ABSTRACT
Rapid molecular diagnostics have great potential to limit the spread of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) (M/XDR-TB). These technologies detect mutations in the Mycobacterium tuberculosis genome that confer phenotypic drug resistance. However, there have been few data published regarding the relationships between the detected M. tuberculosis resistance mutations and M/XDR-TB treatment outcomes, limiting our current ability to exploit the full potential of molecular diagnostics. We analyzed clinical, microbiological, and sequencing data for 451 patients and their clinical isolates collected in a multinational, observational cohort study to determine if there was an association between M. tuberculosis resistance mutations and patient mortality. The presence of an rrs 1401G mutation was associated with significantly higher odds of patient mortality (adjusted odds ratio [OR] = 5.72; 95% confidence interval [CI], 1.65 to 19.84]) after adjusting for relevant patient clinical characteristics and all other resistance mutations. Further analysis of mutations, categorized by the associated resistance level, indicated that the detection of mutations associated with high-level fluoroquinolone (OR, 3.99 [95% CI, 1.10 to 14.40]) and kanamycin (OR, 5.47 [95% CI, 1.64 to 18.24]) resistance was also significantly associated with higher odds of patient mortality, even after accounting for clinical site, patient age, reported smoking history, body mass index (BMI), diabetes, HIV, and all other resistance mutations. Specific gyrA and rrs resistance mutations, associated with high-level resistance, were associated with patient mortality as identified in clinical M. tuberculosis isolates from a diverse M/XDR-TB patient population at three high-burden clinical sites. These results have important implications for the interpretation of molecular diagnostics, including identifying patients at increased risk for mortality during treatment. (This study has been registered at ClinicalTrials.gov under registration no. NCT02170441.)
KEYWORDS: Mycobacterium tuberculosis, diagnostics, molecular genetics, multidrug resistance
INTRODUCTION
Molecular diagnostics, with their ability to rapidly detect mutations in bacterial genes, have great potential to shorten the time between multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) (M/XDR-TB) diagnosis and appropriate treatment. These technologies identify mutations in the genome of Mycobacterium tuberculosis that confer phenotypic drug resistance, as defined by current phenotypic drug susceptibility testing (DST) at a single, “critical concentration” of the relevant drug compounds. However, recent studies have demonstrated that different M. tuberculosis mutations, even those occurring within the same gene region or codon, can confer different levels of phenotypic resistance to antituberculous drugs (as determined by quantitative DST, or by MIC testing, in solid or liquid media) (1–5). While studies correlating particular M. tuberculosis mutations with quantitative phenotypic resistance levels have helped clinicians to better understand the likely clinical relevance of molecular diagnostic assay results, it is still unclear whether specific mutations are directly associated with poor patient clinical outcomes.
As M. tuberculosis diagnostic standards move toward genotypic DST, it is becoming increasingly important to understand the complex clinical relevance of a diverse set of tuberculosis (TB) resistance mutations rather than to rely on their presence only as a predictor of phenotypic resistance as defined by a single drug concentration. However, few studies to date have investigated the associations between specific M. tuberculosis resistance mutations and patient outcomes (6–10) or have evaluated the clinical relevance of these mutations in the broader context of comprehensive M. tuberculosis genotypic resistance profiles (M/XDR-TB genotypes) and clinical variables. The Global Consortium for Drug-resistant TB Diagnostics (GCDD) conducted a large, multisite observational cohort study evaluating the diagnostic performance of different rapid diagnostics (11, 12), including a pyrosequencing assay, for M/XDR-TB diagnosis and followed up with the patients 52 weeks after enrollment to determine clinical outcomes. This study investigated the clinical characteristics associated with mortality and examined the associations between different M. tuberculosis mutations and patient mortality in the GCDD patient population.
RESULTS
Study population.
A total of 1,128 patients with risk factors for DR-TB were enrolled in our initial study from April 2012 to June 2013 (Fig. 1) (12). Clinical characteristics of this patient population at enrollment have been described in detail previously (11). Among the patients in the enrolled population, 518 (45.9%) transferred out or were lost to follow-up before the 52-week follow-up period. Another 159 patients were found to lack relevant clinical data and were excluded from analyses. Complete patient clinical and outcome data were available for 451 (40.0%) of the original 1,128 enrolled patients (Table 1).
FIG 1.
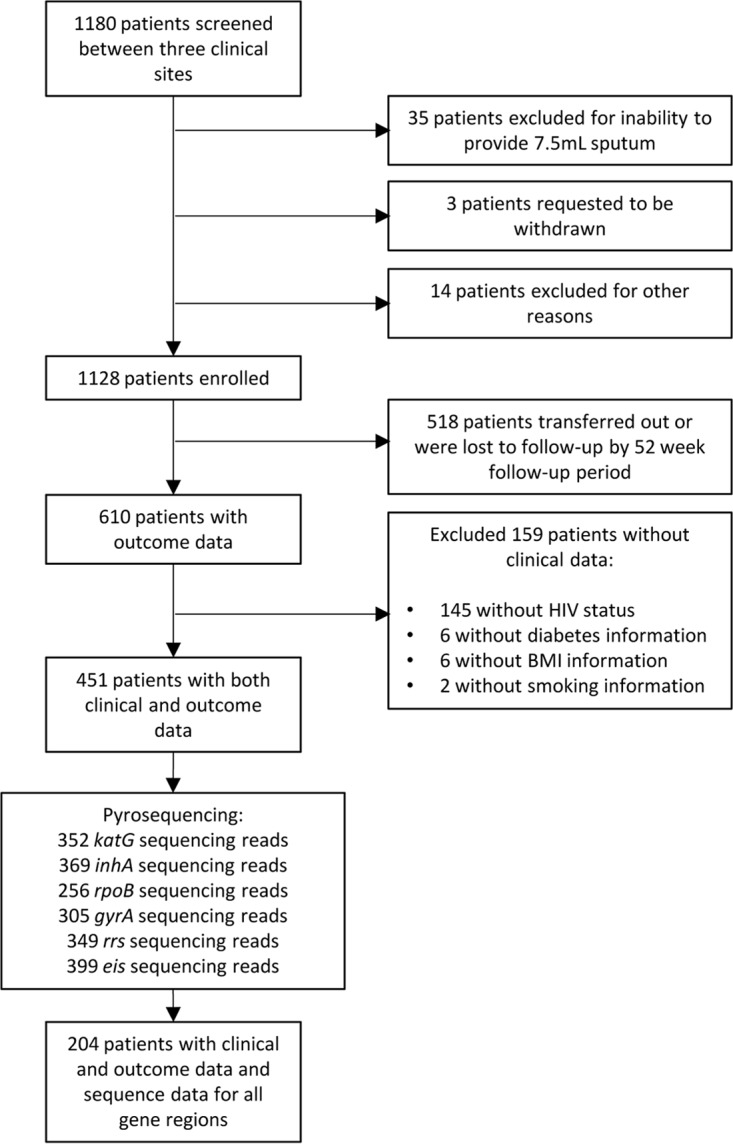
Schematic presentation of data availability for patient outcome analyses.
TABLE 1.
Associations between baseline clinical characteristics and patient mortality by 52-week follow-up period for patients with complete sequencing data for at least one gene of interest (n = 451)
| Characteristic or parameter | No. of patients | No. deceased | % deceased | OR (95% CI)a |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| Total no. of patients | 451 | 88 | 19.5 | ||
| Sex | |||||
| Female | 155 | 31 | 20.0 | 1.00 | 1.00 |
| Male | 296 | 57 | 19.3 | 0.95 (0.59–1.55) | 1.37 (0.78–2.42) |
| Age (yrs) | |||||
| <25 | 90 | 20 | 22.2 | 1.00 | 1.00 |
| 25 to 49 | 271 | 54 | 19.9 | 0.87 (0.49–1.55) | 1.12 (0.58–2.15) |
| ≥50 | 90 | 14 | 15.6 | 0.64 (0.30–1.37) | 1.19 (0.49–2.86) |
| BMI | |||||
| <18.50 | 210 | 57 | 27.1 | 1.00 | 1.00 |
| 18.50 to <25 | 212 | 25 | 11.8 | 0.36 (0.21–0.60)** | 0.40 (0.23–0.69)** |
| ≥25 | 29 | 6 | 20.7 | 0.70 (0.27–1.81) | 0.88 (0.30–2.54) |
| Smoked in previous 3 mo | |||||
| No | 314 | 69 | 22.0 | 1.00 | 1.00 |
| Yes | 137 | 19 | 13.9 | 0.57 (0.33–0.99)* | 1.07 (0.53–2.18) |
| TB treatment | |||||
| New | 135 | 14 | 10.4 | 1.00 | 1.00 |
| Previously treated | 316 | 74 | 23.4 | 2.64 (1.43–4.87)** | 1.24 (0.49–3.12) |
| Site | |||||
| Moldova | 164 | 17 | 10.4 | 1.00 | 1.00 |
| India | 165 | 47 | 28.5 | 3.44 (1.88–6.31)** | 2.81 (1.06–7.47)* |
| South Africa | 122 | 24 | 19.7 | 2.12 (1.08–4.15)* | 0.92 (0.32–2.68) |
| Diabetes | |||||
| No | 430 | 83 | 19.3 | 1.00 | 1.00 |
| Yes | 21 | 5 | 23.8 | 1.31 (0.47–3.67) | 1.43 (0.45–4.50) |
| HIV positive | |||||
| No | 383 | 68 | 17.8 | 1.00 | 1.00 |
| Yes | 68 | 20 | 29.4 | 1.93 (1.08–3.46)* | 2.76 (1.22–6.28)* |
The adjusted model is adjusted for all other variables. *, P ≤ 0.05; **, P ≤ 0.01. Bolded text indicates statistically significant findings.
Associations between baseline clinical characteristics and mortality.
Eighty-eight (19.5%) of the total 451 patients with full clinical and outcome data died by follow-up (Table 1). Body mass index (BMI), clinical site, and HIV were significantly associated with mortality in this population: by 52 weeks, patients with a normal BMI (18.50 to <25) had significantly lower odds of mortality (adjusted odds ratio [OR] = 0.40 [95% CI, 0.23 to 0.69]) than those with a low BMI (<18.50); patients from India had significantly higher odds of mortality (adjusted OR = 2.81 [95% CI, 1.06 to 7.47]) than patients in Moldova; and HIV-positive patients had significantly higher odds of mortality (adjusted OR = 2.76 [95% CI, 1.22 to 6.28]) than HIV-negative patients. No additional relevant clinical variables were identified for the final 204 patients with complete sequencing data (see Table S3 in the supplemental material).
Sequencing results.
Of the 451 specimens with complete clinical and outcome data, M. tuberculosis sequence data were generated only for 256 to 399 specimens for each gene region evaluated (Table 2). The most common resistance mutations, each appearing in 10 or more isolates, were as follows: katG 315ACC (n = 199), inhA −15T (n = 69), rpoB 531TTG (n = 130), gyrA 94GGC (n = 41), gyrA 90GTG (n = 25), gyrA 94GCC (n = 10), rrs 1401G (n = 32), and eis −12T (n = 35). Sequencing results for the final 204 specimens with sequencing data for all gene regions are presented in Table S4.
TABLE 2.
Tuberculosis resistance mutations detected by pyrosequencing for patients with full clinical and outcome data (n = 451)
| Gene and mutation(s) | No. of patients | No. deceased | % deceased |
|---|---|---|---|
| katG | |||
| 315ACC | 199 | 47 | 24 |
| 315ACA | 4 | 3 | 75 |
| No mutation | 149 | 21 | 14 |
| inhA | |||
| −15T | 69 | 15 | 22 |
| −17T | 5 | 2 | 40 |
| −8C | 3 | 2 | 67 |
| No mutation | 292 | 62 | 21 |
| rpoB | |||
| 531TTG | 130 | 35 | 27 |
| 516GTC | 5 | 1 | 20 |
| 526TAC | 5 | 0 | 0 |
| 526GAC | 3 | 0 | 0 |
| 531TGG | 3 | 0 | 0 |
| 511CCG | 2 | 2 | 100 |
| 526TGC | 2 | 1 | 50 |
| 526AAC | 2 | 1 | 50 |
| 533CCG | 2 | 0 | 0 |
| 516TAC | 1 | 0 | 0 |
| 515ATA & 526AAC | 1 | 0 | 0 |
| 513AAA | 1 | 0 | 0 |
| No mutation | 99 | 17 | 17 |
| gyrA | |||
| 94GGC | 41 | 15 | 37 |
| 90GTG | 25 | 9 | 36 |
| 94GCC | 10 | 3 | 30 |
| 91CCG | 6 | 3 | 50 |
| 94AAC | 2 | 2 | 100 |
| 88GCC | 2 | 0 | 0 |
| 95ACC/no mutation | 219 | 37 | 17 |
| rrs | |||
| 1401G | 32 | 16 | 50 |
| No mutation | 317 | 60 | 19 |
| eis | |||
| −12T | 35 | 6 | 17 |
| −10A | 4 | 0 | 0 |
| −14T | 2 | 1 | 50 |
| −37T | 2 | 0 | 0 |
| No mutation | 356 | 76 | 21 |
Associations between resistance mutations and mortality. (i) Individual mutations.
For the 451 patients with full clinical and outcome data, only the rrs 1401G mutation (adjusted OR = 3.40 [95% CI, 1.48 to 7.81]) and the rpoB 531TTG mutation (adjusted OR = 2.32 [95% CI, 1.02 to 5.29]) were significantly associated with higher odds of mortality following adjustment for relevant clinical factors, though the rpoB 511CCG and gyrA 94AAC mutations were identified exclusively in patients who had died by the 52-week follow-up period (Table S5). Only 204 (45.2%) of these initial 451 isolates had full sequencing data for all relevant gene targets (Table S6). The rrs 1401G mutation was the only mutation that remained significantly associated with higher odds of patient mortality (adjusted OR = 5.72 [95% CI, 1.65 to 19.84]) after adjusting for the presence of any other first- and second-line resistance mutations in this restricted population.
(ii) High- versus low-level resistance mutations.
After categorizing mutations into accepted resistance level categories, only the fluoroquinolone (FQ) resistance mutations and kanamycin (KAN) high-MIC resistance mutations were significantly associated with higher odds of patient mortality by 52 weeks following adjustment for patient age, BMI, HIV status, diabetes, reported smoking history, and clinical site (Table 3). Prior to adjustment for other mutations, the detection of either a recognized high-level (adjusted OR1 = 4.38 [95% CI, 1.43 to 13.42]) or a recognized low-level (adjusted OR1 = 3.41 [95% CI, 1.18 to 9.86]) FQ resistance mutation was significantly associated with increased odds of mortality. Following adjustment, however, only the detection of a recognized high-level (adjusted OR2 = 3.99 [95% CI, 1.10 to 14.40]) FQ resistance mutation was significantly associated with higher odds of patient mortality. Recognized KAN high-level resistance mutations (rrs 1401G mutations) (adjusted OR2 = 5.47 [95% CI, 1.64 to 18.24]) remained significantly associated with increased odds of patient mortality in all models. The proportion of the 204 patients who were treated with corresponding drug compounds and the proportion of those treated patients that died are given by resistance-level category in Table S7.
TABLE 3.
Multivariate associations for categories of resistance mutations and death by 52 weeks (n = 204)
| Phenotype | Total no. of patients | No. deceased | % deceased | Adjusted OR1a (95% CI) | Adjusted OR2b (95% CI) |
|---|---|---|---|---|---|
| INH resistance | |||||
| Susceptible (wild type) | 69 | 12 | 17.4 | 1.00 | 1.00 |
| Highest-MIC mutation | 32 | 9 | 28.1 | 2.67 (0.75–9.52) | 1.39 (0.20–9.64) |
| High-MIC mutation | 97 | 26 | 26.8 | 1.82 (0.61–5.44) | 1.41 (0.29–6.81) |
| Low-MIC mutation | 6 | 2 | 33.3 | 2.52 (0.31–20.17) | 0.71 (0.05–9.49) |
| RIF resistance | |||||
| Susceptible (wild type) | 73 | 13 | 17.8 | 1.00 | 1.00 |
| High-MIC mutation | 120 | 33 | 27.5 | 2.04 (0.75–5.55) | 0.99 (0.22–4.41) |
| Low-MIC mutation | 6 | 2 | 33.3 | 1.98 (0.28–13.89) | 2.80 (0.36–21.59) |
| Unknown MIC mutation | 5 | 1 | 20.0 | 0.95 (0.08–10.73) | 0.38 (0.02–6.82) |
| FQ resistance | |||||
| Susceptible (wild type) | 139 | 23 | 16.5 | 1.00 | 1.00 |
| High-MIC mutation | 32 | 14 | 43.8 | 4.38 (1.43–13.42)* | 3.99 (1.10–14.40)* |
| Low-MIC mutation | 31 | 12 | 38.7 | 3.41 (1.18–9.86)* | 2.16 (0.62–7.51) |
| Unknown MIC mutation | 2 | 0 | |||
| KAN resistance | |||||
| Susceptible (wild type) | 158 | 31 | 19.6 | 1.00 | 1.00 |
| High-MIC mutation | 23 | 14 | 60.9 | 6.20 (2.21–17.44)** | 5.47 (1.64–18.24)** |
| Low-MIC mutation | 23 | 4 | 17.4 | 1.58 (0.43–5.80) | 1.02 (0.23–4.47) |
The adjusted OR1 model is adjusted for HIV status, age, diabetes, BMI, reported smoking history, and site. Site data are significant (P < 0.05) in all models except the model evaluating FQ resistance. *, P ≤ 0.05; **, P ≤ 0.01. Bolded text indicates statistically significant findings.
The adjusted OR2 model is adjusted for HIV status, age, diabetes, BMI, reported smoking history, site, and other resistance mutations. None of these other covariates were significant in the final model. *, P ≤ 0.05; **, P ≤ 0.01. Bolded text indicates statistically significant findings.
DISCUSSION
Few studies to date have investigated the associations between specific M. tuberculosis resistance mutations and patient clinical outcomes or have evaluated these relationships in the broader context of both patient clinical variables and M/XDR-TB genotypes. We found that the detection of mutations previously documented to confer high-level FQ and KAN resistance in M. tuberculosis isolates (gyrA 94AAC and 94GGC and rrs 1401G) was significantly associated with higher odds of TB patient mortality within a multisite, observational cohort study, even after adjusting for the presence of other mutations and relevant clinical factors. Our results suggest that these specific mutations are independently associated with patient mortality in diverse DR-TB populations and that patients harboring M. tuberculosis isolates with these recognized high-level resistance mutations should be considered at increased risk for treatment failure and death.
Notably, the detection of an rrs 1401G mutation was significantly associated with TB patient mortality, even following adjustment for relevant clinical factors and other mutations. This finding supports that of Leung et al., who found a strong correlation between detection of the rrs 1401G mutation and KAN treatment failure, though the analysis did not account for other mutations or relevant clinical variables that could influence the relationship (8). Our study results suggest that the association between the 1401G mutation and negative clinical outcomes is independent of site, patient characteristics, or other M. tuberculosis mutations. Instead, the association is likely related to the high-level KAN resistance conferred by rrs 1401G mutations. Although MIC analyses were not performed in our study, the rrs 1401G mutation has been well demonstrated to be associated with high MICs of second-line injectables (KAN MICs of >20 to 80 μg/ml) (1, 13), corresponding to resistance levels that may exceed the clinical efficacy of KAN and other injectable drugs (14). Therefore, it is likely that second-line treatment regimens would be ineffective for M. tuberculosis infections with this mutation. In comparison, we found no significant associations between the detection of eis promoter mutations and patient mortality, even though these mutations are often considered to confer resistance by phenotypic DST at a critical concentration of 2.5 μg/ml (15). However, as only two patients with these mutations were actually treated with KAN in the final patient population included in this study (see Table S7 in the supplemental material), additional studies will be necessary to confirm whether or not these mutations confer clinically relevant levels of phenotypic KAN resistance.
The detection of high-MIC, FQ resistance mutations was also significantly associated with patient mortality in this study, even after adjusting for clinical covariates and other M. tuberculosis mutations. Although the gyrA 94AAC mutation was found exclusively among patients who had died by study completion, no other single gyrA mutation was significantly associated with patient mortality in these adjusted models, likely due to the fact that these models had low power to identify significant associations. Collectively, however, the data indicated that the recognized high-MIC FQ resistance mutations (94AAC and 94GGC) were significantly associated with higher odds of patient mortality, even after taking into account relevant clinical factors, site, and other M. tuberculosis mutations. Our findings are consistent with those of Rigouts et al., who determined that high-MIC gyrA mutations at codon 94 predicted poor MDR-TB treatment outcome in patients with no overt comorbidities (6). This association is likely related to the high levels of phenotypic resistance to both ofloxacin (OFX) and moxifloxacin (MFX) (MICs of 8 to 16 μg/ml and 1 to 8 μg/ml, respectively) conferred by 94AAC and 94GGC mutations (3, 16, 17). Together, these findings suggest that TB treatment decisions, currently based on phenotypic DST at set critical concentrations particular to each drug being tested (i.e., 0.25 mg/liter MFX and 2.0 mg/liter OFX at the time of this study), could potentially be improved with molecular diagnostics that identify patients at increased mortality risk through the detection of specific M. tuberculosis second-line resistance mutations.
Unlike the second-line resistance mutations, none of the first-line resistance mutations were significantly associated with patient mortality after accounting for the presence of all other resistance mutations. While the rpoB 511CCG mutation was identified exclusively in patients who had died by study completion, a significant association between this mutation and patient mortality could not be demonstrated due to low sample size. To date, there has been only limited and indirect evidence that this recognized low-MIC mutation is directly associated with poor TB patient clinical outcomes (10, 18), though the association may be worth further evaluation. Previous studies have reported the recurrence, persistence, or progression of TB disease for patients with low-level rifampin (RIF) resistance mutations (19), even among those on first-line treatment regimens (9, 10, 20), suggesting that patients with these mutations might need to be treated with second-line drugs or high-dose RIF. For the isoniazid (INH) resistance mutations, our results parallel those of Jacobson et al., who did not find any significant associations between specific INH resistance mutations and poor patient outcomes after accounting for relevant clinical variables (21), and of Dantes et al., who did not find specific INH resistance mutations to be associated with clinical outcome in INH monoresistant TB cases (22). Our results support these previous findings and suggest that first-line resistance mutations do not significantly contribute to DR-TB patient mortality.
There were several limitations to our study. First, we did not perform MIC analyses to specifically correlate mutations with conferred resistance levels. However, the association between specific mutations and drug resistance has been well characterized for all drugs included in this study, and so it is unlikely that this limitation greatly affected the findings. Another limitation was that we were unable to incorporate TB treatment data into outcome analyses. Due to the complexity of treatment data between the three clinical sites, we assumed that all treatment regimens, based on the standard of care and given the phenotypic DST results, were appropriate for a given TB infection. Although generally larger proportions of patients with high-level second-line resistance mutations died than patients with high-level first-line resistance mutations following treatment with the respective drug compounds (Table S7), the corresponding drug treatment numbers for each resistance mutation category were small. Future studies will be necessary to definitively evaluate the contribution of different treatment regimens to M/XDR-TB patient outcomes for patients with specific M. tuberculosis mutations. Another limitation of this study was that we did not consider additional negative patient outcomes, such as treatment failure or relapse, in our analyses. There is a chance that certain mutations identified in this study might have been associated with these other outcomes, but not mortality, by 52 weeks. We took a conservative approach to this study, selecting mortality as the only outcome variable, and so it is likely that our findings are understated for all mutations, including the high-level, second-line resistance mutations. Additionally, a significant proportion of patients were excluded from outcome analyses due to missing clinical and/or sequencing data, and so sample sizes were small for our analysis of the different genotypes. Although this limited the power of our analyses, our comparison of the final 204 patients to the initial 451 patients with follow-up data (Table S3 and Table S4) demonstrated that the two groups were comparable, and so it is unlikely that the missing data biased our results. Another limitation was that our sequencing assay was restricted in its ability to provide a complete genetic resistance profile of the DR-TB specimens evaluated, meaning that we might have failed to identify uncommon markers of resistance, such as rare katG mutations (23) or tlyA or gidB mutations (24). Our failure to sequence these additional gene regions potentially biased results toward the null, as some of the patients with genotypically drug-susceptible infections who died in our study might, in fact, have harbored these undetected resistance mutations. Finally, the clinical sites included in this study generally serve high-burden, low-income populations. Our findings for these populations may not apply to low-burden or high-income populations, where there might be marked variations in drug treatment regimens used or different comorbidity profiles that could not be controlled for in these analyses.
Despite these limitations, our report provides strong evidence that recognized gyrA and rrs high-level resistance mutations are significantly and independently associated with patient mortality. Future studies, incorporating TB drug treatment data in a prospective trial, will be necessary to confirm our findings, to offer further evidence of the role of particular M. tuberculosis genotypes relative to specific treatments and dosing, and ultimately to provide more-specific data to guide interpretation of molecular diagnostic test results as diagnostic standards move toward genotypic DST.
MATERIALS AND METHODS
Note.
This publication investigates the associations between M. tuberculosis isolate sequence and patient clinical and outcome data collected in the course of a larger, diagnostic study conducted by the GCDD (11). Methods for this larger study have been published previously (12). Outcome data have not been previously reported for the GCDD study cohort.
Study population.
Three epidemiologically diverse clinical sites (Chisinau, Moldova; Port Elizabeth, South Africa; and Mumbai, India) were selected for this study. In Mumbai, consecutive patients were enrolled at P.D. Hinduja Hospital, a drug-resistant TB (DR-TB) referral center. In Moldova, consecutive patients were enrolled at four regional TB hospitals, with all samples processed at the Chisinau Phthisiopneumology Institute. In Port Elizabeth, consecutive patients were enrolled at one regional hospital and six primary health care facilities. Newly presenting TB patients over 5 years of age were eligible for the study if they provided informed consent, were smear positive or were suspected of having active pulmonary TB, and had one or more factor indicative of DR-TB (12). Patients were excluded by request or inability to provide sufficient sputum (7.5 ml).
Drug susceptibility testing and patient treatment.
Mycobacterial Growth Indicator Tube (MGIT) 960 cultures were performed in validated, clinical reference laboratories, with all specimens tested for phenotypic resistance to isoniazid (INH), rifampin (RIF), the fluoroquinolones (FQ) moxifloxacin (MFX) and ofloxacin (OFX), amikacin (AMK), kanamycin (KAN), and capreomycin (CAP) using standard manufacturer protocols and critical concentrations recommended by the World Health Organization at the time of our study (25–28). The phenotypic results provided the basis for TB treatment decisions at each site, which were determined and administered by local TB clinicians without GCDD input or recommendation (11).
Clinical data collection.
Patient clinical data were gathered from a combination of patient interviews and chart reviews (12). Interviews were conducted at baseline and at the 52-week follow-up period. All interviews collected information about patient age, race, ethnicity, gender, TB risk factors, and clinical history. Chart reviews were conducted at baseline, at 30 days, and at the 52-week follow-up. Chart reviews noted TB treatment history and HIV status. The outcome of interest for this study was all-cause patient mortality, which was determined based upon data from any or all of these sources. Patients lost to follow-up, and those who transferred out of the study prior to the 52-week follow-up period, were excluded from analyses.
DNA extraction, PCR, and pyrosequencing.
M. tuberculosis DNA was extracted from each decontaminated, concentrated sputum (sediment) sample by heating the cell suspensions in a water bath at 100°C (29). PCR and pyrosequencing were conducted for all targets (see Table S1 in the supplemental material) as previously reported (29, 30). Briefly, a PyroMark Q96 ID system (Qiagen, Valencia, CA) was used for pyrosequencing. Variants were identified automatically using IdentiFire software (Qiagen, Valencia, CA) (29). Queries that did not match library sequences were resequenced in duplicate reactions. Queries that did not resolve upon resequencing were deemed genotypically indeterminate and were not included in analyses.
Mutations and resistance levels.
Following primary analyses, identified wild-type sequences or specific mutations were categorized as susceptible or as showing low-, high-, or very-high-level resistance to the corresponding antituberculous drugs based upon evidence documented in the literature (1–5, 31–36), as shown in Table S2. Only isolates with complete genotypic profiles across all the regions associated with XDR-TB were included in secondary analyses (e.g., for genotypic INH resistance determination, sequencing results had to be obtained for both katG and inhA).
Data analysis.
Data analyses were performed in Stata (version 13.1; Stata Corp., College Station, TX). For comparison of categorical variables, chi-square tests were used. Associations of patient mortality with explanatory clinical variables were expressed as odds ratios (ORs). Confounding effects were investigated using multivariable logistic regression. In the analysis of patient mortality, first all individual mutations and then mutations collated by resistance levels (Table S2) were used as explanatory variables in multivariable logistic regression models. Potential confounders included site, age, gender, BMI, HIV status, diabetes, reported smoking history, and other M. tuberculosis resistance mutations. Age, HIV, diabetes, and smoking history are well-established confounders for negative TB treatment outcomes and were retained in the final model (37–40), along with any variables that showed confounding effects. Mortality analyses were conducted both prior to and following restriction of our data to patients with complete genetic data for all targets, in order to ensure that the final patient population was representative of the initial study population. All tests were performed using a significance level of 5%.
Human research conduct.
Our study, registered with ClinicalTrials.gov (ClinicalTrials registration no. NCT02170441), was reviewed and approved by the Institutional Review Boards of the University of California, San Diego, and the participating institutions at all sites. All participants provided written informed consent. Participation did not alter the standard of care.
Supplementary Material
ACKNOWLEDGMENTS
T.C.R. receives salary support from the Foundation for Innovative New Diagnostics (FIND), a nonprofit organization. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. No other coauthors report any conflicts of interest.
This work was supported by the National Institutes of Health (U01-AI082229, P30-AI036214, and R01-AI111435 to T.C.R. and T32-HL098062 to M.S.). This work was also supported by the Global Consortium for Drug-resistant TB Diagnostics (GCDD; http://gcdd.ucsd.edu).
We thank Antonino Catanzaro for his oversight and input on this study. We thank the laboratory and clinical staff at P.D. Hinduja Hospital and Medical Research Center in Mumbai, India; the Institute of Phthisiopneumology in Chisinau, Moldova; Stellenbosch University and the six Primary Health Care Facilities and Regional Hospital in Port Elizabeth, South Africa; and the University of California, San Diego, for their work and contribution to the GCDD study. We thank the South Africa MRC Centre for TB Research, the DST/NRF Centre of Excellence for Biomedical Tuberculosis Research CoE (CBTBR), and the Division of Molecular Biology and Human Genetics and University of Stellenbosch for providing the infrastructure for this study in South Africa.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00152-17.
REFERENCES
- 1.Kambli P, Ajbani K, Nikam C, Sadani M, Shetty A, Udwadia Z, Georghiou SG, Rodwell TC, Catanzaro A, Rodrigues C. 2016. Correlating rrs and eis promoter mutations in clinical isolates of Mycobacterium tuberculosis with phenotypic susceptibility levels to the second-line injectables. Int J Mycobacteriol 5:1–6. doi: 10.1016/j.ijmyco.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kambli P, Ajbani K, Sadani M, Nikam C, Shetty A, Udwadia Z, Georghiou SB, Rodwell TC, Catanzaro C, Rodrigues C. 2015. Defining multidrug-resistant tuberculosis: correlating GenoType MTBDRplus assay results with minimum inhibitory concentrations. Diagn Microbiol Infect Dis 82:49–53. doi: 10.1016/j.diagmicrobio.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kambli P, Ajbani K, Sadani M, Nikam C, Shetty A, Udwadia Z, Rodwell TC, Catanzaro A, Rodrigues C. 2015. Correlating minimum inhibitory concentrations of ofloxacin and moxifloxacin with gyrA mutations using the genotype MTBDRsl assay. Tuberculosis 95:137–141. doi: 10.1016/j.tube.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaunbrecher MA, Sikes RD Jr, Metchock B, Shinnick TM, Posey JE. 2009. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 106:20004–20009. doi: 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamieson FB, Guthrie JL, Neemuchwala A, Lastovetska O, Melano RG, Mehaffy C. 2014. Profiling of rpoB mutations and MICs for rifampin and rifabutin in Mycobacterium tuberculosis. J Clin Microbiol 52:2157–2162. doi: 10.1128/JCM.00691-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigouts L, Coeck N, Gumusboga M, de Rijk WB, Aung KJ, Hossain MA, Fissette K, Rieder HL, Meehan CJ, de Jong BC, Van Deun A. 2016. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J Antimicrob Chemother 71:314–323. doi: 10.1093/jac/dkv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huyen MN, Cobelens FG, Buu TN, Lan NT, Dung NH, Kremer K, Tiemersma EW, van Soolingen D. 2013. Epidemiology of isoniazid resistance mutations and their effect on tuberculosis treatment outcomes. Antimicrob Agents Chemother 57:3620–3627. doi: 10.1128/AAC.00077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung KL, Yip CW, Yeung YL, Wong KL, Chan WY, Chan MY, Kam KM. 2010. Usefulness of resistant gene markers for predicting treatment outcome on second-line anti-tuberculosis drugs. J Appl Microbiol 109:2087–2094. doi: 10.1111/j.1365-2672.2010.04840.x. [DOI] [PubMed] [Google Scholar]
- 9.Williamson DA, Roberts SA, Bower JE, Vaughan R, Newton S, Lowe O, Lewis CA, Freeman JT. 2012. Clinical failures associated with rpoB mutations in phenotypically occult multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 16:216–220. doi: 10.5588/ijtld.11.0178. [DOI] [PubMed] [Google Scholar]
- 10.Shah NS, Grace Lin SY, Barry PM, Cheng YN, Schecter G, Desmond E. 2016. Clinical impact on tuberculosis treatment outcomes of discordance between molecular and growth-based assays for rifampin resistance, California 2003–2013. Open Forum Infect Dis 3:ofw150. doi: 10.1093/ofid/ofw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catanzaro A, Rodwell T, Catanzaro D, Garfein RS, Jackson RL, Seifert M, Georghiou SB, Trollip A, Groessl E, Hillery N, Crudu V, Victor TC, Rodrigues C, Lin GS, Valafar F, Desmond E, Eisenach K. 2015. Performance comparison of three rapid tests for the diagnosis of drug-resistant tuberculosis. PLoS One 10:e0136861. doi: 10.1371/journal.pone.0136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillery N, Groessl EJ, Trollip A, Catanzaro D, Jackson L, Rodwell TC, Garfein RS, Lin SY, Eisenach K, Ganiats TG, Park D, Valafar F, Rodrigues C, Crudu V, Victor TC, Catanzaro A. 2014. The Global Consortium for Drug-resistant Tuberculosis Diagnostics (GCDD): design of a multi-site, head-to-head study of three rapid tests to detect extensively drug-resistant tuberculosis. Trials 15:434. doi: 10.1186/1745-6215-15-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowajassatakul A, Prammananan T, Chaiprasert A, Phunpruch S. 2014. Molecular characterization of amikacin, kanamycin and capreomycin resistance in M/XDR-TB strains isolated in Thailand. BMC Microbiol 14:165. doi: 10.1186/1471-2180-14-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böttger EC. 2011. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol Infect 17:1128–1134. doi: 10.1111/j.1469-0691.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 2012. Updated interim critical concentrations for first-line and second-line DST. http://www.stoptb.org/wg/gli/assets/documents/Updated%20critical%20concentration%20table_1st%20and%202nd%20line%20drugs.pdf.
- 16.Khanna A, Raj VS, Tarai B, Sood R, Pareek PK, Upadhyay DJ, Sharma P, Rattan A, Saini KS, Singh H. 2010. Emergence and molecular characterization of extensively drug-resistant Mycobacterium tuberculosis clinical isolates from the Delhi region in India. Antimicrob Agents Chemother 54:4789–4793. doi: 10.1128/AAC.00661-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirgel FA, Warren RM, Streicher EM, Victor TC, van Helden PD, Bottger EC. 2012. gyrA mutations and phenotypic susceptibility levels to ofloxacin and moxifloxacin in clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 67:1088–1093. doi: 10.1093/jac/dks033. [DOI] [PubMed] [Google Scholar]
- 18.Van Deun A, Aung KJ, Hossain A, de Rijk P, Gumusboga M, Rigouts L, de Jong BC. 2015. Disputed rpoB mutations can frequently cause important rifampicin resistance among new tuberculosis patients. Int J Tuberc Lung Dis 19:185–190. doi: 10.5588/ijtld.14.0651. [DOI] [PubMed] [Google Scholar]
- 19.Ho J, Jelfs P, Sintchencko V. 2013. Phenotypically occult multidrug-resistant Mycobacterium tuberculosis: dilemmas in diagnosis and treatment. J Antimicrob Chemother 68:2915–2920. doi: 10.1093/jac/dkt284. [DOI] [PubMed] [Google Scholar]
- 20.van Ingen J, Aarnoutse R, de Vries G, Boeree MJ, van Soolingen D. 2011. Low-level rifampicin-resistant Mycobacterium tuberculosis strains raise a new therapeutic challenge. Int J Tuberc Lung Dis 15:990–992. doi: 10.5588/ijtld.10.0127. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. 2011. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin Infect Dis 53:369–372. doi: 10.1093/cid/cir406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dantes R, Metcalfe J, Kim E, Kato-Maeda M, Hopewell PC, Kawamura M, Nahid P, Cattamanchi A. 2012. Impact of isoniazid resistance-conferring mutations on the clinical presentation of isoniazid monoresistant tuberculosis. PLoS One 7:e37956. doi: 10.1371/journal.pone.0037956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres JN, Paul LV, Rodwell TC, Victor TC, Amallraja AM, Elghraoui A, Goodmanson AP, Ramirez-Busby SM, Chawla A, Zadorozhny M, Sirgel FA, Catanzaro D, Rodrigues C, Gler MT, Crudu V, Catanzaro A, Valafar F. 2015. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg Microbes Infect 4:e42. doi: 10.1038/emi.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC. 2012. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 7:e33275. doi: 10.1371/journal.pone.0033275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SY, Desmond E, Bonato D, Gross W, Siddiqi S. 2009. Multicenter evaluation of Bactec MGIT 960 system for second-line drug susceptibility testing of Mycobacterium tuberculosis complex. J Clin Microbiol 47:3630–3634. doi: 10.1128/JCM.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues C, Jani J, Shenai S, Thakkar P, Siddiqi S, Mehta A. 2008. Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the Bactec MGIT 960 system. Int J Tuberc Lung Dis 12:1449–1455. [PubMed] [Google Scholar]
- 27.Siddiqi S. 2006. MGIT procedure manual for Bactec MGIT 960 TB system. FIND Diagnostics. http://www.finddx.org/wp-content/uploads/2016/02/mgit_manual_nov2006.pdf.
- 28.World Health Organization. 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. http://www.who.int/tb/publications/2008/whohtmtb_2008_392/en/. [PubMed]
- 29.Lin SY, Rodwell TC, Victor TC, Rider EC, Pham L, Catanzaro A, Desmond EP. 2014. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacterium tuberculosis in clinical isolates and clinical specimens. J Clin Microbiol 52:475–482. doi: 10.1128/JCM.01821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georghiou SB, Seifert M, Catanzaro D, Garfein RS, Valafar F, Crudu V, Rodrigues C, Victor TC, Catanzaro A, Rodwell TC. 2016. Frequency and distribution of tuberculosis resistance-associated mutations between Mumbai, Moldova, and Eastern Cape. Antimicrob Agents Chemother 60:3994–4004. doi: 10.1128/AAC.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalla Costa ER, Ribeiro MO, Silva MS, Arnold LS, Rostirolla DC, Cafrune PI, Espinoza RC, Palaci M, Telles MA, Ritacco V, Suffys PN, Lopes ML, Campelo CL, Miranda SS, Kremer K, da Silva PE, Fonseca LDS, Ho JL, Kritski AL, Rossetti ML. 2009. Correlations of mutations in katG, oxyR-ahpC and inhA genes and in vitro susceptibility in Mycobacterium tuberculosis clinical strains segregated by spoligotype families from tuberculosis prevalent countries in South America. BMC Microbiol 9:39. doi: 10.1186/1471-2180-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva MS, Senna SG, Ribeiro MO, Valim AR, Telles MA, Kritski A, Morlock GP, Cooksey RC, Zaha A, Rossetti ML. 2003. Mutations in katG, inhA, and ahpC genes of Brazilian isoniazid-resistant isolates of Mycobacterium tuberculosis. J Clin Microbiol 41:4471–4474. doi: 10.1128/JCM.41.9.4471-4474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo H, Seet Q, Denkin S, Parsons L, Zhang Y. 2006. Molecular characterization of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis from the USA. J Med Microbiol 55:1527–1531. doi: 10.1099/jmm.0.46718-0. [DOI] [PubMed] [Google Scholar]
- 34.Heym B, Honore N, Truffot-Pernot C, Banerjee A, Schurra C, Jacobs WR Jr, van Embden JD, Grosset JH, Cole ST. 1994. Implications of multidrug resistance for the future of short-course chemotherapy of tuberculosis: a molecular study. Lancet 344:293–298. doi: 10.1016/S0140-6736(94)91338-2. [DOI] [PubMed] [Google Scholar]
- 35.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. 1998. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother 42:2066–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Lu J, Wang Y, Pang Y, Zhao Y. 2014. Prevalence and molecular characterization of fluoroquinolone-resistant Mycobacterium tuberculosis isolates in China. Antimicrob Agents Chemother 58:364–369. doi: 10.1128/AAC.01228-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. 2009. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 80:634–639. [PMC free article] [PubMed] [Google Scholar]
- 38.El-Sadr WM, Perlman DC, Denning E, Matts JP, Cohn DL. 2001. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis 32:623–632. doi: 10.1086/318706. [DOI] [PubMed] [Google Scholar]
- 39.Teale C, Goldman JM, Pearson SB. 1993. The association of age with the presentation and outcome of tuberculosis: a five-year survey. Age Ageing 22:289–293. doi: 10.1093/ageing/22.4.289. [DOI] [PubMed] [Google Scholar]
- 40.Gajalakshmi V, Peto R, Kanaka TS, Jha P. 2003. Smoking and mortality from tuberculosis and other disease in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet 362:507–515. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


