as the lowest drug concentration at which short, stubby, and highly branched hyphal clusters were observed compared to the growth control well.
Reproducibility.
In order to assess the reproducibility of YO with echinocandins, all experiments were repeated two times on different days. The reproducibility was calculated as the proportion of replicate YO color endpoints that where the exactly same (absolute agreement) and within ±1 2-fold dilution (essential agreement) for each isolate using the optimal conditions defined above.
Agreement.
The 2-fold differences and the level of agreement between the two methods were calculated for each species/drug/incubation time combination as the proportion of the YO color endpoints determined for each strain that fell within ±1 and ±2 2-fold dilutions of the corresponding MECs of the CLSI BMD method.
ACKNOWLEDGMENTS
We thank Thermo Fisher Scientific, Cleveland, OH, for kindly providing the Sensititre YeastOne panels and inoculum broth for the performance of the colorimetric method.
REFERENCES
- 1.Bernal-Martínez L, Alastruey-Izquierdo A, Cuenca-Estrella M. 2016. Diagnostics and susceptibility testing in Aspergillus. Future Microbiol 11:315–328. doi: 10.2217/fmb.15.140. [DOI] [PubMed] [Google Scholar]
- 2.Gardiner RE, Souteropoulos P, Park S, Perlin DS. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med Mycol 43(Suppl 1):S299–S305. doi: 10.1080/13693780400029023. [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Perkhofer S, Howard SJ, Garcia-Effron G, Vishukumar A, Perlin D, Lass-Flörl C. 2008. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob Agents Chemother 52:3504–3511. doi: 10.1128/AAC.00190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madureira A, Bergeron A, Lacroix C, Robin M, Rocha V, de Latour RP, Ferry C, Devergie A, Lapalu J, Gluckmana E, Socié G, Ghannoum M, Ribaud P. 2007. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int J Antimicrob Agents 30:551–554. doi: 10.1016/j.ijantimicag.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart SR, Zimbeck AJ, Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Pappas PG, Chiller T. 2011. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob Agents Chemother 55:3944–3946. doi: 10.1128/AAC.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel R, Mendrick C, Knapp CC, Grist R, McNicholas PM. 2007. Clinical evaluation of the Sensititre YeastOne plate for testing susceptibility of filamentous fungi to posaconazole. J Clin Microbiol 45:2000–2001. doi: 10.1128/JCM.00287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinea J, Peláez T, Alcalá L, Bouza E. 2006. Comparison of Sensititre YeastOne® with the NCCLS M38-A microdilution method to determine the activity of amphotericin B, voriconazole, and itraconazole against clinical isolates of Aspergillus fumigatus. Diagn Microbiol Infect Dis 56:53–55. doi: 10.1016/j.diagmicrobio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A. 2006. Comparison of three commercial assays and a modified disk diffusion assay with two broth microdilution reference assays for testing Zygomycetes, Aspergillus spp., Candida spp., and Cryptococcus neoformans with posaconazole and amphotericin B. J Clin Microbiol 44:3616–3622. doi: 10.1128/JCM.01187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linares MJ, Charriel G, Solis F, Rodriguez F, Ibarra A, Casal M. 2005. Susceptibility of filamentous fungi to voriconazole tested by two microdilution methods. J Clin Microbiol 43:250–253. doi: 10.1128/JCM.43.1.250-253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro C, Serrano MC, Flores B, Espinel-Ingroff A, Martín-Mazuelos E. 2004. Comparison of the Sensititre YeastOne colorimetric antifungal panel with a modified NCCLS M38-A method to determine the activity of voriconazole against clinical isolates of Aspergillus spp. J Clin Microbiol 42:4358–4360. doi: 10.1128/JCM.42.9.4358-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Mazuelos E, Pemán J, Valverde A, Chaves M, Serrano MC, Cantón E. 2003. Comparison of the Sensititre YeastOne colorimetric antifungal panel and Etest with the NCCLS M38-A method to determine the activity of amphotericin B and itraconazole against clinical isolates of Aspergillus spp. J Antimicrob Chemother 52:365–370. doi: 10.1093/jac/dkg384. [DOI] [PubMed] [Google Scholar]
- 12.Meletiadis J, Mouton JW, Meis JFGM, Bouman BA, Verweij PE. 2002. Comparison of the Etest and the sensititre colorimetric methods with the NCCLS proposed standard for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 40:2876–2885. doi: 10.1128/JCM.40.8.2876-2885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J Clin Microbiol 47:3323–3325. doi: 10.1128/JCM.01155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinel-Ingroff A, Fothergill A, Fuller J, Johnson E, Pelaez T, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for caspofungin and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:2855–2859. doi: 10.1128/AAC.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinidad M, Castro C, Martos AI, Romero A, Canton E, Martin-Mazuelos E. 2009. Evaluation of Sensititre Yeast One in susceptibility testing of echinocandins against Aspergillus spp. Mycoses 52:35. doi: 10.1111/j.1439-0507.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 16.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2008. Comparative in vitro pharmacodynamics of caspofungin, micafungin, and anidulafungin against germinated and nongerminated Aspergillus conidia. Antimicrob Agents Chemother 52:321–328. doi: 10.1128/AAC.00699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antachopoulos C, Meletiadis J, Sein T, Roilides E, Walsh TJ. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob Agents Chemother 51:881–887. doi: 10.1128/AAC.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocha EMF, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 51:4174–4176. doi: 10.1128/AAC.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]