ABSTRACT
This study was conducted to determine the effect of testing parameters on the in vitro activity of gepotidacin, a new triazaacenaphthylene antibacterial agent for the treatment of conventional and biothreat pathogens. CLSI methods, and variations of those methods, were used to test 10 Staphylococcus aureus, 10 Streptococcus pneumoniae, 10 Haemophilus influenzae, and 5 Escherichia coli isolates by MIC and 30 S. aureus, 15 S. pneumoniae, and 15 S. pyogenes isolates by disk diffusion (DD) methods. Levofloxacin and linezolid were tested as comparator agents for MIC and DD methods, respectively. Broth microdilution (BMD), macrodilution (MD), and agar dilution (AD) methods were compared. Variations in media, temperature, incubation time, CO2 level, and inoculum concentration were tested by all methods, and variations in pH, calcium, magnesium, zinc, potassium, thymidine, and polysorbate 80 levels were tested by BMD and DD. The addition of albumin, serum, and lung surfactant was studied by BMD. The variables that impacted the results the most were high inoculum and pH 5.5 (no growth of H. influenzae and S. pneumoniae by BMD). Gepotidacin AD MIC levels were increased and disk zone diameters were decreased for all species in 10% CO2 incubation. The following variables had a minimal effect on gepotidacin results: pH, agar method, atmospheric condition, temperature, and addition of serum and albumin for broth methods. There were also some slight differences in gepotidacin disk results between disk manufacturers and some agar types and also with potassium and thymidine for S. pneumoniae. For all other variations, gepotidacin MIC and disk results were considered comparable to reference results.
KEYWORDS: MIC, antimicrobial susceptibility, disk diffusion, gepotidacin, testing variables
INTRODUCTION
Gepotidacin (GSK2140944), first in the novel triazaacenaphthylene class of bacterial type IIA topoisomerase inhibitors, inhibits bacterial DNA replication by a novel mechanism and has in vitro activity against susceptible and drug-resistant pathogens associated with a range of conventional and biothreat infections.
In performing antimicrobial susceptibility testing, it is important to control various testing conditions. Prior in vitro studies for numerous antimicrobial agents have shown the effect of various factors on the susceptibility test result (1–14). Standardized methods, such as the MIC and disk diffusion method established by the Clinical and Laboratory Standards Institute (CLSI), were developed in order to define important test variables and optimal testing conditions (15–17). This study was undertaken to determine the reproducibility of the reference methods when testing the same organism over several days and also to determine the effect of various testing parameters on the in vitro activity of gepotidacin and a comparative agent by MIC against Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, and Haemophilus influenzae and by disk diffusion against S. aureus, S. pneumoniae, and Streptococcus pyogenes.
RESULTS
MIC.
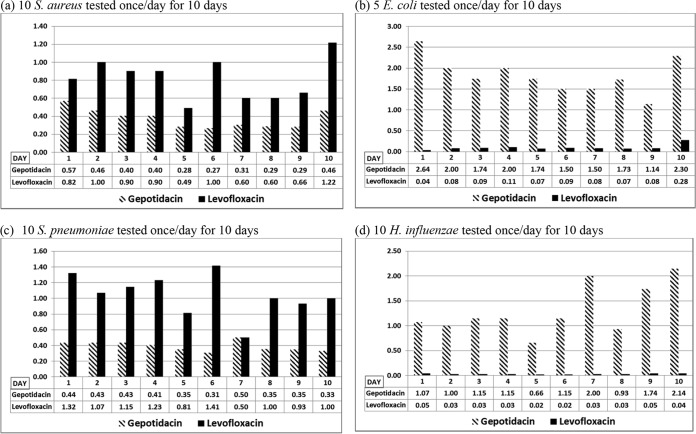
For each MIC method and method variable tested, growth was satisfactory according to CLSI M07, unless otherwise indicated. Gepotidacin mean MIC results by the reference broth microdilution (BMD) method varied by approximately 1 dilution over 10 days, with the exception of H. influenzae mean MIC results, which varied by approximately 2 dilutions (Fig. 1). Gepotidacin BMD MIC results for the four quality control (QC) strains were within CLSI established ranges, with the exception of 1 out-of-range result each for H. influenzae ATCC 49247 (gepotidacin MIC of 2 μg/ml) and S. pneumoniae ATCC 49619 (gepotidacin MIC of 0.5 μg/ml). Levofloxacin mean MIC results varied by approximately 1 dilution for S. aureus, S. pneumoniae, and H. influenzae and by approximately 2 dilutions for E. coli. All levofloxacin BMD MIC results for the four quality control strains were within CLSI established ranges. While the majority of gepotidacin macrodilution (MD) and agar dilution (AD) MIC results were within 1 doubling dilution of the BMD MIC results for all study isolates, S. aureus gepotidacin AD MIC results were approximately 1.2 doubling dilutions higher than BMD MIC results (Table 1).
FIG 1.
Reproducibility of CLSI broth microdilution method (geometric mean MICs [μg/ml] of gepotidacin and levofloxacin for multiple isolates tested on multiple days during the study period).
TABLE 1.
In vitro activity of gepotidacin and levofloxacin: comparison of the CLSI MIC broth microdilution reference method to variations in testing conditions
| Parameter or condition | Comparative variable | Mean dilution differencea (% essential agreementb) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Gepotidacin |
Levofloxacin |
||||||||
| S. aureus (n = 10) | S. pneumoniae (n = 10) | H. influenzae (n = 10) | E. coli (n = 5) | S. aureus (n = 7c) | S. pneumoniae (n = 10) | H. influenzae (n = 10) | E. coli (n = 5) | ||
| MIC method | MD | √ | √ | √ | √ | √ | √ | √ | 0.4d (80.0) |
| AD | 1.2 (60.0) | √ | √ | √ | √ | √ | √ | √ | |
| Mediume | ISB | √ | NA | NA | √ | √ | NA | NA | √ |
| BSB | NA | √ | NA | NA | NA | √ | NA | NA | |
| BSB + NAD | NA | NA | √ | NA | NA | NA | √ | NA | |
| EUB | NA | NA | √ | NA | NA | NA | √ | NA | |
| Temperature (°C) | 30 | √ | √ | √ | √ | −1.2 (71.4) | √ | −0.4 (80.0) | √ |
| 40 | √ | −1.0 (80.0) | −2.5d (30.0) | √ | √ | √ | √ | √ | |
| Incubation time (h) | 16 | √ | √ | √ | √ | √ | √ | √ | √ |
| 24e | √ | √ | √ | √ | √ | √ | √ | √ | |
| 48 | √ | √ | √ | √ | √ | √ | √ | √ | |
| Inoculum (CFU/ml) | 104 | √ | √ | √ | √ | √ | √ | √ | √ |
| 106 | √ | √ | √ | √ | √ | √ | √ | √ | |
| 107 | 6.9d (0.0) | 6.6d (0.0) | 3.9d (0.0) | 4.8d (0.0) | 1.9d (85.7) | 6.0d (0.0) | 6.7d (0.0) | 1.6d (40.0) | |
| Atmosphere (% CO2) | 5 | √ | √ | √ | √ | √ | √ | √ | √ |
| 10 | √ | √ | √ | √ | √ | √ | √ | √ | |
| Calcium (μg/ml) | 50 | √ | √ | √ | √ | √ | √ | √ | √ |
| 100 | √ | √ | √ | √ | √ | √ | √ | √ | |
| Magnesium (μg/ml) | +25 | √ | √ | √ | √ | √ | 1.4 (60.0) | √ | √ |
| +50 | √ | √ | √ | √ | 1.5 (42.9) | √ | √ | √ | |
| Potassium (mmol/liter) | +12.5 | √ | √ | √ | √ | −0.1 (85.7) | √ | √ | √ |
| +25 | √ | √ | √ | √ | √ | √ | √ | √ | |
| +50 | √ | √ | √ | √ | √ | √ | √ | √ | |
| Zinc (mmol/liter) | +2 | √ | √ | √ | √ | √ | √ | √ | √ |
| +5 | √ | √ | √ | √ | 0.6 (85.7) | √ | √ | √ | |
| +10 | √ | √ | √ | √ | −0.2 (85.7) | √ | √ | √ | |
| Thymidine (μg/ml) | +1 | √ | √ | √ | √ | √ | √ | √ | |
| +5 | √ | √ | √ | √ | √ | √ | √ | √ | |
| pH | 5.5 | 1.4 (40.0) | NG | NG | 1.4 (60.0) | √ | NG | NG | √ |
| 6.5 | √ | 1.8 (20.0) | √ | √ | √ | √ | √ | √ | |
| 8.5 | √ | −1.6 (50.0) | −2.6 (0.0) | −1.2 (40.0) | √ | √ | √ | √ | |
| % serum | 25 | −0.7 (80.0) | √ | √ | √ | √ | √ | √ | √ |
| 50 | −0.5 (80.0) | −1.6 (50.0) | √ | −1.0 (80.0) | 0.6 (85.7) | √ | √ | √ | |
| Albumin (μg/dl) | +4 | 1.0 (60.0) | √ | √ | 0.4 (80.0) | √ | √ | √ | √ |
| % lung surfactant | 1 | √ | √ | √ | 0.6 (80.0) | √ | √ | √ | √ |
| 5 | √ | √ | √ | √ | 0.01 (85.7) | √ | √ | √ | |
| % polysorbate 80 | 0.002 | √ | √ | √ | √ | √ | √ | √ | √ |
Dilution differences were calculated for each MIC by subtracting the log2 + 10 test MIC from the log2 + 10 reference MIC, and mean dilution differences were determined for each method. MD, macrodilution; AD, agar dilution; ISB, IsoSensitest broth; BSB, ISB–5% LHB; BSB-NAD, ISB–5% LHB–20 mg/liter NAD; EUB, CAMHB–5% LHB–20 mg/liter NAD (NAD). √, essential agreement ≥ 90%; NG, no growth; NA, not applicable.
Data represent percentages of MICs by the reference and comparative methods within ±1 doubling dilution.
Some strains not included because reference MICs were off the scale.
Value might have been greater than reported due to the presence of an isolate(s) with an off-scale MIC value(s).
For S. pneumoniae and H. influenzae, the reference condition was 24 h of incubation time (h) and the tested variable was 20 h.
Mean dilution differences and essential agreement percentages for those method variations that were shown to have essential agreement percentages of <90% are shown in Tables 1 and 2. Although incubation temperatures of 30°C and 40°C did not impact MIC results for most species tested, gepotidacin MIC results were lower for S. pneumoniae and H. influenzae incubated at 40°C than for the reference BMD at 35°C (mean dilution differences, −1.0 and −2.5, respectively). There was no difference in BMD MIC results attributed to atmospheric conditions; however, higher gepotidacin AD MIC results were obtained for S. aureus incubated in 5% CO2 and in 10% CO2 than were obtained with the reference AD under ambient conditions (mean dilution differences, 1.6 and 1.7, respectively). Gepotidacin AD MIC results were also higher for S. pneumoniae incubated in 10% CO2 (mean dilution difference, 0.7) and for E. coli incubated in 5% CO2 and 10% CO2 (mean dilution difference, 0.8) than those obtained under the reference AD conditions for each organism. MIC results were especially impacted as a result of the use of various inoculum concentrations. For all species tested (with the exception of H. influenzae and agar dilution, a combination which was not tested), the use of an inoculum concentration of 107 CFU/ml for BMD and 2 × 106 CFU/spot for AD resulted in gepotidacin and levofloxacin BMD MIC results that were significantly higher than the MIC results for the reference BMD and AD methods (the majority of MIC results were off the scale at above the highest dilution tested and were indicative of a >16-fold increase in MIC). Changes in pH also had an impact on gepotidacin BMD MIC results. For S. aureus and E. coli, a pH of 5.5 resulted in increased gepotidacin MIC results compared to reference MIC results (mean dilution difference, 1.4). For S. pneumoniae and H. influenzae, a pH of 5.5 resulted in no growth in the gepotidacin- or levofloxacin-containing wells or in the positive-growth control well. At a pH of 6.5, S. pneumoniae gepotidacin MIC results were higher than reference MIC results (mean dilution difference, 1.8). At a pH of 8.5, E. coli, S. pneumoniae, and H. influenzae gepotidacin MIC results were lower than the reference MIC results (mean dilution differences, −1.2, −1.6, and −2.6, respectively). The addition of 25% serum decreased gepotidacin MIC results for S. aureus (mean dilution difference, −0.7), and the addition of 50% serum also decreased gepotidacin MIC results for E. coli, S. pneumoniae, and S. aureus compared to reference MIC results (mean dilution differences, −1.0, −1.6, and −0.5, respectively). The addition of 4 μg/dl of albumin increased gepotidacin MICs compared to reference MIC results (increase of 1 mean dilution).
TABLE 2.
In vitro activity of gepotidacin and levofloxacin: comparison of the CLSI MIC agar dilution reference method to variations in testing conditions
| Parameter or condition | Comparative variable | Mean dilution differencea (% essential agreementb) |
|||||
|---|---|---|---|---|---|---|---|
| Gepotidacin |
Levofloxacin |
||||||
| S. aureus (n = 10) | S. pneumoniae (n = 10) | E. coli (n = 5) | S. aureus (n = 7c) | S. pneumoniae (n = 10) | E. coli (n = 4c) | ||
| Mediume | ISA | 0.7 (80.0) | NA | √ | √ | NA | −0.4 (80.0)d |
| BSA | NA | √ | NA | NA | √ | NA | |
| EUA | NA | √ | NA | NA | √ | NA | |
| Temperature (°C) | 30 | √ | √ | √ | √ | √ | √ |
| 40 | √ | √ | √ | √ | √ | √ | |
| Incubation time (h) | 16 | √ | √ | √ | √ | √ | √ |
| 24e | √ | √ | √ | √ | √ | √ | |
| 48 | √ | √ | √ | √ | √ | √ | |
| Inoculum (CFU/spot) | 2 × 103 | √ | √ | √ | √ | √ | √ |
| 2 × 105 | √ | √ | √ | √ | √ | √ | |
| 2 × 106 | 5.8d (0.0) | 2.8d (0.0) | 3.4d (0.0) | 5.1d (0.0) | 3.1d (0.0) | 8.4d (20.0) | |
| Atmosphere (% CO2) | 5e | 1.6 (30.0) | √ | 0.8 (80.0) | 1.3 (66.7) | √ | √ |
| 10 | 1.7 (40.0) | 0.7 (80.0) | 0.8 (80.0) | 1.7 (66.7) | √ | √ | |
ISA, IsoSensitest agar; BSA, MHA–5% defribrinated horse blood; EUA, MHA–5% defribrinated horse blood–20 mg/liter NAD.
Dilution differences were calculated for each MIC by subtracting the log2 + 10 test MIC from the log2 + 10 reference MIC, and mean dilution differences were determined for each method.
Percentage of MICs by the reference and comparative methods within ± 1 doubling dilution.
Some strains not included because reference MICs were off the scale.
Value may be greater than reported due to the presence of an isolate(s) with an off-scale MIC value(s).
For S. pneumoniae 24 h and 5% CO2 incubation is the reference condition and 20 h and ambient is the tested variable.
√ = Essential agreement ≥ 90%; NA, not applicable.
Disk diffusion.
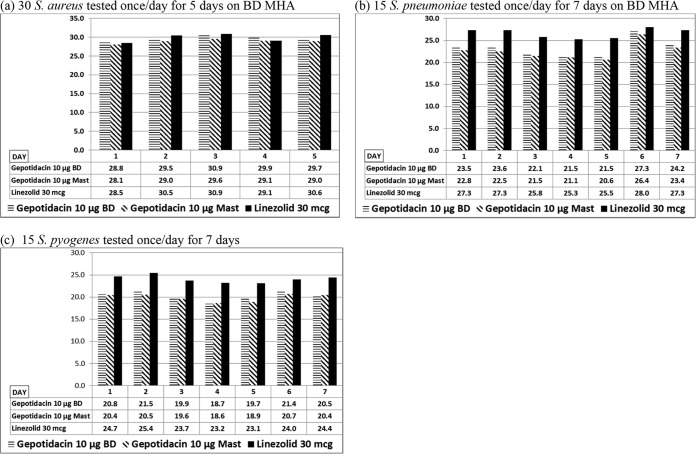
For each disk method and method variable tested, growth was satisfactory according to CLSI M02, unless otherwise indicated. The reference gepotidacin (10 μg) and linezolid (30 μg) mean disk zone diameters differed over 7 days by 2.8 and 2.4 mm for S. aureus, 6.7 and 2.7 mm for S. pneumoniae, and 2.9 and 2.3 mm for S. pyogenes, respectively (Fig. 2). Mean reference method BD zone diameters for S. aureus tested over 5 days and for S. pneumoniae tested over 7 days were an average of 0.8 mm larger than MAST (Hardy Diagnostics, Santa Maria, CA) zone diameters, and BD zone diameters for S. pyogenes tested over 7 days were an average of 0.5 mm larger than MAST zone diameters. All gepotidacin 10 μg BD disk results for the two quality control strains were within CLSI established ranges. There were two outlier gepotidacin (10 μg) MAST disk results (21 mm) for S. pneumoniae ATCC 49619.
FIG 2.
Reproducibility of CLSI disk diffusion method (mean zone diameters [mm] of gepotidacin and linezolid by CLSI disk diffusion for multiple isolates tested on multiple days during the study period).
Mean zone diameter differences and essential agreement percentages for those method variations which were shown to have essential agreement percentages of <90% are shown in Table 3. Mean zone diameter differences and essential agreement percentages are not shown for method variations that were in agreement with those determined by the reference method (essential agreement, ≥ 90%). Among the results from the medium and method study, gepotidacin zone diameters for S. aureus determined by the use of a MAST disk on Oxoid Mueller-Hinton agar (MHA) were lower than the reference zone diameters (mean zone difference, −2.8 mm). Gepotidacin zone diameters for S. pyogenes determined by the use of a MAST disk on MHA–5% defibrinated horse blood (Cleveland Scientific, Bath, OH)–20 mg/liter NAD (EUA) were larger than reference zone diameters (mean zone difference, 1.7 mm). With the exception of S. aureus with the MAST disk, gepotidacin zone diameters were larger with ISA using the British Society for Antimicrobial Chemotherapy (BSAC) inoculum for all isolates (mean zone differences: S. aureus, 2.1 mm; S. pneumoniae = 3.6 and 3.7 mm; S. pyogenes, 5.5 and 5.3 mm [for BD and MAST disks, respectively]). A pH of 5.5 reduced gepotidacin zone diameters for S. aureus (mean zone differences, −4.0 and −5.2 mm for BD and MAST disks, respectively). Growth was diminished and gepotidacin zone diameters for streptococci were increased with media at pH 5.5 (mean zone differences: S. pneumoniae, 6.1 and 5.4 mm for BD and MAST disks, respectively; S. pyogenes, 3.0 for the gepotidacin BD disk). Gepotidacin zone diameters for S. aureus on media with a pH of 8.5 were increased compared to reference zone diameters (mean zone differences, 13.6 and 12.7 mm for BD and MAST disks, respectively), and gepotidacin zone diameters for S. pyogenes were also increased (mean zone difference, 2.3 mm). Zinc, added at a concentration of 5 mmol/liter, increased zone diameters for gepotidacin and S. aureus (mean zone difference, 0.9 mm for the BD disk). The inoculum had a significant impact on disk diffusion results. Gepotidacin zone diameters were larger at inoculum concentrations of 106 CFU/ml for all isolates (mean zone differences ranging from 4.5 to 6.1 mm) and 107 CFU/ml (mean zone differences ranging from 3.0 to 3.9 mm). With the exception of the MAST disk for S. pyogenes, a reduction in gepotidacin zone diameters for all isolates occurred when plates were incubated in a 10% CO2 atmosphere (mean zone differences ranging from −3.4 to −5.6). Gepotidacin zone diameters were also reduced at 5% CO2 for S. pneumoniae by the BD disk method (mean zone difference, −1.1 mm). Gepotidacin zone diameters for S. pneumoniae were reduced with addition of 1 μg/ml thymidine (mean zone differences, −3.7 and −3.6 mm for BD and MAST disks, respectively) and 5 μg/ml thymidine (mean zone differences, −3.1 and −3.4 mm for BD and MAST disks, respectively). A reduced incubation time of 14 h resulted in larger gepotidacin zone diameters for S. pyogenes (mean zone differences, 4.4 and 3.9 mm for BD and MAST disks, respectively). An incubation temperature of 40°C decreased gepotidacin zone diameters for S. aureus and S. pneumoniae (mean zone differences ranging from −0.9 to −3.1 mm); however, in contrast, a temperature of 40°C resulted in increased gepotidacin zone diameters for S. pyogenes (mean zone differences, 5.2 and 4.7 mm for BD and MAST disks, respectively). When plates were incubated at a reduced temperature of 30°C, gepotidacin zone diameters also increased for S. pyogenes (mean zone differences, 4.2 and 3.6 mm for BD and MAST disks, respectively). Agar supplementation with 12.5, 25, and 50 mmol/liter of potassium decreased gepotidacin zone diameters for S. pneumoniae (mean zone differences ranging from 0 to −0.4 mm), with the exception of the addition of 50 mmol/liter potassium for the MAST disk, which increased the mean gepotidacin zone diameter by 0.13 mm.
TABLE 3.
In vitro activity of gepotidacin and linezolid: comparison of the CLSI disk diffusion reference method to variations in testing conditions
| Parameter or condition | Comparative variable | Mean zone diam (mm) differencea (% essential agreementb) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus (n = 30) |
S. pneumoniae (n = 15) |
S. pyogenes (n = 15) |
||||||||
| Gepotidacin (10-μg disk) |
Linezolid (30-μg disk) | Gepotidacin (10-μg disk) |
Linezolid (30-μg disk) | Gepotidacin (10-μg disk) |
Linezolid (30-μg disk) | |||||
| BD | MAST | BD | MAST | BD | MAST | |||||
| Medium | Oxoid MHAc | √ | −2.8 (83.3) | √ | √ | √ | √ | √ | √ | √ |
| Hardy MHAc | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| ISA (CLSI inoculum)d,e | 0.1 (86.7) | √ | √ | √ | √ | √ | √ | √ | √ | |
| ISA (BSAC inoculum)d,f | 2.1 (83.3) | √ | 4.5 (56.7) | 3.6 (86.7) | 3.7 (80.0) | 4.4 (66.7) | 5.5 (33.3) | 5.3 (26.7) | 7.1 (6.7) | |
| EUA | NA | NA | NA | √ | √ | √ | √ | 1.7 (86.7) | √ | |
| Temperature (°C) | 30 | √ | √ | √ | √ | √ | 4.8 (40.0) | 4.2 (66.7) | 3.6 (80.0) | 3.5 (73.3) |
| 40 | −0.9 (80.0) | −0.9 (80.0) | −4.2 (50.0) | −3.1 (80.0) | −2.8 (80.0) | −2.8 (86.7) | 5.2 (53.3) | 4.7 (53.3) | √ | |
| Incubation time (h)g | 14 | √ | √ | √ | √ | √ | √ | 4.4 (60.0) | 3.9 (73.3) | 3.7 (66.7) |
| 24 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 48 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Inoculum (CFU/ml) | 106 | 4.5 (66.7) | 4.5 (46.7) | 5.6 (36.7) | 6.6 (6.7) | 6.1 (13.3) | 6.4 (6.7) | 5.5 (33.3) | 5.6 (20.0) | 6.9 (0.0) |
| 107 | 3.2 (83.3) | 3.0 (83.3) | 3.2 (80.0) | 3.9 (66.7) | 3.5 (66.7) | 4.1 (46.7) | 3.8 (80.0) | 3.8 (73.3) | 4.9 (33.3) | |
| 109 | √ | √ | −2.6 (80.0) | √ | √ | −3.0 (86.7) | √ | √ | √ | |
| Atmosphere (% CO2) | 5h | √ | √ | √ | −1.1 (80.0) | √ | √ | √ | √ | √ |
| 10 | −3.6 (63.3) | −3.9 (60.0) | √ | −5.6 (33.3) | −5.5 (20.0) | −2.1 (86.7) | −3.4 (86.7) | √ | √ | |
| Calcium (μg/ml) | 50 | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 100 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Magnesium (μg/ml) | +12.5 | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| +25 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Potassium (mmol/liter) | +12.5 | √ | √ | √ | −0.4 (86.7) | −0.2 (86.7) | (73.3) | √ | √ | √ |
| +25 | √ | √ | √ | −0.1 (80.0) | 0.0 (80.0) | 0.1 (73.3) | √ | √ | √ | |
| +50 | √ | √ | √ | −0.1 (66.7) | 0.13 (73.3) | √ | √ | √ | √ | |
| Zinc (mmol/liter) | +2 | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| +5 | 0.9 (86.7) | √ | √ | √ | √ | √ | √ | √ | √ | |
| +10 | √ | √ | √ | √ | √ | 2.9 (86.7) | √ | √ | √ | |
| Thymidine (μg/ml) | +1 | √ | √ | √ | −3.7 (53.3) | −3.1 (66.7) | √ | √ | √ | √ |
| +5 | √ | √ | √ | −3.6 (73.3) | −3.4 (66.7) | −2.3 (86.7) | √ | √ | √ | |
| pH | 5.5 | −4.0 (50.0) | −5.2 (30.0) | 3.2 (60.0) | 6.1 (20.0) | 5.4 (33.3) | 12.0 (13.3) | √ | 3.0 (73.3) | 2.1 (86.7) |
| 6.5 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 8.5 | 13.6 (0.0) | 12.7 (0.0) | 8.2 (30.0) | √ | 2.3 (80.0) | √ | √ | √ | √ | |
| % polysorbate 80 | 0.002 | √ | √ | √ | √ | √ | √ | √ | √ | √ |
Mean zone diameters were determined for each comparative variable method and subtracted from the mean zone diameter of the reference results from the same day of testing. MHA, Mueller-Hinton agar; ISA, IsoSensitest agar; EUA, MHA–5% defribrinated horse blood–20 mg/liter NAD. √, essential agreement ≥ 90%; NA, not applicable.
Data represent percentages of zone diameters by the reference method and comparative method within ± 4 mm.
For S. pneumoniae and S. pyogenes, MHA was supplemented with 5% sheep blood (SBA).
For S. pneumoniae and S. pyogenes, ISA was supplemented with 5% defibrinated horse blood (BSA).
The CLSI inoculum was a 0.5 McFarland standard.
The BSAC inoculum dilutions used were 1:10 for S. aureus and S. pneumoniae and 1:100 for beta-hemolytic streptococci.
For S. pneumoniae and S. pyogenes, the reference condition was 20 to 24 h of incubation time and the comparative variables were 16, 18, and 48 h.
For S. pneumoniae and S. pyogenes, the reference condition was 5% CO2 incubation time and the comparative variable was ambient atmosphere.
Result summary.
When the study isolates were tested according to the reference methods on multiple days during the course of the study, average gepotidacin MIC results for all isolates were within an approximately 2-dilution range and average gepotidacin disk results (with the exception of S. pneumoniae results) were within an approximately 2-mm range. Greater variations in gepotidacin reference disk diffusion results were observed for S. pneumoniae (average, 5.8-mm range). For all species tested, a high inoculum level was shown to impact gepotidacin and comparator MIC and disk diffusion results to the greatest degree. A variation of the media pH was also shown to affect results; pH 5 affected the growth of streptococcus and H. influenzae in general, as well as S. aureus and E. coli results; pH 8.5 affected gepotidacin results for all species, with the exception of S. aureus MIC and S. pyogenes disk results. Incubation in 10% CO2 for agar dilution and disk diffusion affected gepotidacin results for all species. Other factors that had a minimal effect on gepotidacin results were agar dilution for S. aureus, incubation at 40°C for all species, with the exception of S. aureus and E. coli by BMD, and the addition of serum to BMD for S. aureus and S. pneumoniae and albumin for S. aureus. In addition, disk results differed slightly between disk manufacturers (larger zones for BD disks), on the various media (smaller zones on Oxoid MHA, larger on EUA media, and larger on ISA with BSAC inoculum), and with addition of potassium and thymidine for S. pneumoniae. For the majority of the study isolates, all other variables tested had no significant effect on gepotidacin MIC or disk results.
DISCUSSION
Reference susceptibility testing methods are standardized; for some drugs, however, this biological assay can be significantly impacted by slight changes in testing conditions. While the standardized method includes accepted ±1-dilution variability, drug- and organism-specific studies are necessary in order to understand this variation. This is especially important when testing and reporting results from clinical isolates and determining susceptibility interpretive criteria (18). In this study, the impact of testing variables on gepotidacin susceptibility was evaluated. For most organisms, there was good agreement (within ±1 dilution or ±4 mm) between results on multiple days when gepotidacin and comparators were tested by the reference standardized broth microdilution, agar dilution, and disk diffusion methods. Any minor MIC or zone differences attributed to day-to-day variation were addressed by performing the reference method on the same day as the variable testing method.
Polysorbate 80 is used as a surfactant for testing of some antibacterial agents and may also be used in commercial antimicrobial susceptibility systems at a concentration of 0.002 μg/ml; therefore, it is important to understand any impact on the in vitro activity of new antimicrobial agents when polysorbate 80 is utilized. Gepotidacin and levofloxacin BMD MICs and gepotidacin and linezolid zone diameters were not impacted by the addition of 0.002 μg/ml polysorbate 80 in this study. Another important variable to consider is the effect of lung surfactant on the MIC. The effect of lung surfactant should be studied for any agent that is being developed for respiratory indications, as the impact of this variable was initially studied and determined to be an issue for the use of daptomycin (19). Gepotidacin and levofloxacin MIC results were not affected by the addition of 1% and 5% of the lung surfactant utilized in this study.
With exception of the 16-h read time variable for the S. aureus and E. coli BMD method, the levels of each variable tested in this study were in contrast to those recommended by and utilized in standard published method guidelines, such as those from CLSI and EUCAST (15–17) (http://www.eucast.org/ast_of_bacteria/). Therefore, if the standard method is followed, the reproducibility of gepotidacin MIC and disk results should be similar to what was observed in this study for the reference methods. For most laboratories, reproducibility is assessed with QC organisms and surveillance of MIC and zone diameter distributions for clinical isolates. If QC results are out of range or trending, the factors that may be impacting the susceptibility results should be assessed. For gepotidacin, the inoculum is an important factor to consider, although a substantial (2 log10) increase in the inoculum concentration would be required for a significant MIC change. Since gepotidacin agar dilution MIC results for S. aureus were approximately 1.5 dilutions higher than the broth microdilution results, this should be taken into consideration in evaluating agar dilution results, especially if MIC results are near a susceptible/resistant breakpoint.
As gepotidacin is tested and used clinically, it is important to understand the impact of the various factors in performing susceptibility tests. The results of this study further emphasize the importance of adhering to standardized testing methods in conducting susceptibility testing for antimicrobials, including gepotidacin.
MATERIALS AND METHODS
The reference MIC methods (BMD, AD, and MD) as well as the different media were initially tested for both MIC and disk methods. CLSI BMD and disk diffusion methods were then modified according to specific method variables. The variables tested were those that could potentially affect gepotidacin susceptibility results as experienced with other antimicrobial agents. Lung surfactant was added to determine if there was any impact on the in vitro activity of gepotidacin in consideration of potential respiratory indications. The MIC study was performed initially, and the disk diffusion study followed; therefore, not all isolates or variables tested were similar. The bacterial species chosen for testing were those targeted for potential indications for gepotidacin at the time that the studies were conducted. The studies were performed over several days, and the reference method was tested once on each day of testing along with the method variations studied that day for each isolate. In addition to the assessment of the impact of the variable modifications, this allowed assessment of the reproducibility of the reference broth microdilution (BMD) and disk diffusion (DD) methods over time. The reference method and variation of method procedures by organism species are summarized in Table 4 and 5.
TABLE 4.
Reference method and variation of MIC methods studied
| Parameter or condition | Broth microdilution (BMD) result |
Agar dilution (AD) result |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test organism(s) | Reference method | Variables studied |
Test organism(s) | Reference method | Variables studied |
|||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||||
| MIC method | All | BMD | MD | AD | ||||||
| Medium | S. aureus, E. coli | CAMHB | ISB | S. aureus, E. coli | MHA | ISA | ||||
| S. pneumoniae | LHB | BSB | S. pneumoniae | SBA | EUA | BSA | ||||
| H. influenzae | HTM | EUB | BSBNAD | |||||||
| Temperature (°C) | All | 35 | 30 | 40 | All | 35 | 30 | 40 | ||
| Incubation time (h) | S. aureus, E. coli | 20 | 16a | 48 | S. aureus, E. coli | 20 | 16a | 48 | ||
| S. pneumoniae, H. influenzae | 24 | 16 | 48 | S. pneumoniae | 24 | 16 | 48 | |||
| Inoculum | All | 28 × 105 CFU/ml | 104 CFU/ml | 106 CFU/ml | 107 CFU/ml | All | 2 × 104 CFU/spot | 2 × 103 CFU/spot | 2 × 105 CFU/spot | 2 × 106 CFU/spot |
| Atmosphere | All | Ambient | 5% CO2 | 10% CO2 | S. aureus, E. coli | Ambient | 5% CO2 | 10% CO2 | ||
| S. pneumoniae | 5% CO2 | Ambient | 10% CO2 | |||||||
| pH | All | 7.2–7.4 | 5.5b | 6.5b | 8.5b | |||||
| Calcium (μg/ml) | All | 20–25 | 50 | 100 | ||||||
| Magnesium (μg/ml)c | All | 10–12.5 | +12.5 | +25 | ||||||
| Zinc (mmol/liter)c | All | CAMHB | +2 | +5 | +10 | |||||
| Potassium (mmol/liter)c | All | CAMHB | +12.5 | +25 | +50 | |||||
| Thymidine (μg/ml)c | All | CAMHB | +1 | +5 | ||||||
| % serumd | All | CAMHB | 25b | 50b | ||||||
| Albumin (μg/dl)d | All | CAMHB | +4 | |||||||
| % polysorbate 80e | All | CAMHB | 0.002 | |||||||
| % lung surfactantf | All | CAMHB | 1 | 5 | ||||||
BMD, broth microdilution; MD, macrodilution; AD, agar dilution; CAMHB, cation adjusted Mueller-Hinton broth; LHB, lysed horse blood (5%); HTM, Haemophilus test media; ISB, IsoSensitest broth; BSB, ISB–5% LHB; EUB, CAMHB–5% LHB–20 mg/liter NAD; BSB-NAD, ISB–5% LHB–20 mg/liter NAD; MHA, Mueller-Hinton agar; ISA, IsoSensitest agar; SBA, MHA–5% sheep blood agar; EUA, MHA–5% defribrinated horse blood–20 mg/liter NAD; BSA, MHA–5% defribrinated horse blood.
The minimum incubation time for the reference CLSI BMD and AD methods is 16 h.
The pH was adjusted to 5.5 and 6.5 with HCl and to 8.5 with NaOH.
The indicated additional component was added to the media at the stated concentration(s); the final concentration in the media was not measured.
Normal human pooled serum and human albumin (Innovative Research, Novi, MI) were added to the media at the stated concentrations.
Polysorbate 80 (Sigma-Aldrich, St. Louis, MO) was added to the media at concentration of 0.002%.
Surfactant (Survanta; Abbott Pharmaceuticals) was added to CAMHB to achieve the stated surfactant concentrations (19).
TABLE 5.
Reference method and Variation of Disk Diffusion methods Studied
| Parameter or condition | Disk diffusion (gepotidacin [10-μg] BD and MAST and linezolid [30-μg] BD disks) result |
||||||
|---|---|---|---|---|---|---|---|
| Test organism(s) | Reference method | Variables studied |
|||||
| 1 | 2 | 3 | 4 | 5 | |||
| Medium | S. aureus | MHA (BD) | MHA (Oxoid) | MHA (Hardy) | ISA (CLSI inoculuma) | ISA (BSAC inoculumb) | |
| S. pneumoniae, S. pyogenes | SBA (BD) | SBA (Oxoid) | SBA (Hardy) | EUA | BSA (CLSI inoculumb) | BSA (BSAC inoculumb) | |
| Temperature (°C) | All | 35 | 30 | 40 | |||
| Incubation time (h) | S. aureus | 16–18 | 14 | 24 | 48 | ||
| S. pneumoniae, S. pyogenes | 20–24 | 16 | 18 | 48 | |||
| Inoculum (CFU/ml) | All | 108 | 106 | 107 | 109 | ||
| Atmosphere | S. aureus | Ambient | 5% CO2 | 10% CO2 | |||
| S. pneumoniae, S. pyogenes | 5% CO2 | Ambient | 10% CO2 | ||||
| pH | All | 7.2–7.4 | 5.5c | 6.5c | 8.5c | ||
| Calcium (μg/ml) | All | 20–25 | 50 | 100 | |||
| Magnesium (μg/ml)d | All | 10–12.5 | +12.5 | +25 | |||
| Zinc (mmol/liter)d | All | MHA | +2 | +5 | +10 | ||
| Potassium (mmol/liter)d | All | MHA | +12.5 | +25 | +50 | ||
| Thymidine (μg/ml)d | All | MHA | +1 | +5 | |||
| % polysorbate 80e | All | MHA | 0.002 | ||||
MHA, Mueller-Hinton agar; SBA, MHA–5% sheep blood agar; ISA, IsoSensitest agar; EUA, MHA–5% defribrinated horse blood–20 mg/liter NAD; BSA, MHA–5% defribrinated horse blood.
The CLSI inoculum was a 0.5 McFarland standard.
The BSAC inoculums used were 1:10 for S. aureus and S. pneumoniae (107 CFU/ml) and 1:100 for beta-hemolytic streptococci (106 CFU/ml).
The pH was adjusted to 5.5 and 6.5 with HCl and to 8.5 with NaOH.
The indicated additional component was added to the media at the stated concentration(s); the final concentration in the media was not measured.
Polysorbate 80 (Sigma-Aldrich, St. Louis, MO) was added to the media at concentration of 0.002%.
MIC.
Initially, 10 S. aureus isolates (5 methicillin-susceptible and 5 methicillin-resistant strains, including 1 glycopeptide-intermediate and 1 glycopeptide-resistant strain), 5 E. coli isolates (including 1 cefixime-resistant, 1 extended β-lactamase-producing, and 1 carbapenemase-producing strain), 10 S. pneumoniae isolates (3 penicillin-susceptible, 3 penicillin-intermediate, and 4 penicillin-resistant strains, including 2 multidrug-resistant strains), and 10 H. influenzae isolates (5 β-lactamase-negative strains, including 1 ampicillin-resistant strain, and 5 β-lactamase-positive strains, including 1 tetracycline-resistant strain) were tested using 3 different MIC methods (BMD, MD, and AD) for gepotidacin and levofloxacin (15, 16). The effects of the choice of media and other method variables were then determined by broth microdilution and agar dilution methods. Quality control strains (S. aureus ATCC 29213, E. coli ATCC 25922, S. pneumoniae ATCC 49619, and H. influenzae ATCC 49247) were tested each day for all reference and variable MIC methods, and the results were compared to CLSI expected ranges (16).
Broth microdilution.
The effect of media was determined for the BMD method by using cation-adjusted Mueller-Hinton broth (CAMHB; Becton Dickinson, Sparks, MD) and IsoSensitest broth (ISB; Oxoid, Basingstoke, United Kingdom) against S. aureus and E. coli; CAMHB–5% lysed horse blood (LHB; Cleveland Scientific, Bath, OH) and ISB–5% lysed horse blood (BSB) against S. pneumoniae; and Haemophilus Test Medium (HTM; Remel, Lenexa, KS), CAMHB–5% lysed horse blood–20 mg/liter NAD (EUB; Sigma-Aldrich, St. Louis, MO), and ISB–5% lysed horse blood–20 mg/liter NAD (BSB-NAD) against H. influenzae. The following significant variables were studied for all study isolates by BMD: temperature, incubation time, atmospheric conditions, and inoculum concentration, pH, calcium, magnesium, zinc, potassium, thymidine, polysorbate 80, albumin, serum, and lung surfactant levels. All medium additives were filter sterilized and added to the broth following autoclave sterilization of the broth. At the higher inoculum concentrations of 106 and 107 CFU/ml, although there was slight turbidity of the wells prior to incubation, MIC endpoints were easily determined on the basis of the presence of large buttons of growth following incubation.
Agar dilution.
The effect of media was also determined for the AD method by using Mueller-Hinton agar (MHA; Becton Dickinson, Sparks, MD) and IsoSensitest agar (ISA; Oxoid, Basingstoke, United Kingdom) against S. aureus and E. coli and MHA–5% sheep blood (SBA), MHA–5% defibrinated horse blood (Cleveland Scientific, Bath, OH)–20 mg/liter NAD (EUA), and ISA–5% defibrinated horse blood (BSA) against S. pneumoniae. The following significant variables were studied for all study isolates by AD: temperature, incubation time, atmospheric condition, and inoculum concentration.
Data analysis.
The mean doubling dilution difference of the MIC determined using each method (including AD, MD, media, and other MIC variables) from the reference method MIC (tested on the same day) and the essential agreement (percentage of the variable method MIC within ±1 dilution of the reference method MIC) were calculated. The dilution difference was determined by converting the MIC to log2 + 10 values, and the reference value was subtracted from the variable method value to obtain the dilution difference (e.g., if reference MIC was 0.5 μg/ml [log2 + 10 = 9] and the variable method MIC was 1.0 μg/ml [log2 + 10 = 10], the dilution difference was 1 [10 − 9]). Mean dilution differences and essential agreement percentages for method variations that demonstrated <90% essential agreement compared to the reference method MIC determined on the same day of testing are shown in Tables 1 and 2. Mean dilution differences and essential agreement percentages are not shown for method variations that were in agreement with the reference method MIC (essential agreement, ≥90%).
Disk diffusion.
Disk diffusion testing was performed against 30 S. aureus isolates (the same 10 strains tested in the MIC study and an additional 10 methicillin-susceptible and 10 methicillin-resistant strains), 15 S. pneumoniae isolates (the same 10 strains tested in the MIC study and an additional 2 penicillin-susceptible strains, 2 penicillin-intermediate strains, and 1 penicillin-resistant strain), and 15 S. pyogenes isolates (including 3 macrolide- and 3 tetracycline-resistant strains) according to CLSI methods with gepotidacin (10 μg) disks from 2 manufacturers (BD disks [Becton Dickinson, Sparks, MD] and MAST disks [Hardy Diagnostics, Santa Maria, CA]) and linezolid (30 μg) disks (Becton Dickinson, Sparks, MD) (17). All agar plates used in this study were prepared by the testing laboratory. The effect of media was determined by using Mueller-Hinton agar (MHA; Becton Dickinson, Sparks, MD; Oxoid, Basingstoke, United Kingdom; Hardy Diagnostics, Santa Maria, CA) and IsoSensitest agar (ISA; Oxoid, Basingstoke, United Kingdom) against S. aureus and MHA–5% sheep blood (SBA; MHA from Becton Dickinson, Sparks, MD, Oxoid, Basingstoke, United Kingdom, and Hardy Diagnostics, Santa Maria, CA, and sheep blood from Cleveland Scientific, Bath, OH), MHA–5% defibrinated horse blood (BSA; MHA from Becton Dickinson, Sparks, MD, and horse blood from Cleveland Scientific, Bath, OH), and BSA with addition of 20 mg/liter NAD (EUA; Sigma-Aldrich, St. Louis, MO) against S. pneumoniae and S. pyogenes. Quality control strains S. aureus ATCC 25923, S. aureus ATCC 29213, and S. pneumoniae ATCC 49619 were tested each day for all reference and variable disk diffusion methods for all disks and compared to CLSI reference QC ranges (with the exception of S. aureus ATCC 29213, which was tested for informational purposes) (16). The following significant variables were studied: media, temperature, incubation time, atmospheric conditions, inoculum concentrations, and pH, calcium, magnesium, zinc, potassium, thymidine, and polysorbate 80 levels. All medium additives were filter sterilized and added to the agar following autoclave sterilization of the agar. Data analysis included calculation of the difference in mean zone diameter for each variable method from the reference method mean zone diameter (tested on the same day) and calculation of essential agreement (percentage of variable method zone diameters within ±4 mm of the reference method zone diameters based on the investigator's criteria).
ACKNOWLEDGMENTS
This work was supported by GlaxoSmithKline and funded by contract HDTRA1-07-9-0002 from the Defense Threat Reduction Agency. L.A.M. and N.E.S.-O. are employees of and hold shares in GlaxoSmithKline.
REFERENCES
- 1.Jones RN. 1996. Medium and supplement effects on the antimicrobial activity of quinupristin/dalfopristin tested by agar dilution and Etest methods. Diagn Microbiol Infect Dis 26:99–102. doi: 10.1016/S0732-8893(96)00179-4. [DOI] [PubMed] [Google Scholar]
- 2.Libertin CR, Leal F, Stein DS. 1988. Group G streptococci: susceptibility patterns and the effect of the inoculum size and growth phase on the bactericidal activity of penicillin. Diagn Microbiol Infect Dis 9:33–40. doi: 10.1016/0732-8893(88)90058-2. [DOI] [PubMed] [Google Scholar]
- 3.Konig C, Simmen HP, Blaser J. 1993. Effect of pathological changes of pH, pO2 and pCO2 on the activity of antimicrobial agents in vitro. Eur J Clin Microbiol Infect Dis 12:519–526. doi: 10.1007/BF01970957. [DOI] [PubMed] [Google Scholar]
- 4.Hwang JM, Piccinini TE, Lammel CJ, Hadley WK, Brooks GF. 1986. Effect of storage temperature and pH on the stability of antimicrobial agents in MIC trays. J Clin Microbiol 23:959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reimer LG, Stratton CW, Reller LB. 1981. Minimum inhibitory and bactericidal concentrations of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob Agents Chemother 19:1050–1055. doi: 10.1128/AAC.19.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeth LM, King A, Knight H, May J, Miller LA, Poupard JA. 2000. Comparison of cation-adjusted Mueller-Hinton broth with Iso-Sensitest broth for the NCCLS broth microdilution method. J Antimicrob Chemother 46:369–376. doi: 10.1093/jac/46.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Koeth LM, Leclercq R, Olsson-Liljequist B. 2003. Comparison of daptomycin and vancomycin MIC results by DIN, CLSI, SFM and SRGA methods for 297 Gram-positive organisms. Int J Antimicrobial Agents 23:17–24. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs MR, Bajaksouzian S, Windau A, Appelbaum PC, Lin G, Felmingham D, Dencer C, Koeth L, Singer ME, Good CE. 2002. Effects of various test media on the activities of 21 antimicrobial agents against Haemophilus influenzae. J Clin Microbiol 40:3269–3276. doi: 10.1128/JCM.40.9.3269-3276.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washington JA II, Snyder RJ, Kohner PC, Wiltse CG, Ilstrup DM, McCall JT. 1978. Effect of cation content of agar on the activity of gentamicin, tobramycin, and amikacin against Pseudomonas aeruginosa. J Infect Dis 137:103–111. doi: 10.1093/infdis/137.2.103. [DOI] [PubMed] [Google Scholar]
- 10.Auckenthaler R, Michéa-Hamzehpour M, Pechère JC. 1986. In-vitro activity of newer quinolones against aerobic bacteria. J Antimicrob Chemother 17(Suppl B):29–39. doi: 10.1093/jac/17.suppl_B.29. [DOI] [PubMed] [Google Scholar]
- 11.Daly JS, Dodge RA, Glew RH, Soja DT, DeLuca BA, Hebert S. 1997. Effect of zinc concentration in Mueller-Hinton agar on susceptibility of Pseudomonas aeruginosa to imipenem. J Clin Microbiol 35:1027–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennie RP, Koeth L, Jones RN, Fritsche TR, Knapp CC, Killian SB, Goldstein BP. 2007. Factors influencing broth microdilution antimicrobial susceptibility test results for dalbavancin, a new glycopeptide agent. J Clin Microbiol 45:3151–3154. doi: 10.1128/JCM.02411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlton CL, Hindler JA, Turnidge J, Humphries RM. 2014. Precision of vancomycin and daptomycin MICs for methicillin-resistant Staphylococcus aureus and effect of subculture and storage. J Clin Microbiol 52:3898–3905. doi: 10.1128/JCM.01571-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilius AM, Beyer JM, Flamm RK, Tanaka SK. 1997. Variability in susceptibilities of Haemophilus influenzae to clarithromycin and azithromycin due to medium pH. J Clin Microbiol 35:1311–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 9th ed Approved standard M07-A9 CLSI, Wayne, PA. [Google Scholar]
- 16.CLSI. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed Approved standard, CLSI publication M100-S26 CLSI, Wayne, PA. [Google Scholar]
- 17.CLSI. 2012. Performance standards for antimicrobial disk susceptibility tests, 11th ed Approved standard M02-A11 CLSI, Wayne, PA. [Google Scholar]
- 18.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]