ABSTRACT
Serological diagnosis of Zika virus is challenging due to high cross-reactivity of Zika virus with other flavivirus antibodies. Recently, a Zika NS1-based enzyme-linked immunosorbent assay (ELISA) was developed and shown to be highly specific for Zika antibody detection; however, sensitivity was evaluated for only a small number of confirmed Zika-infected patients. In this study, we measured the sensitivity and kinetics of Zika IgM and IgG antibodies using the Zika NS1-based ELISA in 105 samples from 63 returning travelers infected with Zika virus (proven by PCR or neutralization assay) from Israel, Czech Republic, Italy, Belgium, Germany, and Chile. Zika virus IgM was detected from 2 to 42 days post-symptom onset (PSO) with an overall sensitivity of 79% in the first month and 68% until 2 months PSO, while IgG antibodies were detected from 5 days to 3 years PSO with 79% sensitivity. Interestingly, significant differences in IgM sensitivity and IgM detection period were observed between Israeli and European/Chilean Zika-infected travelers, adding to the complexity of Zika infection diagnosis and suggesting that other diagnostic methods should be complemented to reduce false-negative results.
KEYWORDS: Zika, NS1, ELISA, travelers, sensitivity
INTRODUCTION
Zika virus (ZIKV) is a Mosquito-borne flavivirus that has spread in the last year to >50 countries and territories throughout the Americas (1). Laboratory diagnosis of ZIKV infection is based on detection of Zika RNA in body fluids, such as serum, urine, saliva, and whole blood, or detection of IgM and IgG antibodies against ZIKV in serum. However, Zika diagnosis is challenging due to high cross-reactivity of Zika with other flavivirus antibodies and the limited time that ZIKV RNA is detected in body fluids (2–5).
ZIKV has a single positive-sense RNA genome that is translated into 3 structural (C, PrM, and E) and 7 nonstructural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins. Nonstructural protein 1 (NS1) forms a homodimer inside the cells and is necessary for viral replication and late infection. NS1 is also secreted by infected cells into the bloodstream, interacting with and stimulating components of the immune system to produce NS1 antibodies (6).
Recently, an enzyme-linked immunosorbent assay (ELISA) based on the detection of antibodies against ZIKV NS1 antigen has been developed and shown to be a highly specific tool for the serodiagnosis of ZIKV infections, eliminating cross-reactions with antibodies to dengue virus (DENV) and other flaviviruses (7). In this study, we investigated the sensitivity of detection and the kinetics of Zika IgM and IgG antibodies in Zika-infected returning travelers, evaluating them with Zika NS1-based ELISA.
RESULTS
In order to examine the sensitivity of the Zika NS1-based ELISA, we obtained 105 samples from 63 ZIKV-infected travelers returning from areas where ZIKV is endemic. Thirty-three samples were obtained from residents of Israel, 38 from the Czech Republic, 13 from central Italy, 9 from Belgium, 6 from Chile, and 6 from Germany. Diagnosis of all Zika patients was performed in their resident country either by detection of ZIKV RNA in whole blood, urine, semen, or serum samples (18 patients) (4), by neutralization assay (26 patients), or by both Zika RNA detection and neutralization (19 patients) (Table 1). Some of the positive PCR patients were also sequenced (8). All samples from Zika-positive patients were subjected to Zika NS1-based ELISA in the patient's resident country.
TABLE 1.
Results of Zika NS1-based ELISA from 63 travelers with neutralization or RT-PCR positive Zika virus infection
| Patient no. | Country of origin | Country of acquisition | Gender/age (yr)a | RT-PCR resultb | Neutralization resultb | 1st sampleb |
2nd sampleb |
3rd sampleb |
4th sampleb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time from onset (days) | IgM | IgG | Time from onset (days) | IgM | IgG | Time from onset (days) | IgM | IgG | Time from onset (days) | IgM | IgG | ||||||
| 1 | Czech Republic | Martinique | F/38 | Neg | Pos | 3 | Pos | Neg | 33 | Equ | Pos | ||||||
| 2 | Czech Republic | Martinique | F/43 | Neg | Pos | 5 | Equ | Neg | 59 | Neg | Pos | ||||||
| 3 | Czech Republic | Dominican Republic | F/49 | Pos | Pos | 5 | Neg | Neg | 10 | Pos | Pos | 34 | Pos | Pos | |||
| 4 | Czech Republic | Martinique | M/58 | Pos | Pos | 14 | Pos | Neg | 27 | Pos | Pos | 42 | Pos | Pos | |||
| 5 | Czech Republic | Martinique | M/44 | Neg | Pos | 13 | Pos | Equ | 38 | Pos | Pos | ||||||
| 6 | Czech Republic | Martinique | M/41 | Neg | Pos | 19 | Pos | Pos | 34 | Pos | Pos | ||||||
| 7 | Czech Republic | Martinique | F/42 | NDc | Pos | 31 | Pos | Pos | |||||||||
| 8 | Czech Republic/Slovakia | Barbados, St. Vincent, St. Lucia | M/33 | Neg | Pos | 8 | Pos | Equ | 31 | Pos | Pos | ||||||
| 9 | Czech Republic | Dominican Republic | F/64 | Pos | Pos | 4 | Pos | Neg | |||||||||
| 10 | Czech Republic | Guadeloupe | F/43 | Pos | Pos | 3 | Neg | Neg | 7 | Pos | Equ | 15 | Pos | Pos | 35 | Pos | Pos |
| 11 | Czech Republic/Slovakia | Guadeloupe | M/41 | Pos | Pos | 3 | Neg | Neg | 17 | Pos | Pos | ||||||
| 12 | Czech Republic | Nicaragua | M/31 | Pos (semen) | Pos | 11 | Pos | Pos | 15 | Pos | Pos | 61 | Neg | Pos | |||
| 13 | Czech Republic/Israel | Mexico | M/26 | Neg | Pos | 14 | Pos | Neg | |||||||||
| 14 | Czech Republic | Nicaragua | F/31 | Pos | Pos | 4 | Neg | Neg | 6 | Pos | Neg | 21 | Equ | Pos | |||
| 15 | Czech Republic | Mexico | F/23 | Neg | Pos | 26 | Pos | Pos | |||||||||
| 16 | Czech Republic/France | Martinique | M/40 | Pos | Equ | 3 | Neg | Neg | |||||||||
| 17 | Czech Republic | Martinique | M/7 | ND | Pos | 33 | Neg | Pos | |||||||||
| 18 | Czech Republic | Nicaragua | F/34 | ND | Pos | 182 | Neg | Equ | |||||||||
| 19 | Czech Republic | Colombia | M/32 | ND | Pos | ∼360 | Neg | Equ | |||||||||
| 20 | Czech Republic | Colombia | F/27 | ND | Pos | ∼360 | Neg | Equ | |||||||||
| 21 | Czech Republic | French Polynesia | M/65 | ND | Pos | ∼1,000 | Neg | Pos | |||||||||
| 22 | Belgium | Venezuela | M/45 | Pos | ND | 4 | Neg | Neg | 11d | Pos | Equ | ||||||
| 23 | Belgium | Venezuela | M/4 | Pos | ND | 3d | Neg | Neg | |||||||||
| 24 | Belgium | Brazil | F/28 | Pos | ND | 11d | Pos | Pos | 22 | Pos | Pos | ||||||
| 25 | Belgium | Martinique | M/66 | Neg | Pos | 20 | Pos | Pos | |||||||||
| 26 | Belgium | Martinique | F/66 | Neg | Pos | 23 | Pos | Pos | |||||||||
| 27 | Belgium | Martinique | M/46 | Neg | Pos | 19 | Pos | Pos | |||||||||
| 28 | Belgium | Suriname | F/20 | Neg | Pos | ±80 | Neg | Pos | |||||||||
| 29 | Chile | Brazil | F/25 | Pos | ND | 1 | Neg | Neg | |||||||||
| 30 | Chile | Brazil | M/35 | Pos | ND | 3 | Neg | Neg | |||||||||
| 31 | Chile | Venezuela | F/17 | Pos | ND | 4 | Pos | Neg | |||||||||
| 32 | Chile | Colombia | M/40 | Pos | ND | 6 | Pos | Neg | |||||||||
| 33 | Chile | Dominican Republic | M/39 | Pos | ND | 2 | ND | ND | 240 | ND | Neg | ||||||
| 34 | Chile | Costa Rica | M/25 | Pos | ND | 2 | Neg | Neg | |||||||||
| 35 | Italy | Thailand | M/32 | Neg | Pos | 5 | Pos | ND | 39 | Equ | ND | 110 | Neg | ND | |||
| 36 | Italy | Italye | F/30 | Neg | Pos | 2 | Pos | ND | 36 | Pos | ND | 90 | Neg | ND | |||
| 37 | Italy | Brazil | M/74 | ND | Pos | 40 | Pos | ND | |||||||||
| 38 | Italy | Haiti | M/34 | ND | Pos | 16 | Pos | ND | |||||||||
| 39 | Italy | Venezuela | F/42 | Pos | Pos | 7 | Pos | Pos | 14 | Pos | Pos | 49 | Equ | Pos | |||
| 40 | Italy | Martinique, Guadalupe | F/34 | Pos | Equ | 4 | Equ | ND | |||||||||
| 41 | Italy | Dominican Republic | F/51 | Pos | Pos | 7 | Equ | ND | |||||||||
| 42 | Italy | Venezuela | F/32 | Neg | Pos | 75 | Neg | ND | |||||||||
| 43 | Italy | Venezuela | M/30 | ND | Pos | 79 | Neg | ND | |||||||||
| 44 | Italy | Brazil | F/37 | ND | Pos | 62 | Neg | ND | |||||||||
| 45 | Israel | Colombia | F/50 | Pos | Pos | 5 | Pos | Pos | 20 | Neg | Pos | ||||||
| 46 | Israel | Colombia | F/32 | Pos | Pos | 10 | Pos | Pos | 53 | Neg | Pos | ||||||
| 47 | Israel | Vietnam | M/61 | Pos | Neg | 10 | Pos | Neg | 58 | Neg | Neg | ||||||
| 48 | Israel | Dominican Republic | M/30 | Pos | Pos | 26 | Pos | Pos | 46 | Neg | Pos | ||||||
| 49 | Israel | Guatemala, Mexico | F/30 | Pos | Neg | 26 | Neg | Neg | 48 | Neg | Neg | ||||||
| 50 | Israel | Colombia | M/38 | Neg | Pos | 34 | Equ | Pos | |||||||||
| 51 | Israel | Jamaica | M/23 | Pos | Pos | 11 | Pos | Equ | 25 | Pos | Pos | 49 | Neg | Pos | |||
| 52 | Israel | USA (Miami) | F/30 | Pos | Pos | 8 | Pos | Neg | |||||||||
| 53 | Israel | Costa Rica | M/27 | Pos | Pos | 7 | Pos | Neg | 18 | Equ | Equ | 37 | Neg | Pos | |||
| 54 | Israel | Mexico | M/26 | Pos | Pos | 27 | Equ | Pos | 52 | Neg | Pos | ||||||
| 55 | Israel | Mexico | F/30 | Pos | Pos | 12 | Pos | Equ | 15 | Pos | Pos | 29 | Equ | Pos | 50 | Neg | Pos |
| 56 | Israel | Mexico | M/37 | Pos | Pos | 5 | Neg | Neg | 14 | Pos | Neg | 28 | Pos | Pos | 59 | Neg | Neg |
| 57 | Israel | Costa Rica | M/26 | Pos | Pos | 6 | Neg | Neg | 11 | Pos | Neg | ||||||
| 58 | Israel | Costa Rica | M/20 | Neg | Pos | 29 | Pos | Pos | |||||||||
| 59 | Israel | Mexico/Cuba | F/21 | Pos | ND | 6 | Neg | Neg | 16 | Pos | Pos | ||||||
| 60 | Germany | Martinique | M/56 | Pos | ND | 3 | Pos | Neg | 8 | Pos | Pos | ||||||
| 61 | Germany | Martinique | F/53 | Pos | ND | 12 | Equ | Neg | 16 | ND | Pos | ||||||
| 62 | Germany | Guadeloupe | M/33 | Pos | ND | 11 | Pos | Neg | |||||||||
| 63 | Germany | Guadeloupe | F/29 | Pos | ND | 6 | Pos | Neg | |||||||||
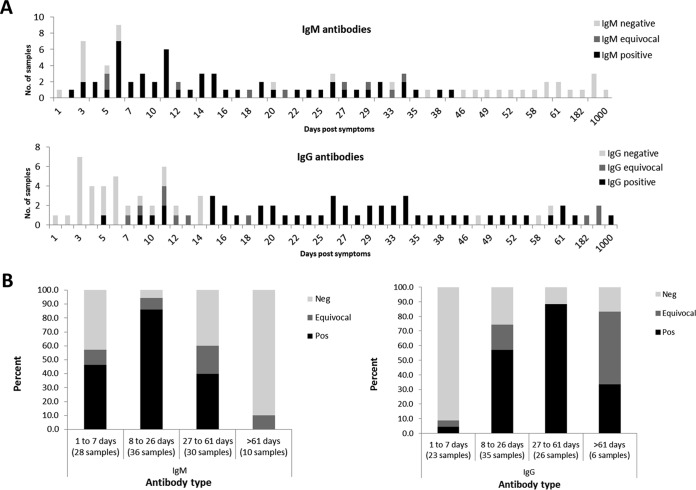
Table 1 summarizes Zika NS1 ELISA IgM and IgG antibody data obtained from all patients. Our results (Table 1 and Fig. 1A) demonstrate that IgM antibodies can be detected from as early as 2 days until 42 days from the onset of symptoms while IgG antibodies are first detected at day 5 and can still be present 3 years post-symptom onset (PSO), although this was observed for only 1 patient (Fig. 1A). Overall, IgM sensitivity during the first month (from day 2 to 26 days PSO) and until 61 days PSO was found to be 79% and 68%, respectively, while IgG sensitivity starting from day 8 post-symptom onset was 79% (Fig. 1B). The combined sensitivity of IgM and IgG was 81% from day 1 and 88% from day 5 PSO.
FIG 1.
Overall performance of Zika NS1-based ELISA. A total of 105 samples from ZIKV-infected travelers returning from areas where ZIKV is endemic were tested with IgM and IgG Zika NS1-based ELISA. Number of samples for each day post-symptom onset (A) and percentage of samples obtained 1 to 7, 8 to 26, 27 to 61, and >61 days post-symptom onset (B) are presented. Negative (Neg), positive (Pos), and equivocal IgM and IgG antibodies are indicated.
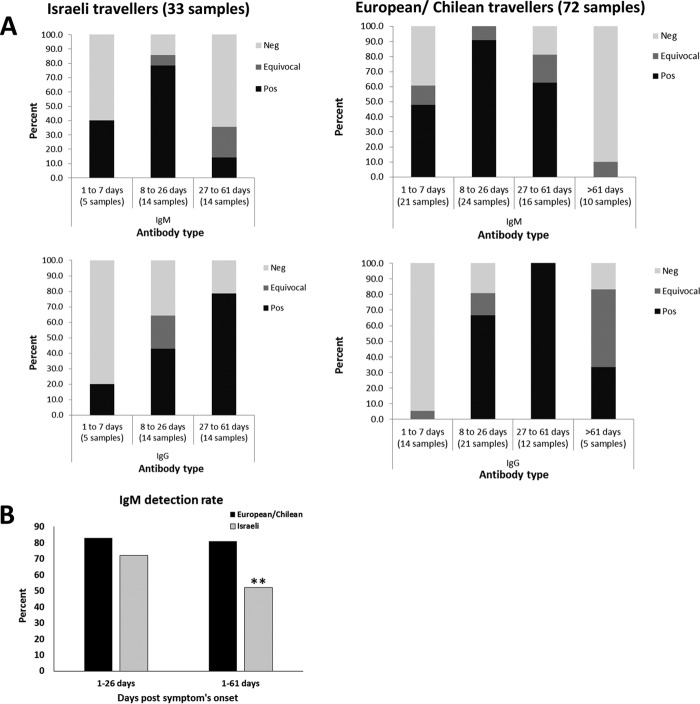
Interestingly, a marked difference was observed in the IgM sensitivities of Israeli and European/Chilean travelers (Fig. 2). In the first month (until 26 days PSO), the sensitivity of IgM antibodies was 83% in European/Chilean travelers, which was not significantly higher than the 72% sensitivity observed for Israeli travelers. However, a significant difference was observed between Israeli and European/Chilean travelers in the first 61 days PSO with sensitivities of 81% and 52%, respectively (Fig. 2B). The IgG sensitivity of samples obtained more than 7 days PSO was 85% in European/Chilean travelers, which is not significantly different from the 68% sensitivity observed for Israeli travelers. In both groups, the highest detection rate was observed between 8 and 26 days PSO for IgM and 27 and 61 days PSO for IgG (Fig. 2). It is important to note that several samples from Zika-infected Israeli travelers that were tested in 3 different laboratories yielded similar results, reducing the possibility for technical variation factor between labs. Comparable results and differential sensitivities between the 2 groups of travelers were also observed when taking into account only samples from Zika real-time PCR (RT-PCR)-positive patients (without including samples from patients detected by neutralization assay).
FIG 2.
Differential sensitivity of IgM antibodies in Zika NS1-based ELISA. Samples obtained from travelers from Israel and European/Chilean countries with neutralization or RT-PCR-positive ZIKV infection were subjected to IgM and IgG Zika NS1-based ELISA. (A) Percent of negative (Neg), positive (Pos), and equivocal IgM and IgG antibody results from samples obtained 1 to 7, 8 to 26, 27 to 61, and >61 days post-symptom onset are presented. Number of samples in each time frame is indicated. (B) IgM detection rate of samples obtained from Zika-Infected patients; 1 to 26 and 1 to 61 days post-symptom onset in European/Chilean and Israeli travelers are presented. **, P value of <0.05.
DISCUSSION
Accumulating evidence suggests that ZIKV infection during pregnancy is a cause of congenital brain abnormalities, including microcephaly (9). Recently, congenital ZIKV infection without microcephaly at birth has been reported (10) as has postnatal development of microcephaly in infants presumed to be infected congenitally (11). Consequently, follow-up ultrasound examinations may not detect all Zika infection abnormalities, and other highly specific and sensitive diagnostic methods for Zika infection must be implemented. Since ZIKV RNA, like that of other flaviviruses, can be detected for only a short time post-symptom onset (although we have shown that in rare cases Zika RNA in whole blood can still be detected 2 months after onset of symptoms [4]), a negative PCR result should be followed by serological testing.
The serological tests that are currently available for Zika diagnosis are designed to detect IgM and IgG antibodies for either the envelope or the NS1 proteins. The envelope-based tests are sensitive but lack specificity due to high cross-reactivity with other flaviviruses, primarily dengue (3), and therefore are considered nonspecific and require confirmation with neutralization assay, which is time-consuming and unavailable in many laboratories (12). The new method of NS1-based ELISA was demonstrated to be highly specific with almost no cross-reactivity with other flaviviruses (7). Additionally, in a recent publication, high IgM (100%) and lower IgG (60%) sensitivities were observed in RT-PCR-confirmed ZIKV-infected travelers returning from areas where ZIKV is endemic (13); however, these data were collected from only 8 samples, and antibody kinetics were not shown. Here, we examined the performance of IgM and IgG antibody detection by the NS1 ELISA for 105 samples obtained from 63 travelers returning from countries where Zika is endemic. Our results point to 79% sensitivity of IgM in the first 26 days PSO, suggesting that false-negative results in IgM detection may occur. Similarly, 79% sensitivity was observed for IgG testing starting from day 8 PSO.
A major advantage of our study was the availability of several follow-up samples from many of the Zika-infected patients and the high number of samples tested (105), which allowed us to assess the overall duration of detectable IgM and IgG antibodies against NS1. The detection time frame of IgM antibodies suggests that NS1 IgM testing is useful only in the first month after symptom onset, whereas IgG antibodies should be tested at least 8 days PSO in order to allow maximum sensitivity.
Of particular interest is the significant difference in IgM sensitivity of the Zika NS1-based ELISA observed in this study between Israeli and European/Chilean travelers. Recently, only 41.7% anti-NS1 IgM sensitivity was detected in RT-PCR-confirmed ZIKV infection among residents of areas where ZIKV is endemic (13). In Israel, a 41% prevalence of West Nile virus (WNV) antibodies has been observed in adults (14), and therefore, many Israeli travelers may have WNV background immunity. Due to cross-reactivity with ZIKV, we could not test the actual WNV immunity in this cohort. Based on these data, it is not farfetched to speculate that ZIKV infection in Israeli travelers may generate lower levels of IgM antibodies compared to European/Chilean travelers and as a result IgM antibodies are lower than the threshold of the NS1-based ELISA in samples from Israeli travelers, particularly when starting to decline. This may be the reason for the difference in sensitivities observed specifically between 1 and 2 months after symptom onset. Conversely, the high flavivirus background present in several European countries, such as the 26.3% seroprevalence of tick-borne encephalitis (TBE) in several areas of the Czech Republic (15), indicates that other factors may impact the differential NS1 antibody sensitivity observed between Israeli and European/Chilean travelers. Future studies should investigate the performance of NS1-based Zika serology kits for Zika-infected patients with known flavivirus history to assess the effect of different flavivirus backgrounds on Zika diagnosis.
The limited sensitivity of the NS1 IgM and IgG tests measured in our study suggests that some of the Zika-infected patients may be undetected using this assay alone. Combined testing of both IgM and IgG antibodies for all samples obtained from Zika-suspected patients more than 5 days PSO has an 88% detection rate and, therefore, should be recommended. However, since analysis of Zika RNA in both urine (2) and whole blood (4) has been shown to be effective in the first weeks after symptom onset, our results confirm that both molecular and ELISA NS1 serological tests should be performed in acute (up to 2 months) ZIKV-suspected patients.
From the beginning of the outbreak, Zika turned out to be an elusive virus and a complicated algorithm is required for optimal diagnosis of infection. Despite its high specificity (6, 11), the study performed here underscores the limitations of the current Zika NS1-based ELISA. Therefore, other serological diagnosis tests that would increase the sensitivity of Zika antibody detection should be developed.
MATERIALS AND METHODS
Zika patients and samples.
A total of 105 samples from 63 ZIKV-infected travelers returning from areas where ZIKV is endemic were tested with ZIKV NS1-based ELISA. The median age of the 29 female patients was 32 and overall age ranged between 17 and 66, while the 34 male patients had a median age of 35 and ranged between 4 and 74. Symptoms were mostly comprised of rash, fever, and arthralgia or myalgia. PSO is defined as the number of days since the start of symptoms. None of the patients were pregnant. All samples arrived to the lab at 4°C, were tested for Zika serology, and were consequently stored at −20°C or −80°C.
All patient samples were tested for ZIKV infection with RT-PCR, NS1-based ELISA, and neutralization assay in their country of origin as depicted in Table 1. Patients were tested at the Central Virology Laboratory in Israel; at the Institute of Public Health in Ostrava, Czech Republic; at the Istituto Superiore di Sanità in Rome, Italy; at the Institute of Tropical Medicine in Antwerp, Belgium; at the University Medical Centre in Hamburg, Germany; and at the Pontificia Universidad Catolica de Chile in Chile.
Zika RT-PCR.
Zika RNA in samples obtained from Israeli travelers was detected as previously described (4). In Italy, Zika RNA was detected as published in reference 3, in Belgium as published in reference 16, and in the Czech Republic by the RealStar ZIKV RT-PCR kit (Altona diagnostics GMBH, Hamburg, Germany).
Zika neutralization assay.
For neutralization, samples tested in Israel were subjected to microneutralization assay (17) with 100 50% tissue culture infective dose (TCID50) Zika MR-677. Samples from the Czech Republic were tested by virus neutralization assay in micromodification (18) with 100 TCID50 Zika MR-677. Samples from Belgium were tested with a virus neutralization (VNT) using Vero cells and 90% neutralizing titer (NT90) (16), while plaque reduction neutralization (PRNT) was performed for samples from Italy (15).
ZIKV NS1-based ELISA.
Zika-positive patients were subjected to Euroimmun ZIKV ELISA (Euroimmun, Lübeck, Germany) according to the manufacturer's recommendations. In brief, serum samples were diluted 1:101 in sample buffer and incubated at 37°C for 60 min. Before IgM detection, serum samples were preincubated with sample buffer containing rheumatoid factor absorbent as recommended. After several incubation and washing steps, the optical density (OD) was measured in a Tecan Sunrise system (Austria, GMBH). A signal-to-cutoff ratio was calculated, and values of <0.8 were regarded as negative, values of ≥0.8 to <1.1 were regarded as borderline, and value of ≥1.1 were regarded as positive.
Statistical analysis.
Fisher's exact test was used to compare results of Israeli and European/Chilean travelers. Statistical significance was defined as a P value of <0.05 as indicated.
ACKNOWLEDGMENT
We declare no conflicts of interest.
REFERENCES
- 1.Fauci AS, Morens DM. 2016. Zika virus in the Americas–yet another arbovirus threat. N Engl J Med 374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 2.Gourinat AC, O'Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. 2015. Detection of Zika virus in urine. Emerg Infect Dis 21:84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lustig Y, Mendelson E, Paran N, Melamed S, Schwartz E. 2016. Detection of Zika virus RNA in whole blood of imported Zika virus disease cases up to 2 months after symptom onset, Israel, December 2015 to April 2016. Euro Surveill 21(26):pii=30269 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22515. [DOI] [PubMed] [Google Scholar]
- 5.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. 2015. Detection of Zika virus in saliva. J Clin Virol 68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Rastogi M, Sharma N, Singh SK. 2016. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol J 13:131. doi: 10.1186/s12985-016-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huzly D, Hanselmann I, Schmidt-Chanasit J, Panning M. 2016. High specificity of a novel Zika virus ELISA in European patients after exposure to different flaviviruses. Euro Surveill 21(16):pii=30203 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21450. [DOI] [PubMed] [Google Scholar]
- 8.Meltzer E, Lustig Y, Leshem E, Levy R, Gottesman G, Weissmann R, Rabi DH, Hindiyeh M, Koren R, Mendelson E, Schwartz E. 2016. Zika virus disease in traveler returning from Vietnam to Israel. Emerg Infect Dis 22:1521–1522. doi: 10.3201/eid2208.160480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. 2016. Zika and the risk of microcephaly. N Engl J Med 375:1–4. doi: 10.1056/NEJMp1605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franca GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, Nunes ML, Castro MC, Serruya S, Silveira MF, Barros FC, Victora CG. 2016. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 11.Moura da Silva AA, Ganz JS, Sousa PD, Doriqui MJ, Ribeiro MR, Branco MD, Queiroz RC, Pacheco MJ, Vieira da Costa FR, Silva FS, Simoes VM, Pacheco MA, Lamy-Filho F, Lamy ZC, Soares de Britto EAMT. 2016. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis 22:1953–1956. doi: 10.3201/eid2211.160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landry ML, St George K. 2017. Laboratory diagnosis of Zika virus infection. Arch Pathol Lab Med 141:60–67. doi: 10.5858/arpa.2016-0406-SA. [DOI] [PubMed] [Google Scholar]
- 13.Steinhagen K, Probst C, Radzimski C, Schmidt-Chanasit J, Emmerich P, van Esbroeck M, Schinkel J, Grobusch MP, Goorhuis A, Warnecke JM, Lattwein E, Komorowski L, Deerberg A, Saschenbrecker S, Stocker W, Schlumberger W. 2016. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: a multicohort study of assay performance, 2015 to 2016. Euro Surveill 21(50):pii=30426 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen D, Zaide Y, Karasenty E, Schwarz M, LeDuc JW, Slepon R, Ksiazek TG, Shemer J, Green MS. 1999. Prevalence of antibodies to West Nile fever, sandfly fever Sicilian, and sandfly fever Naples viruses in healthy adults in Israel. Public Health Rev 27:217–230. [PubMed] [Google Scholar]
- 15.Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, Trotta M, Rizzo C, Mantella A, Rezza G, Bartoloni A. 2016. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill 21(8):pii=30148 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21395. [DOI] [PubMed] [Google Scholar]
- 16.De Smet B, Van den Bossche D, van de Werve C, Mairesse J, Schmidt-Chanasit J, Michiels J, Arien KK, Van Esbroeck M, Cnops L. 2016. Confirmed Zika virus infection in a Belgian traveler returning from Guatemala, and the diagnostic challenges of imported cases into Europe. J Clin Virol 80:8–11. doi: 10.1016/j.jcv.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Lustig Y, Mannasse B, Koren R, Katz-Likvornik S, Hindiyeh M, Mandelboim M, Dovrat S, Sofer D, Mendelson E. 2016. Superiority of West Nile virus RNA detection in whole blood for diagnosis of acute infection. J Clin Microbiol 54:2294–2297. doi: 10.1128/JCM.01283-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litzba N, Zelena H, Kreil TR, Niklasson B, Kuhlmann-Rabens I, Remoli ME, Niedrig M. 2014. Evaluation of different serological diagnostic methods for tick-borne encephalitis virus: enzyme-linked immunosorbent, immunofluorescence, and neutralization assay. Vector Borne Zoonotic Dis 14:149–159. doi: 10.1089/vbz.2012.1287. [DOI] [PubMed] [Google Scholar]