ABSTRACT
Staphylococcus hyicus and Staphylococcus agnetis are two coagulase-variable staphylococcal species that can be isolated from bovine milk and are difficult to differentiate. The objectives of this study were to characterize isolates of bovine milk origin from a collection that had previously been characterized as coagulase-positive S. hyicus based on phenotypic species identification methods and to develop a PCR-based method for differentiating S. hyicus, S. agnetis, and Staphylococcus aureus. Isolates (n = 62) were selected from a previous study in which milk samples were collected from cows on 15 dairy herds. Isolates were coagulase tested and identified to the species level using housekeeping gene sequencing. A multiplex PCR to differentiate S. hyicus, S. agnetis, and S. aureus was developed. Pulsed-field gel electrophoresis was conducted to strain type the isolates. Based on gene sequencing, 44/62 of the isolates were determined to be either S. agnetis (n = 43) or S. hyicus (n = 1). Overall, 88% (37/42) of coagulase-positive S. agnetis isolates were found to be coagulase positive at 4 h. The herd-level prevalence of coagulase-positive S. agnetis ranged from 0 to 2.17%. Strain typing identified 23 different strains. Six strains were identified more than once and from multiple cows within the herd. Three strains were isolated from cows at more than one time point, with 41 to 264 days between samplings. These data suggest that S. agnetis is likely more prevalent on dairy farms than S. hyicus. Also, some S. agnetis isolates in this study appeared to be contagious and associated with persistent infections.
KEYWORDS: Staphylococcus, cattle, milk
INTRODUCTION
Staphylococci are the most common bacteria isolated from the cow's mammary gland and are frequently associated with subclinical or mild clinical mastitis (1–3). Staphylococci can be differentiated into groups using the coagulase test. Hemolytic coagulase-positive isolates are often presumptively identified as Staphylococcus aureus, a so-called “major” contagious mastitis pathogen, and coagulase-negative staphylococci are frequently grouped together as “minor” pathogens (4).
Currently, there are two coagulase-variable staphylococcal species that have been associated with bovine mastitis, Staphylococcus hyicus and Staphylococcus agnetis. Staphylococcus hyicus was the first staphylococcal species to be described as coagulase variable, with coagulase production being found in approximately 24 to 56% of strains (5). The coagulation reaction of S. hyicus was described as often having weak activity, requiring 18 to 24 h for detection (5). Staphylococcus hyicus has been associated with chronic intramammary infections (IMI) that cause subclinical or mild clinical mastitis in dairy cattle (6, 7). Historically, S. hyicus was found to be one of the most common organisms isolated from mammary gland secretions and teat canal keratin samples from primigravid heifers (8). When identified as a coagulase-negative staphylococcal (CNS) species, the reported prevalence of S. hyicus on dairy farms has been highly varied, with some researchers finding it to be one of the most prevalent species identified (7), while being rarely identified by others (3, 9). Coagulase-positive S. hyicus has been described as a low-prevalence mastitis pathogen and has been found to be a greater problem in herds with a low prevalence of S. aureus IMI (6). Staphylococcus agnetis is a newly described species that is genetically very similar to S. hyicus, making it difficult to differentiate from S. hyicus with routine phenotypic and genotypic tests. It was first reported in 2012 associated with subclinical and mild clinical bovine mastitis (10).
The phenotypic tests used to differentiate staphylococcal species have likely led to classification errors. Prior to 1986, Staphylococcus chromogenes and S. hyicus were not recognized as different staphylococcal species (11), and phenotypic classification methods, such as API 20 (API test; bioMérieux, Hazelwood, MO, USA), have been shown to have low specificity for detecting S. hyicus (12). Only since 2012 has S. agnetis been recognized as a species and mastitis agent (10); thus, prior to this date, most coagulase-positive non-aureus Staphylococcus spp. were likely classified as S. hyicus. Further, from a practical standpoint, when a staphylococcal species is identified as coagulase positive, it is often presumptively identified as S. aureus, and thus S. hyicus and S. agnetis may be misclassified by practitioners or dairy farmers performing their own milk microbiology (6, 13), particularly since approximately one-fifth to one-fourth of S. aureus isolates from bovine mastitis do not present any detectable beta-hemolytic activity in primary culture (14).
Recently, genotypic methods to identify staphylococcal species have been described as being superior to phenotypic methods (12). Analysis of the 16S rRNA gene sequence is the most commonly used method for the identification and classification of bacteria (15). However, the usefulness of 16S rRNA gene sequencing is limited when applied to certain staphylococcal species because of the high degree of similarity between closely related species (15, 16). For example, 16S rRNA gene sequences of S. agnetis isolates were found to exhibit 99.7% identity to S. hyicus ATCC 11249T and 99.1% identity to S. chromogenes ATCC 43764 (10). Using the recommended cutoff value of 98.7% to differentiate a species (17), these results would be inconclusive. Several other genes have been used as targets for sequenced-based species identification of staphylococcal isolates, including rpoB (18), tuf (19), and cpn60 (20). When using rpoB gene sequencing with a cutoff of ≥97% for identification of these species, S. agnetis can be differentiated from the swine-origin type strain of S. hyicus (ATCC 11249), as these isolates exhibit only 93.5% identity. However, the partial sequences of the S. agnetis rpoB genes are 99% similar to those of some bovine-origin S. hyicus strains, e.g., strain KSOS1-02, an S. hyicus isolate from bovine milk, thus leading to further confusion in making a definitive diagnosis. It is unknown if the rpoB gene of S. hyicus of bovine origin is different from that of swine origin or if there is an error in the database, which has been previously reported (21). The tuf gene can be used to differentiate S. agnetis and S. hyicus when following the recommended cutoff values of ≥98.0% identity with >0.8% separation between species (22). However, if this gene is not the primary gene used to identify staphylococcal isolates to the species level, several gene sequencing reactions may need to be performed before arriving at a definitive identification. In addition, access to gene sequencing equipment is needed. Hence, a rapid, accurate, and inexpensive method to differentiate coagulase-positive staphylococci isolated from bovine milk would improve diagnostic accuracy and allow a true understanding of the epidemiology of S. hyicus, S. agnetis, and S. aureus on dairy farms.
Authors have recently published whole-genome sequences for S. hyicus (23), S. agnetis (24), and S. chromogenes (25). Examination of these whole-genome sequences identified unique gene sequences that were postulated to be good diagnostic targets for differentiating S. hyicus from S. agnetis isolated from bovine milk using a multiplex PCR platform. Hence, the objectives of this study were to (i) develop a simple multiplex PCR-based assay to differentiate S. hyicus, S. agnetis, and S. aureus, and (ii) use this assay along with pulsed-field gel electrophoresis strain typing to characterize a collection of coagulase-positive non-aureus Staphylococcus spp. isolated from bovine milk samples from 15 dairy herds in Washington and Idaho. A further objective was to compare the prevalences of S. aureus, S. agnetis, and S. hyicus isolated from milk samples from each herd.
RESULTS
A total of 62 isolates that had been previously identified as coagulase-positive S. hyicus based on coagulase testing and API Staph (26) were studied. Among the 62 isolates received from Washington State University, 15 isolates were found to be coagulase negative and 47 isolates were coagulase positive. Based on rpoB gene sequencing, 13 of the coagulase-negative isolates were identified as species other than S. agnetis or S. hyicus, including S. chromogenes (n = 8), Staphylococcus hominis (n = 2), Staphylococcus epidermidis (n = 1), Staphylococcus simulans (n = 1), and Staphylococcus xylosus (n = 1). One of the coagulase-negative isolates was identified by rpoB (99.8% sequence homology to S. hyicus and 93.5% sequence homology to S. agnetis) and tuf gene sequences as S. hyicus, and the remaining coagulase-negative isolate was definitively identified as S. agnetis by the tuf gene sequence. Among the 47 coagulase-positive isolates, 5 isolates were identified as S. aureus by rpoB gene sequence, and the remaining 42 isolates could not be definitively identified using the rpoB gene sequence, as all sequences were 99% homologous with both S. agnetis and S. hyicus. Using tuf gene sequencing, all of the remaining 42 coagulase-positive staphylococcal (CPS) isolates were definitively identified as S. agnetis. A total of 88% (37/42) of the coagulase-positive S. agnetis isolates were found to be coagulase positive at 4 h. The remaining coagulase-positive S. agnetis isolates (12% [5/42]) exhibited delayed coagulation and were found to be coagulase negative at 4 h and coagulase positive at 24 h.
The tuf gene sequencing results were used as the definitive identification method against which the newly designed multiplex PCR was tested. All 43 S. agnetis isolates and the four S. agnetis control isolates each produced a single 293-bp band on gel electrophoresis following multiplex PCR (Fig. 1), whereas the single isolate that was identified as S. hyicus, based on rpoB and tuf gene sequencing, and the S. hyicus type strain (ATCC 11249) each produced a single 425-bp band. Sanger sequencing and DNA alignment of the aroD gene fragments found 99 to 100% homology of all isolates identified as S. agnetis (Fig. 2). Sanger sequencing of the single S. hyicus test isolate aroD gene showed 99% homology with the S. hyicus type strain (ATCC 11249) aroD gene sequence. Alignment of the S. agnetis and S. hyicus gene fragments that were produced using the multiplex PCR showed that the fragments were only about 58% similar. No electrophoretic band was detectable when the two S. chromogenes control isolates were tested using the multiplex PCR.
FIG 1.
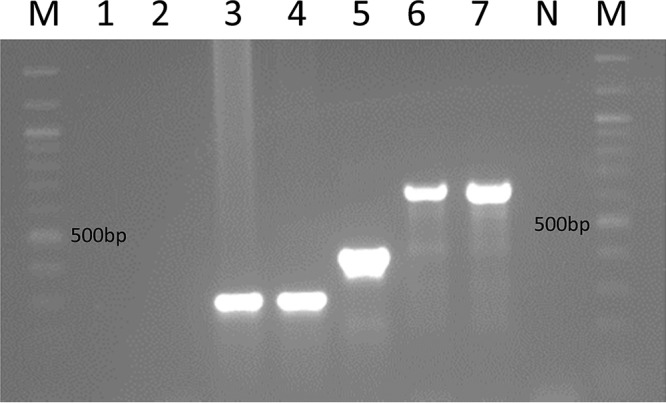
Multiplex PCR amplification profiles. The M lanes contain a 100-bp molecular weight ladder marker. Lane 1, S. chromogenes ATCC 43764T; lane 2, S. chromogenes MU 970; lane 3, coagulase-negative S. agnetis DSM 23656T; lane 4, coagulase-positive S. agnetis 43OE; lane 5, S. hyicus ATCC 11249T; lane 6, S. aureus ATCC 12600T, lane 7, S. aureus ATCC 29740. The N lane contains a negative control.
FIG 2.
Comparison and alignment of aroD gene sequences from all S. agnetis isolates. The dendrogram shows that all isolates were at least 99.25% homologous. The dendrogram includes the S. agnetis type strain (DSM 23656 [6VT]), the reference strain DSM 23658 (55 OE), and two strains (100 VT and 43 OE) originally isolated from cows in Finland (10). A consensus sequence is at the top of the figure, with all other isolates below. All nucleotide bases in agreement with the consensus sequence are noted by a vertical bar (|), and any single-nucleotide changes are noted by the presence of the variant nucleotide base present within that column.
Overall, 40/42 coagulase-positive S. agnetis isolates could be categorized according to one of the sampling periods, including the three herd cultures or cattle at parturition (Table 1). Data on isolate collection time point were missing for 2 isolates; therefore, they were not included in the prevalence data. Overall, all herds had at least one cow with a CPS isolated from the milk (Table 1). Of the 15 herds, 10 herds had at least one S. agnetis strain isolated from milk samples. In 5 herds, no S. agnetis was isolated, and on 1 herd (herd 4), the only recorded CPS isolated from milk samples was an S. agnetis isolate. Overall, the prevalence of S. agnetis was low, ranging from 0.0% to 2.2%. However, the prevalence of S. agnetis was equal to or higher than that of S. aureus at least once among the different sample collection periods on 5/15 (33%) herds (Table 1). The only S. hyicus isolate was from herd 2.
TABLE 1.
Total number and percentage of cows with CPS isolated within each herd from each of the three whole-herd milk samplings and milk samples collected from heifers and cows at calving at 15 commercial dairy herdsa
| Herd no. | Prevalence of CPS at herd cultures |
Prevalence of CPS at parturition |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Herd culture 1 |
Herd culture 2 |
Herd culture 3 |
Heifers |
Cows |
|||||||||||
| Herd sizeb | S. aureus (no. [%]) | S. agnetis (no. [%]) | Herd sizeb | S. aureus (no. [%]) | S. agnetis (no. [%]) | Herd sizeb | S. aureus (no. [%]) | S. agnetis (no. [%]) | nc | S. aureus (no. [%]) | S. agnetis (no. [%]) | nc | S. aureus (no. [%]) | S. agnetis (no. [%]) | |
| 1 | 233 | 5 (2.2) | 1 (0.4) | 262 | 4 (1.5) | 0 | 279 | 4 (1.4) | 2 (0.7) | 105 | 1 (1.0) | 5 (4.8) | 155 | 0 | 0 |
| 2 | 274 | 8 (2.9) | 0 | 261 | 3 (1.2) | 1 (0.4) | 288 | 4 (1.4) | 3 (1.0) | 15 | 0 | 1 (6.7) | 185 | 2 (1.1) | 2 (1.1) |
| 4 | 166 | 0 | 0 | 173 | 0 | 1 (0.6) | 178 | 0 | 0 | 34 | 0 | 0 | 50 | 0 | 0 |
| 5 | 198 | 2 (1.0) | 0 | 215 | 0 | 0 | 213 | 1 (0.5) | 1 (0.5) | 76 | 0 | 0 | 165 | 1 (0.6) | 2 (1.2) |
| 6 | 48 | 1 (2.1) | 0 | 54 | 0 | 0 | 42 | 0 | 0 | 10 | 0 | 0 | 8 | 0 | 0 |
| 7 | 168 | 3 (1.8) | 0 | 154 | 5 (3.3) | 0 | 148 | 2 (1.4) | 0 | 16 | 0 | 0 | 61 | 3 (4.9) | 0 |
| 8 | 82 | 3 (3.7) | 1 (1.2) | 101 | 7 (6.9) | 1 (1.0) | 92 | 3 (3.3) | 2 (2.2) | 2 | 0 | 0 | 17 | 0 | 0 |
| 9 | 220 | 0 | 0 | 244 | 2 (0.8) | 0 | 581 | 4 (0.7) | 0 | 15 | 0 | 0 | 104 | 1 (1.0) | 0 |
| 10 | 315 | 36 (11.4) | 0 | 333 | 35 (10.5) | 1 (0.3) | 308 | 31 (10.1) | 1 (0.3) | 104 | 5 (4.8) | 0 | 53 | 3 (5.7) | 0 |
| 11 | 86 | 3 (3.5) | 0 | 84 | 3 (3.6) | 0 | 78 | 3 (3.9) | 1 (1.3) | 3 | 0 | 0 | 61 | 0 | 0 |
| 12 | 152 | 2 (1.3) | 1 (0.7) | 149 | 4 (2.7) | 1 (0.7) | 165 | 2 (1.2) | 1 (0.6) | 70 | 3 (4.3) | 2 (2.9) | 123 | 1 (0.8) | 0 |
| 13 | 131 | 8 (6.1) | 0 | 146 | 7 (4.8) | 0 | 146 | 6 (4.1) | 0 | 68 | 1 (1.5) | 0 | 117 | 5 (4.3) | 0 |
| 14 | 343 | 10 (2.9) | 0 | 326 | 6 (1.8) | 0 | 374 | 13 (3.5) | 0 | 63 | 7 (11.1) | 0 | 268 | 0 | 0 |
| 15 | 345 | 4 (1.2) | 2 (0.6) | 418 | 2 (0.5) | 2 (0.5) | 464 | 3 (0.7) | 2 (0.4) | 165 | 4 (2.4) | 1 (0.6) | 288 | 1 (0.4) | 1 (0.4) |
| 16 | 374 | 6 (1.6) | 0 | 401 | 6 (1.5) | 0 | 399 | 10 (2.5) | 0 | 162 | 20 (12.4) | 1 (0.6) | 362 | 11 (3.0) | 0 |
| Total | 3,135 | 91 | 5 | 3,321 | 84 | 7 | 3,755 | 86 | 13 | 908 | 41 | 10 | 2,017 | 28 | 5 |
Data are in bold where the prevalence of S. agnetis is greater than or equal to that of S. aureus. No coagulase-positive S. hyicus isolates were identified in this study.
Herd size is the number of lactating cows present in the herd at the time of sample collection.
n = total number of heifers or cows sampled at calving.
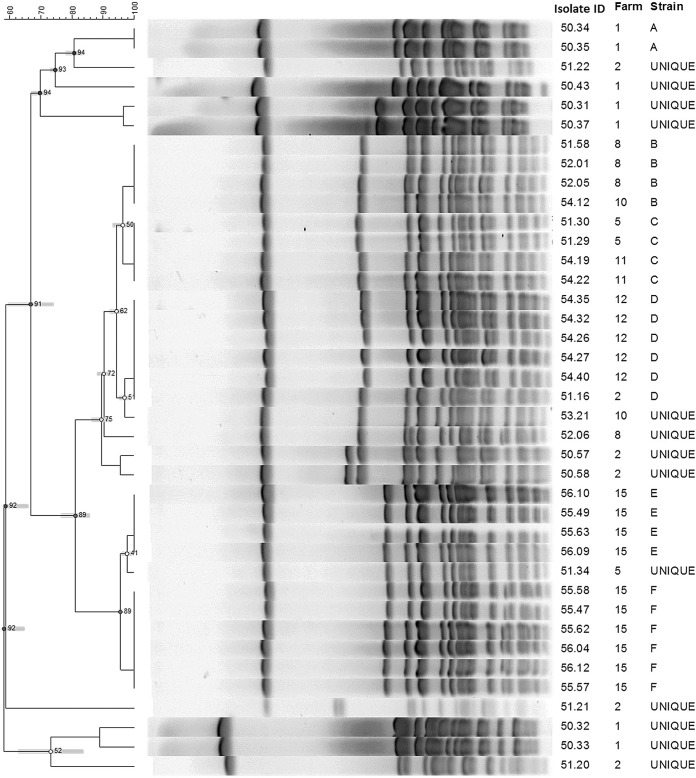
Pulsed-field gel electrophoresis (PFGE) was completed on all 44 non-aureus Staphylococcus isolates (42 coagulase-positive S. agnetis, 1 coagulase-negative S. agnetis, and 1 coagulase-negative S. hyicus). Two of the coagulase-positive S. agnetis isolates were not typeable by PFGE and produced consistent smearing of the electrophoretic bands. Among the 42 typeable isolates, 23 different PFGE banding patterns were identified. Overall, 17 strains were only identified once, and 6 strains were identified more than one time (Table 2). One of the strains found only once was the S. hyicus isolate, which had a different banding pattern from all of the S. agnetis isolates. Additionally, one of the strains only identified once was the coagulase-negative S. agnetis isolate. Further analysis was completed on 39 of the isolates, which included all the S. agnetis isolates that originated from a herd (n = 8 herds) with more than one S. agnetis strain isolated (Fig. 3). Of the 6 strain types that were identified more than once, all 6 types were identified from more than one cow within the herd. Overall, 3/6 (50%) strains that were identified more than once were also identified on more than one herd (Fig. 3). A total of 3 strains were associated with persistent infections, with each of the 3 strains being isolated from at least one cow at more than one time point. The time between samplings and isolation of the same strain ranged from 41 to 264 days.
TABLE 2.
Summary of pulsed-field gel electrophoresis results for all strains identified more than once on a herd, including number of cows infected with each strain and number of cows identified with the strains at multiple sampling points
| Herd no.a | PFGE strain typing ID | No. of isolates within strain type/total S. agnetis isolates within herd (% of total S. agnetis isolates) | No. of cows with that strain type | No. of cows with strain type at multiple time points | Days between sample collections |
|---|---|---|---|---|---|
| 1 | A | 2/8 (25) | 2 | ||
| 8 | B | 3/4 (75) | 2 | 1 | 177 |
| 5 | C | 2/3 (67) | 2 | ||
| 11 | C | 2/2 (100) | 2 | ||
| 12 | D | 5/5 (100) | 4 | 1 | 264 |
| 15 | E | 4/10 (40) | 4 | ||
| 15 | F | 6/10 (60) | 4 | 2 | 41 and 153, respectively |
Herds were included here only if a PFGE strain type was identified more than one time. If a herd is not listed, all strains within that herd were unique based on a PFGE electrophoretic pattern with at least one band difference.
FIG 3.
Comparison of all S. agnetis isolates (n = 39) from 8 commercial dairy herds in which more than one S. agnetis isolate was identified from bovine milk samples. A total of 6 different strains were identified (strains A to F). Strains B, C, and D include isolates from more than one herd. ID, identification.
DISCUSSION
To our knowledge, this is the first study to characterize a collection of coagulase-positive S. agnetis isolates from dairies in the United States and to develop a simple method to differentiate S. hyicus, S. agnetis, and S. aureus. In the original description of S. agnetis, 13 isolates originating from 13 cows on 8 dairies in southern Finland were characterized, including 12 isolates from bovine subclinical or mild clinical mastitis and one isolate from a teat apex (10). Of those original 13 S. agnetis isolates, 3 isolates were found to have a delayed coagulase-positive reaction, and the remaining 10 isolates were coagulase negative. The delayed coagulase reaction of the previously reported S. agnetis isolates (10) matched the coagulase description originally given to S. hyicus (5). In contrast, in the present study, most (88%) of the coagulase-positive isolates were tube coagulase positive at 4 h. The isolates used in the present study originated from a study in which the primary goal was to identify S. aureus (26), but the study protocol included isolation and storage of all coagulase-positive staphylococci during the initial screening of all milk samples. Coagulase-negative staphylococci were not saved for further characterization and thus could not be evaluated in the present study. In the original study, the banked CPS isolates were identified to the species level using biochemical methods, and only those isolates identified as S. aureus were utilized for further study at the time.
The data presented here demonstrate that although the overall prevalence of coagulase-positive S. agnetis was determined to be low, it can be prevalent among CPS isolated from milk in some herds and could be confused with S. aureus, especially since the majority of isolates clotted rabbit plasma after 4 h of incubation similar to S. aureus and differently from the delayed coagulation typically described for S. hyicus (5, 10). Misclassification could impact management decisions, such as unnecessary culling based on the belief that the cow has S. aureus and is a contagious reservoir for infection of other cows.
Based on the original phenotypic species identification of the isolates used in this study, 18 isolates were originally misclassified as S. hyicus. When genotypic species identification was performed, the 18 isolates were found to be staphylococcal species other than S. hyicus or S. agnetis. Some of this error could be due to storage error or loss of the initial organism during the recovery and subculturing of the isolates. Some of the error is also likely due to misclassification reported to occur with phenotypic species identification, as such methods can lead to a higher number of unidentified or misidentified staphylococcal isolates (27). When using phenotypic methods, S. chromogenes can be misidentified as S. hyicus (28), as was seen with 8/18 (44%) of the misclassified isolates in this study. Although phenotypic methods have been shown to be inaccurate for non-aureus Staphylococcus species (29), previous reports have shown the API system to be satisfactory in the identification of S. aureus (30). Therefore, in this study, the S. aureus isolate identities were not further confirmed using genotypic methods.
The aroD gene encodes 3-dehydroquinate dehydratase, an enzyme involved in the shikimate pathway used to synthesize chorismic acid, a central precursor for aromatic compounds (31). This is a housekeeping function for the cell and therefore, the gene should be highly conserved. Selection of a highly conserved gene as a target for species identification is important to allow for broad applicability and consistency in results. Based on the gene sequence analyses reported herein, the aroD gene fragment used for the multiplex PCR appears to be highly conserved, with over 99% homology found within all S. agnetis isolates studied, including isolates from 10 different U.S. herds that were composed of 23 different PFGE strains types, as well as four isolates from Finland.
Because S. hyicus was rarely found in this study, we are unable to determine how conserved the aroD gene is in this species. However, when the single study isolate was compared to a type strain isolate of swine origin, it was found to have 99% sequence homology, suggesting that a conserved region was selected. It should be further noted that the only S. hyicus isolate harvested from cow's milk was not coagulase positive, but it was included in the analyses to demonstrate that the multiplex PCR could differentiate a field S. hyicus strain from S. agnetis and S. aureus. Further, it was included in the PFGE analyses to demonstrate that the electrophoretic banding pattern was different from all of the S. agnetis isolates. Similarly, the coagulase-negative S. agnetis isolate was included in the PFGE analysis to demonstrate that it was genotypically different from the coagulase-positive variants. Note that the data in Table 1 do not include the coagulase-negative S. agnetis isolate or the S. hyicus isolate.
Although S. agnetis was found at a low prevalence among all herds studied, it can be identified at a prevalence equal to or greater than that of S. aureus during some sampling periods. Based on PFGE, some strains of S. agnetis appear to act as contagious strains based on the fact that the same PFGE strain type was isolated from the milk of multiple animals in a single herd. Also, some strains appear to cause a persistent infection, lasting over 150 days in 3 cows. Cow-level somatic cell count and milk yield data were not collected in the original study; thus, it cannot be determined whether the reported strains have an important impact on udder health. The potential for contagious spread does raise concern that some strains of coagulase-positive S. agnetis may be important udder pathogens in some herds, but further studies would be needed to confirm this finding.
The multiplex PCR described herein provides an inexpensive accurate method to differentiate three species of staphylococci that can be difficult to differentiate based on phenotype alone. Further, preliminary evidence is provided that suggests the multiplex PCR has promise for differentiating S. chromogenes from coagulase-negative variants of S. hyicus and S. agnetis. However, more coagulase-negative isolates need to be evaluated to confirm this finding.
One limitation of this study was the lack of a comparable number of S. hyicus field isolates and the lack of a coagulase-positive S. hyicus as a comparator for coagulase-positive S. agnetis in the PCR test. This may be due to S. hyicus, and in particular coagulase-positive S. hyicus, truly being a rare species in dairy herds or just rare in the herds within this study. Several other recent studies in which species identification was done using genotypic methods, have found the prevalence of coagulase-negative S. hyicus to be very low, ranging from 0.5% to 1.3% (32, 33). The data could also be biased, because the original protocol for isolate storage included only coagulase-positive isolates. Thus, the prevalence and importance of coagulase-negative S. hyicus and S. agnetis among staphylococcal species isolated from milk samples on these herds cannot be evaluated. The original misclassification of the two coagulase-negative isolates as coagulase positive (1 S. hyicus and 1 S. agnetis) may be due to the banked isolate not being the originally recorded CPS isolate or differences in interpretation of a weak or delayed coagulase test result. Although these isolates were misclassified, they were not removed from the study due to the fact that they were the staphylococcal species of interest and therefore useful to validate the multiplex PCR, as the total number of isolates in this study was low.
In conclusion, within these herds, S. agnetis was much more prevalent than S. hyicus. Based on PFGE, some strains of S. agnetis were shown to have contagious behavior and cause persistent infections. The use of the described multiplex PCR allows inexpensive and efficient differentiation of the common species of CPS isolated from milk without the need for housekeeping gene sequence analysis, and preliminary evidence suggests it may aid in differentiating CNS variants of S. agnetis and S. hyicus from each other and from S. chromogenes. Future studies will evaluate the utility of the multiplex PCR on CNS variants and also examine the effect of S. agnetis on the host, including somatic cell count.
MATERIALS AND METHODS
Isolate and phenotypic characterization.
Banked coagulase-positive non-aureus Staphylococcus isolates (n = 62) collected from whole-herd milk samplings and milk samples of cows and heifers at calving on 15 dairy herds in Washington and Idaho as part of a previous study (26) to evaluate different cattle importation practices on the prevalence of S. aureus IMI were utilized for the present study. The original study used herds located in the Pacific Northwest (PNW) region of the United States that were identified as being willing to participate in the study by their veterinarian. Veterinarians were contacted by mail using a mailing list provided by the American Association of Bovine Practitioners of all members located in the PNW region. Sixteen herds were initially identified, enrolled, and grouped based on cattle importation practices. One herd (herd 3) was excluded due to lack of sample collection by the herd. In the original study, all lactating cattle had composite mammary foremilk samples (aliquots of milk from all four mammary quarters comingled in a single sample vial) aseptically collected three times at approximately 4-month intervals over a 1-year period. Additional composite foremilk samples were taken from cows and heifers at the time of calving. During sample processing to identify cows with S. aureus IMI, all coagulase-positive staphylococci were stored in phosphate-buffered glycerol at −20°C; no coagulase-negative staphylococci were saved. All coagulase-positive isolates were then identified to the species level using a commercial biochemical test strip (API Staph; bioMérieux, Inc., Hazelwood, MO, USA). Isolates were either identified as S. aureus or S. hyicus by this method. The focus of the present study was the isolates originally identified as coagulase-positive S. hyicus.
Isolates were subcultured from the phosphate-buffered glycerol storage medium onto brain heart infusion agar slants (Remel, Lenexa, KS, USA) at Washington State University and shipped chilled to the University of Missouri for further characterization. At the University of Missouri, isolates were grown on Columbia blood agar (CBA) (Remel) for approximately 24 h at 37°C, and representative colonies of each isolate were coagulase tested using rabbit plasma (Thermo Scientific, Lenexa, KS, USA). Coagulase results were recorded after 4 h and 24 h of incubation at 37°C.
Confirmation of species identification by housekeeping gene sequence analysis.
A single colony was picked from CBA and inoculated into 100 μl of 1× Tris-EDTA (Promega, Madison, WI, USA) for lysate preparation. Standard PCR, with the addition of an initial denaturation step of 15 min at 94°C, was used to amplify the rpoB (18) and tuf (22) genes. The PCR for rpoB gene identification and subsequent sequencing for species identification were done on all isolates, and the PCR for tuf gene identification and subsequent sequencing were conducted only on isolates not definitively identified as S. agnetis or S. hyicus. The resultant PCR products were purified using a commercial kit (Invitrogen, Carlsbad, CA, USA) and submitted to the University of Missouri DNA Core Facility for Sanger sequencing. Sequences were compared to the GenBank database using the publicly available nucleotide-BLAST algorithm (www.ncbi.nlm.nih.gov). For rpoB analysis, species identity was confirmed if the sequence had ≥97% identity with a single species of Staphylococcus in the GenBank database (34). For tuf gene analysis, species were confirmed if sequence identity was ≥98.0% with >0.8% separation between species (22).
Multiplex PCR development.
A novel multiplex PCR was developed to differentiate S. hyicus from S. agnetis by comparing the whole-genome sequences for S. agnetis CBMRN 20813338 (24) and S. hyicus ATCC 11249T (23). The Artemis comparison tool (Wellcome Trust Sanger Institute, Hinxton, UK) was used to identify regions of difference between the two genomes. Based on these genome-wide comparisons, primer sets were designed to amplify a partial segment of the aroD gene for S. hyicus and S. agnetis (Table 3). Standard multiplex PCR, including sets of aroD gene primers to identify S. hyicus and S. agnetis, respectively, and a nuc gene primer set to identify S. aureus (35), was performed using the following thermocycler conditions: 94°C for 15 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min. The run was completed at 72°C for 5 min and then held at 4°C. After amplification, electrophoresis was completed using a 1% agarose gel, and gels were visualized by UV transillumination. A 100-bp ladder was used to determine PCR product size.
TABLE 3.
Primers used for the multiplex PCR to identify to the species level and differentiate S. hyicus, S. agnetis, and S. aureus
| Genea | Primer | Sequence (5′ to 3′) | Amplicon size (bp) | Positive species |
|---|---|---|---|---|
| aroD | aroD-HyF | TAT GGT GTC GAC CAA TCG AAG GCT | 425 | S. hyicus |
| aroD | aroD-HyR | ACC CTA TAG CCC GCT TAC TT | ||
| aroD | aroD-AgF | CGC ATG AGA GAC CAA TAC GCT | 293 | S. agnetis |
| aroD | aroD-AgR | TAG GAC GTA TAG AGG TGG | ||
| nucb | nuc-S | CTG GCA TAT GTA TGG CAA TTG TT | 664 | S. aureus |
| nucb | nuc-AS | TAT TGA CCT GAA TCA GCG TTG TCT |
aroD, 3-dehydroquinate dehydratase gene; nuc, thermonuclease gene.
See reference 35.
Testing the specificity of the novel multiplex PCR assay to differentiate S. hyicus, S. agnetis, and S. aureus.
Isolates obtained from Washington State University that were identified as S. agnetis or S. hyicus by housekeeping gene sequence (n = 44 of 62 isolates) were analyzed using the novel multiplex PCR described above. Positive controls included S. agnetis CBMRN 20813338 and DSM 23656T (6 VT), S. hyicus ATCC 11249T, and S. aureus ATCC 12600T and ATCC 29740. Additional S. agnetis strains were also included as controls, including the reference strain DSM 23658 (55 OE), and two strains (43 OE and 100 VT) reported in the original description of S. agnetis (10). Given that S. chromogenes is genotypically similar to both S. hyicus and S. agnetis, two strains of S. chromogenes (ATCC 43764T and MU 970) were tested to ensure that the multiplex PCR did not misclassify S. chromogenes as S. agnetis, S. hyicus, or S. aureus. All PCR products that amplified with the aroD gene primer sets were purified and sequenced to verify amplification of the targeted gene and to compare sequences.
Pulsed-field gel electrophoresis.
To understand subspecies variation within the tested isolates, pulsed-field gel electrophoresis was performed as previously described (36). Briefly, SmaI (Invitrogen, Carlsbad, CA, USA) digests of staphylococcal DNA were separated by PFGE in a 1% agarose gel (Pulsed Field Certified agarose; Bio-Rad, Hercules, CA) immersed in 0.5% Tris-boric acid-EDTA (TBE) buffer at 15°C for 20 h at 6 V/cm with a 5- to 50-s pulse time on a CHEF-DR III PFGE machine (Bio-Rad). Salmonella BAA-664 was used as a molecular weight size standard and was digested using XbaI (Invitrogen). Standards were included in the first, middle, and last lanes of each gel. Completed gels were stained with ethidium bromide and photographed using UV transillumination.
Data analysis.
All data were descriptive, with isolate species and strain type information expressed as absolute numbers and percentages. For the purpose of comparing the prevalence of detection of S. aureus versus coagulase-positive non-aureus Staphylococcus spp. at the cow level, S. aureus prevalence data from the original study were utilized (Table 1). Using the definitive genus and species identification data for coagulase-positive non-aureus Staphylococcus spp. and the S. aureus data from the original study (26), the cow-level prevalences for each CPS species identified from the three herd cultures and cattle at the time of calving on each herd were calculated. Herd identification numbers from the original study (26) were maintained.
Sequenced products from aroD gene PCR amplification were aligned and compared through the construction of a neighbor-joining dendrogram based on the multiple alignment similarity matrix using computer software (BioNumerics, version 7.5; Applied Maths, Kortrijk, Belgium). For interpretation of PFGE results, isolates that had indistinguishable banding patterns based on having the same number of bands and corresponding bands of the same apparent molecular weight were considered to be the same strain (37). Additionally, PFGE gel images from herds with more than one S. agnetis isolate were analyzed using BioNumerics 7.5 (Applied Math) using Dice similarity coefficient and an unweighted pair group method using average linkages (UPGMA) dendrogram type. Tolerance settings included optimization at 1.0% and position tolerance at 1.0%. Strain types were determined by using a threshold cut point of 100% similarity.
Accession number(s).
The aroD gene fragment sequences from the isolates included in the study were submitted to NCBI GenBank, accession numbers KT750097 to KT750140.
ACKNOWLEDGMENTS
We thank Suvi Taponen at the University of Helsinki for sharing DNA from four S. agnetis isolates in her collection and for reviewing the manuscript.
This research was partially funded with U.S. Department of Agriculture, National Institute for Food and Agriculture Formula funds, under project MO-AHNC0001. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Pitkälä A, Haveri M, Pyörälä S, Myllys V, Honkanen-Buzalski T. 2004. Bovine mastitis in Finland 2001–prevalence, distribution of bacteria, and antimicrobial resistance. J Dairy Sci 87:2433–2441. doi: 10.3168/jds.S0022-0302(04)73366-4. [DOI] [PubMed] [Google Scholar]
- 2.Tenhagen BA, Koster G, Wallmann J, Heuwieser W. 2006. Prevalence of mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Brandenburg, Germany. J Dairy Sci 89:2542–2551. doi: 10.3168/jds.S0022-0302(06)72330-X. [DOI] [PubMed] [Google Scholar]
- 3.Sampimon O, Barkema HW, Berends I, Sol J, Lam T. 2009. Prevalence of intramammary infection in Dutch dairy herds. J Dairy Res 76:129–136. doi: 10.1017/S0022029908003762. [DOI] [PubMed] [Google Scholar]
- 4.National Mastitis Council. 1999. Laboratory handbook on bovine mastitis, National Mastitis Council, Verona, WI [Google Scholar]
- 5.Kloos WE, Schleifer KH. 1986. Genus IV. Staphylococcus Rosenbach 1884, 18AL, p 1013–1035. In Sneath PH, Mair NS, Sharpe ME, Holt JG (ed), Bergey's manual of systematic bacteriology, vol 2 Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 6.Roberson JR, Fox LK, Hancock DD, Gay JM, Besser TE. 1996. Prevalence of coagulase-positive staphylococci, other than Staphylococcus aureus, in bovine mastitis. Am J Vet Res 57:54–58. [PubMed] [Google Scholar]
- 7.Gillespie BE, Headrick SI, Boonyayatra S, Oliver SP. 2009. Prevalence and persistence of coagulase-negative Staphylococcus species in three dairy research herds. Vet Microbiol 134:65–72. doi: 10.1016/j.vetmic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Trinidad P, Nickerson SC, Alley TK. 1990. Prevalence of intramammary infection and teat canal colonization in unbred and primigravid dairy heifers. J Dairy Sci 73:107–114. doi: 10.3168/jds.S0022-0302(90)78652-3. [DOI] [PubMed] [Google Scholar]
- 9.Fry PR, Middleton JR, Dufour S, Perry J, Scholl D, Dohoo I. 2014. Association of coagulase-negative staphylococcal species, mammary quarter milk somatic cell count, and persistence of intramammary infection in dairy cattle. J Dairy Sci 97:4876–4885. doi: 10.3168/jds.2013-7657. [DOI] [PubMed] [Google Scholar]
- 10.Taponen S, Supré K, Piessens V, Van Coillie E, De Vliegher S, Koort JM. 2012. Staphylococcus agnetis sp. nov., a coagulase-variable species from bovine subclinical and mild clinical mastitis. Int J Syst Evol Microbiol 62:61–65. doi: 10.1099/ijs.0.028365-0. [DOI] [PubMed] [Google Scholar]
- 11.Hájek VDL, Mordarski M, Goodfellow M, Pulverer G, Varaldo PE. 1978. 1986. Elevation of Staphylococcus hyicus subsp. chromogenes (Devriese et al., 1978) to species status: Staphylococcus chromogenes (Devriese et al., comb. nov. Syst Appl Microbiol 8:169–173. [Google Scholar]
- 12.Zadoks RN, Watts JL. 2009. Species identification of coagulase-negative staphylococci: genotyping is superior to phenotyping. Vet Microbiol 134:20–28. doi: 10.1016/j.vetmic.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Roberson JR, Fox LK, Hancock DD, Besser TE. 1992. Evaluation of methods for differentiation of coagulase-positive staphylococci. J Clin Microbiol 30:3217–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boerlin P, Kuhnert P, Hussy D, Schaellibaum M. 2003. Methods for identification of Staphylococcus aureus isolates in cases of bovine mastitis. J Clin Microbiol 41:767–771. doi: 10.1128/JCM.41.2.767-771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker K, Harmsen D, Mellmann A, Meier C, Schumann P, Peters G, von Eiff C. 2004. Development and evaluation of a quality-controlled ribosomal sequence database for 16S ribosomal DNA-based identification of Staphylococcus species. J Clin Microbiol 42:4988–4995. doi: 10.1128/JCM.42.11.4988-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gribaldo S, Cookson B, Saunders N, Marples R, Stanley J. 1997. Rapid identification by specific PCR of coagulase-negative staphylococcal species important in hospital infection. J Med Microbiol 46:45–53. doi: 10.1099/00222615-46-1-45. [DOI] [PubMed] [Google Scholar]
- 17.Jousson O, Di Bello D, Vanni M, Cardini G, Soldani G, Pretti C, Intorre L. 2007. Genotypic versus phenotypic identification of staphylococcal species of canine origin with special reference to Staphylococcus schleiferi subsp. coagulans. Vet Microbiol 123:238–244. doi: 10.1016/j.vetmic.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Drancourt M, Raoult D. 2002. rpoB gene sequence-based identification of Staphylococcus species. J Clin Microbiol 40:1333–1338. doi: 10.1128/JCM.40.4.1333-1338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heikens E, Fleer A, Paauw A, Florijn A, Fluit AC. 2005. Comparison of genotypic and phenotypic methods for species-level identification of clinical isolates of coagulase-negative staphylococci. J Clin Microbiol 43:2286–2290. doi: 10.1128/JCM.43.5.2286-2290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwok AY, Su SC, Reynolds RP, Bay SJ, Av-Gay Y, Dovichi NJ, Chow AW. 1999. Species identification and phylogenetic relationships based on partial HSP60 gene sequences within the genus Staphylococcus. Int J Syst Bacteriol 49:1181–1192. doi: 10.1099/00207713-49-3-1181. [DOI] [PubMed] [Google Scholar]
- 21.Woo PC, Teng JL, Wu JK, Leung FP, Tse H, Fung AM, Lau SK, Yuen KY. 2009. Guidelines for interpretation of 16S rRNA gene sequence-based results for identification of medically important aerobic Gram-positive bacteria. J Med Microbiol 58:1030–1036. doi: 10.1099/jmm.0.008615-0. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SM, Kim MS, Park KU, Song J, Kim EC. 2011. tuf gene sequence analysis has greater discriminatory power than 16S rRNA sequence analysis in identification of clinical isolates of coagulase-negative staphylococci. J Clin Microbiol 49:4142–4149. doi: 10.1128/JCM.05213-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calcutt MJ, Foecking MF, Hsieh HY, Adkins PR, Stewart GC, Middleton JR. 2015. Sequence analysis of Staphylococcus hyicus ATCC 11249T, an etiological agent of exudative epidermitis in swine, reveals a type VII secretion system locus and a novel 116-kilobase genomic island harboring toxin-encoding genes. Genome Announc 3(1):e01525-14. doi: 10.1128/genomeA.01525-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calcutt MJ, Foecking MF, Fry PR, Hsieh HY, Perry J, Stewart GC, Scholl DT, Messier S, Middleton JR. 2014. Draft genome sequence of bovine mastitis isolate Staphylococcus agnetis CBMRN 20813338. Genome Announc 2(5):e00883-14. doi: 10.1128/genomeA.00883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fry PR, Calcutt MJ, Foecking MF, Hsieh HY, Suntrup DG, Perry J, Stewart GC, Middleton JR. 2014. Draft genome sequence of Staphylococcus chromogenes strain MU 970, isolates from a case of chronic bovine mastitis. Genome Announc 2(4):e00835-14. doi: 10.1128/genomeA.00835-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middleton JR, Fox LK, Gay JM, Tyler JW, Besser TE. 2002. Use of pulsed-field gel electrophoresis for detecting differences in Staphylococcus aureus strain populations between dairy herds with different cattle importation practices. Epidemiol Infect 129:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampimon OC, Zadoks RN, De Vliegher S, Supré K, Haesebrouck F, Barkema HW, Sol J, Lam TJ. 2009. Performance of API Staph ID 32 and Staph-Zym for identification of coagulase-negative staphylococci isolated from bovine milk samples. Vet Microbiol 136:300–305. doi: 10.1016/j.vetmic.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Bes M, Guerin-Faublee V, Meugnier H, Etienne J, Freney J. 2000. Improvement of the identification of staphylococci isolated from bovine mammary infections using molecular methods. Vet Microbiol 71:287–294. doi: 10.1016/S0378-1135(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 29.Park JY, Fox LK, Seo KS, McGuire MA, Park YH, Rurangirwa FR, Sischo WM, Bohach GA. 2011. Comparison of phenotypic and genotypic methods for the species identification of coagulase-negative staphylococcal isolates from bovine intramammary infections. Vet Microbiol 147:142–148. doi: 10.1016/j.vetmic.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jasper DE, Infante F, Dellinger JD. 1985. Efficacy of the API Staph-Ident system for identification of Staphylococcus species from milk. Am J Vet Res 46:1263–1267. [PubMed] [Google Scholar]
- 31.Servos S, Chatfield S, Hone D, Levine M, Dimitriadis G, Pickard D, Dougan G, Fairweather N, Charles I. 1991. Molecular cloning and characterization of the aroD gene encoding 3-dehydroquinase from Salmonella Typhi. J Gen Microbiol 137:147–152. doi: 10.1099/00221287-137-1-147. [DOI] [PubMed] [Google Scholar]
- 32.De Visscher A, Piepers S, Haesebrouck F, De Vliegher S. 2016. Intramammary infection with coagulase-negative staphylococci at parturition: species-specific prevalence, risk factors, and effect on udder health. J Dairy Sci 99:6457–6469. doi: 10.3168/jds.2015-10458. [DOI] [PubMed] [Google Scholar]
- 33.Taponen S, Nykäsenoja S, Pohjanvirta T, Pitkälä A, Pyörälä S. 2016. Species distribution and in vitro antimicrobial susceptibility of coagulase-negative staphylococci isolated from bovine mastitic milk. Acta Vet Scand 58:12. doi: 10.1186/s13028-016-0193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellmann A, Becker K, von Eiff C, Keckevoet U, Schumann P, Harmsen D. 2006. Sequencing and staphylococci identification. Emerg Infect Dis 12:333–336. doi: 10.3201/eid1202.050962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graber HU, Casey MG, Naskova J, Steiner A, Schaeren W. 2007. Development of a highly sensitive and specific assay to detect Staphylococcus aureus in bovine mastitic milk. J Dairy Sci 90:4661–4669. doi: 10.3168/jds.2006-902. [DOI] [PubMed] [Google Scholar]
- 36.Middleton JR, Fox LK, Gay JM, Tyler JW, Besser TE. 2002. Influence of Staphylococcus aureus strain-type on mammary quarter milk somatic cell count and N-acetyl-beta-d-glucosaminidase activity in cattle from eight dairies. J Dairy Sci 85:1133–1140. doi: 10.3168/jds.S0022-0302(02)74175-1. [DOI] [PubMed] [Google Scholar]
- 37.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]