Abstract
Enrichment was performed to isolate organisms that could utilize reduced phosphorus compounds as their sole phosphorus sources. One isolate that grew well with either hypophosphite or phosphite was identified by 16S rRNA gene analysis as a strain of Alcaligenes faecalis. The genes required for oxidation of hypophosphite and phosphite by this organism were identified by using transposon mutagenesis and include homologs of the ptxD and htxA genes of Pseudomonas stutzeri WM88, which encode an NAD-dependent phosphite dehydrogenase (PtxD) and 2-oxoglutarate-dependent hypophosphite dioxygenase (HtxA). This organism also has the htxB, htxC, and htxD genes that comprise an ABC-type transporter, presumably for hypophosphite and phosphite transport. The role of these genes in reduced phosphorus metabolism was confirmed by analyzing the growth of mutants in which these genes were deleted. Sequencing data showed that htxA, htxB, htxC, and htxD are virtually identical to their homologs in P. stutzeri at the DNA level, indicating that horizontal gene transfer occurred. However, A. faecalis ptxD is very different from its P. stutzeri homolog and represents a new ptxD lineage. Therefore, this gene has ancient evolutionary roots in bacteria. These data suggest that there is strong evolutionary selection for the ability of microorganisms to oxidize hypophosphite and phosphite.
Inorganic phosphate and its organic esters and anhydrides are the predominant forms of phosphorus found in biological systems. These compounds, which are required metabolites in all living organisms, each contain P in its most oxidized state (+5 valence). Nevertheless, it is now clear that many organisms produce and consume reduced P compounds. For example, many bacteria can catalyze the oxidation of reduced P compounds, such as hypophosphite (+1 valence) and phosphite (+3 valence), to phosphate, which allows use of these compounds as sole P sources (3, 7, 12, 14, 15). In addition, at least one organism, Desulfotignum phosphitoxidans, is known to use the oxidation of phosphite as its sole energy source for growth (18).
Three discrete metabolic pathways that allow the use of phosphite as a source of phosphorus have been characterized. The first of these pathways, mediated by the enzyme carbon-phosphorus lyase (C-P lyase), is found in numerous bacterial species. The phnCDEFGHIJKLMNOP operon, which encodes C-P lyase and a phosphorus transport system, was originally discovered as a genetic locus in Escherichia coli that is responsible for the oxidation of organic phosphonic acids (21). However, it was later shown that C-P lyase also allows the oxidation of inorganic phosphite (14). E. coli also contains a second system for phosphite oxidation, which surprisingly turns out to be a mediated by the enzyme alkaline phosphatase (encoded by phoA). Although this enzyme is much better known for its ability to hydrolyze phosphate esters, it is also apparently able to hydrolyze phosphite to phosphate and molecular H2. Strangely, this activity is a unique property of the E. coli enzyme and is not a property of the phosphatases from Bacillus subtilis and Pseudomonas stutzeri (25). A third pathway that allows phosphite oxidation is encoded by the ptxABCDE operon of P. stutzeri. This locus encodes a binding protein-dependent phosphite transporter (PtxABC) and the enzyme phosphite:NAD oxidoreductase (PtxD).
Much less is known about hypophosphite oxidation. The only characterized system is that encoded by the htx operon of P. stutzeri. This locus encodes the enzyme hypophosphite:2-oxoglutarate dioxygenase (HtxA), a binding protein-dependent hypophosphite transporter (HtxBCDE), and a C-P lyase (HtxFGHIJKLMNP). The htx genes, together with the ptx locus, comprise a biochemical pathway that allows the use of hypophosphite as a sole P source. Consistent with this proposed function, we have recently shown that both htx and ptx operons of P. stutzeri are regulated in response to phosphate starvation by the two-component regulatory system encoded by phoBR (22).
The diversity of the known pathways for the oxidation of reduced P compounds, combined with the abundance of P-oxidizing organisms that have been isolated from the environment, led us to question whether there are other, as-yet-uncharacterized pathways. Here we describe isolation and characterization of the hypophosphite-oxidizing organism Alcaligenes faecalis WM2072, which has a unique genetic arrangement of phosphorus oxidation genes that provides evidence that there has been horizontal gene transfer of this trait and ancient evolution of phosphite oxidation.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The bacterial strains used in this study and their derivations are shown in Table 1. E. coli DH5α (9) was used as a host for plasmids derived from pMAL-c2X (New England Biolabs, Beverly, Mass.) or pBluescript SK(+) (Stratagene, La Jolla, Calif.). E. coli strain DH5α/λpir (16) was used as the host for cloning transposon insertions created with Tn5-RL27 (11) and Tn5-RL55. The media used in this study have been described previously (15, 19). All strains were grown at 37°C. Antibiotics were used at the following concentrations for E. coli: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; tetracycline, 12 μg/ml; carbenicillin, 50 μg/ml; and chloramphenicol, 12 μg/ml. For A. faecalis WM2072, the following antibiotic concentrations were used: kanamycin, 50 or 100 μg/ml; 12 μg/ml, tetracycline; and chloramphenicol, 20 μg/ml. The chromogenic substrates for alkaline phosphatase, XP (5-bromo-4-chloro-3-indolyl-phosphate), and β-galactosidase, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), were added to solid media at concentrations of 160 and 80 μM, respectively.
TABLE 1.
Bacterial strains used in this study
| Strain or plasmid | Relevant characteristic(s) or description and/or construction | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 | 9 |
| DH5α/λpir | λpir φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 hsdR17 deoR thi-1 supE44 gyrA96 relA1 | 16 |
| BW20767 | RP4-2-Tc::Mu-1kan::Tn7 integrant leu-63::IS10 recA1 zbf-5 creB510 hsdR17 endA1 thi uidA(ΔMluI)::pir+ | 13 |
| A. faecalis strains | ||
| WM2072 | Wild type; Hpt+ Pt+ isolate from pig lagoon | This study |
| WM3425 | ΔptxD; integration and segregation with pMW12 | This study |
| WM3544 | ΔhtxA; integration and segregation with pMW14 | This study |
| WM3627 | ΔhtxBCD; integration and segregation with pMW15 | This study |
| WM3652 | ΔptxE; integration and segregation with pMW18 | This study |
| WM3818 | Δhtx/ptx; integration and segregation with pMW19 | This study |
| This study | ||
| Plasmids | ||
| pMAL-c2X | Apr; malE fusion protein expression vector | New England Biolabs |
| PBluescript SK(+) | Apr cloning vector | Stratagene |
| pRL27 | Tn5-RL27 (Kmr-oriR6K) delivery vector | 11 |
| pWM405 | 11 | |
| pRL47 | Recircularized SstI-cut pWM405 after treatment with T4 DNA polymerase and deoxynucleoside triphosphates | This study |
| pRL17 | Kmr; circularized Tn5-RL27 | 11 |
| pRL48 | aph-oriR6K containing KpnI-XbaI fragment of pRL17 inserted into KpnI-XbaI-cut pRL47 | This study |
| p34S-Cm | Apr/Cmr; carries cat gene cassette | |
| pRL50 | aph-containing SstI fragment of pRL48 replaced with the cat-containing SstI fragment of p34S-Cm | This study |
| pRL10 | AprtetAp-tnp oriT | 11 |
| pRL55 | Apr Tn5-RL55 (Cmr-oriR6K) delivery vector; BamHI-cut PCR fragment from pRL10 (primers pRL10-1 and pRL10-2) ligated to BglII-cut PCR fragment from pRL50 (primer Tn5-OE1) | This study |
| pAW19 | Kmr/AprsacB suicide vector | 24 |
| pMW6 | HindIII-digested PCR of ptxD inserted into HindIII and XmnI sites of pMAL-c2X | This study |
| pMW12 | NotI- and EcoRI-digested PCR of ptxD upstream region (primers AptxDupF and AptxDupR) and SpeI- and EcoRI-digested PCR of ptxD downstream region (primers AptxDDNF and AptxDDNR) inserted into NotI- and SpeI-digested pAW19 | This study |
| pMW14 | NotI- and EcoRI-digested PCR of htxA upstream region (primers AhtxAdelY and AhtxARUP) and SacI- and EcoRI-digested PCR of htxA downstream region (primers AhtxAdelX and AhtxAFDN) inserted into NotI- and SacI-digested pAW19 | This study |
| pMW15 | NotI- and EcoRI-digested PCR of htxBCD upstream region (primers htxBDUPF and htxBDUPR) and SacI- and EcoRI-digested PCR of htxBCD downstream region (primers htxBDDNF and htxBDDNR) inserted into NotI- and SacI-digested pAW19 | This study |
| pMW18 | NotI- and EcoRI-digested PCR of ptxE upstream region (primers delptxEUPF and delptxEUPR) and SpeI- and EcoRI-digested PCR of ptxE downstream region (primers delptxEDNF and delptxEDNR) inserted into NotI- and SpeI-digested pAW19 | This study |
| pMW19 | NotI- and EcoRI-digested PCR of htxA upstream region (primers AhtxAdelY and AhtxAUPR) and SpeI- and EcoRI-digested PCR of ptxE downstream region (primers delptxEDNF and delptxEDNR) inserted into NotI- and SpeI-digested pAW19 | This study |
| pMW20 | BamHI-digested PCR of WM2072 16S rRNA gene inserted into BamHI-digested pBluescript SK(+) | This study |
P oxidation phenotypes.
P oxidation phenotypes were scored by growth on 20 mM succinate-MOPS (morpholinepropanesulfonic acid) medium containing the appropriate P source at a concentration of 0.5 mM (19). Because phosphate is required for growth, organisms cannot grow on this medium unless they have the ability to oxidize the supplied P compound to phosphate. Because the amount of P required for growth is relatively small, the contaminating levels of phosphate found in many medium components, especially agar, allow slight background growth of all strains in such media. To control for this variable, the strains were always compared to suitable positive and negative controls streaked on the same plate. To remove contaminating phosphate, the agar and all glassware were rinsed with multiple changes of ultrapure deionized water prior to use. Solutions of all P compounds were prepared immediately prior to use and filter sterilized.
DNA methods.
Standard methods were used throughout this study for isolation and manipulation of plasmid DNA (2). The plasmids and primers used in this study are described in Tables 1 and 2. Chromosomal DNA was isolated from A. faecalis by the cetyltrimethylammonium bromide method (2). For hybridization experiments, genomic DNA was digested with appropriate restriction endonucleases, separated by agarose gel electrophoresis, and blotted onto nylon membranes. Hybridizing bands were detected by using probes labeled with a DIG-High Prime kit (Roche, Indianapolis, Ind.) according to the manufacturer's specifications. DNA sequencing reactions were carried out by using the BigDye sequencing reagent (Applied Biosystems, Foster City, Calif.) according to the manufacturer's recommendations, and the results were analyzed at the W. M. Keck Center for Comparative and Functional Genomics at the University of Illinois (Urbana, Ill.). The initial sequences of each transposon insertion mutant were always determined by using primers tpnRL17-1 and tpnRL13-2 (11). The remaining sequences were obtained with internal primers designed from the initial and subsequent sequences.
TABLE 2.
Primers used in this study
| Primer | Sequence | Restriction site |
|---|---|---|
| Tn5-OE1 | 5′-GAATTCAGATCTCAGCTGTCTCTTATACACATCTCAACC-3′ | BglII |
| pRL10-1 | 5′-GAATTCGGATCCTTGGTCTGACAGTTACCAATGC-3′ | BamHI |
| pRL10-2 | 5′-GAATTCGGATCCCGCCAAGCTATTTAGGTGACA-3′ | BamHI |
| ΔptxDUPF | 5′-GGCGCGCCGCGGCCGCGATTACGGTACTGGCGGTTG-3′ | NotI |
| ΔptxDUPR | 5′-GGCGCGCCGCGGCCGCGATTACGGTACTGGCGGTTG-3′ | EcoRI |
| ΔptxDDNF | 5′-GGCGCGCCGAATTCCTGAGTGATTAATCTCGATC-3′ | EcoRI |
| ΔptxDDNR | 5′-GGCGCGCCACTAGTTCGCCCATATCTTTCACTCC-3′ | SpeI |
| ΔhtxARUP | 5′-GGCGCGCCGAATTCGAGATATTCGCGTTGCTGCTC-3′ | EcoRI |
| ΔhtxAdelY | 5′-GCGGATCCGCGGCCGCGGCGAGGCCCTACTACCTAT-3′ | NotI |
| ΔhtxAFDN | 5′-GGCGCGCCGAATTCCCGTTGTCGATGTACGGTG-3′ | EcoRI |
| ΔhtxAdelX | 5′-GGCGCGCCGAGCTCCGTGCGTCAGTCCACCTTG-3′ | SacI |
| htxBDUPF | 5′-GGCGCGCCGCGGCCGCCAGTTTGCCCTCCCGATTAT-3′ | NotI |
| htxBDUPR | 5′-GGCGCGCCGAATTCGAATTTCGAAAACAGAGTAA-3′ | EcoRI |
| htxBDDNF | 5′-GGCGCGCCGAATTCGCGTAGTTCTTCCTCAGAGGTC-3′ | EcoRI |
| htxBDDNR | 5′-GGCGCGCCGAGCTCACGCTTCGAGGATACTGAGG-3′ | SacI |
| delptxEUPF | 5′-GGCGCGCCGCGGCCGCCGGGATGATGGTGTTTATGC-3′ | NotI |
| delptxEUPR | 5′-GGCGCGCCGAATTCCTTAAGCGACTCTTCAAGCTTC-3′ | EcoRI |
| delptxEDNF | 5′-GGCGCGCCGAATTCATCGGCTTGTGAGCTCGG-3′ | EcoRI |
| delptxEDNR | 5′-GGCGCGCCACTAGTATTACCCTGAAACCCGCG-3′ | SpeI |
Cloning and analysis of 16S rRNA gene.
The 16S rRNA gene from A. faecalis was amplified by PCR from genomic DNA with Fail Safe DNA polymerase (Epicentre Madison, Wis.) by using primers 5′-TTGGATCCAGAGTTTGATCMTGGCTCAG-3′ and 5′-GTTGGATCCACGGYTACCTTGTTACGAYT-3′, cloned into BamHI-digested pBluescript SK(+) (Stratagene) to generate pMW20, and sequenced as described above. To identify the species, the 16S rRNA gene sequence was compared to other sequences in Ribosomal Database Project II (http://rdp.cme.msu.edu) by using the analysis tools provided at the internet site (5).
Transposon mutagenesis and cloning of transposon insertions.
The transposon delivery vectors pRL27 and pRL55 were transferred into A. faecalis recipients by conjugation from E. coli strain BW20767 as previously described (11). Exconjugants were selected on agar-solidified 20 mM succinate-MOPS media with 0.5 mM phosphate containing kanamycin or chloramphenicol. These transposon-induced mutants were then replica plated onto succinate-MOPS medium containing the appropriate antibiotic with either hypophosphite or phosphite to identify organisms that failed to utilize these alternative P sources. Additionally, mutants were plated onto succinate-MOPS medium with the appropriate antibiotic containing 0.1 mM phosphate and XP to identify organisms that had lost the ability to synthesize a phosphate starvation-inducible phosphatase (identifiable as white colonies due to their inability to cleave the chromogenic phosphatase substrate). The transposon insertion sites for selected mutants were determined by molecular cloning and DNA sequencing as previously described (11). Sequences were compared to the protein sequence database (GenBank) by using the BlastX algorithm (1). For each mutant, the junction between the transposon sequence (the Tn5 inverted repeat sequence ending with GTG TAT AAG AGA CAG) and the genomic DNA sequence and the 9-bp target duplication (a characteristic of Tn5 insertions) were identified.
Directed mutagenesis.
Mutants containing in-frame deletions of various A. faecalis genes were constructed by using a two-step integration-segregation strategy similar to that described previously (13). In-frame deletion mutations were constructed by cloning ca. 1-kbp PCR fragments carrying the sequences immediately upstream and downstream of the desired deletion junction into the suicide vector pAW19 (24). After cloning in the upstream and downstream regions for each deletion, the lacZ gene under control of the tetA promoter was cloned into each plasmid. The plasmids were transferred into A. faecalis by conjugation with E. coli BW20767 donors carrying the appropriate plasmids. Recombinants in which the plasmid, which could not replicate in A. faecalis, had integrated into the chromosome by homologous recombination were selected on the basis of kanamycin resistance encoded on the plasmid. Conjugation was performed as described previously (12). Transconjugants were grown nonselectively to allow loss of the plasmid by recombination and then screened for recombinants that had lost the plasmid backbone. Cleavage of the chromogenic substrate X-Gal conferred by the lacZ gene was used to screen for the presence of plasmid integrants and segregants because the sacB-dependent sucrose-sensitive selection normally used with pAW19 (24) proved to be ineffective in A. faecalis. Approximately 50% of these recombinants contained the desired deletion mutation, while in the remainder the wild-type allele was restored. The following plasmids were used in strain construction: pMW12 for deletion of ptxD, pMW14 for deletion of htxA, pMW15 for deletion of htxBCD, pMW18 for deletion of ptxE, and pMW19 for deletion of the entire htx region (Table 1). All deletion mutants were verified to have the predicted chromosome structures by DNA hybridization experiments as described above.
Purification and enzymatic activity of MBP-PtxD from A. faecalis and P. stutzeri.
Construction of pMW6, which carries an N-terminal malE::ptxD translational fusion to the second codon of the A. faecalis ptxD gene, is described in Table 1. The vector used for overexpression of a maltose-binding protein-PtxD fusion (MBP-PtxD) from P. stutzeri was described previously (6). The methods used for overexpression and purification of MBP-PtxD proteins and for phosphite dehydrogenase assays were described previously (6).
Nucleotide sequence accession numbers.
The GenBank nucleotide accession numbers for sequences determined in this study are AY548382 for the htx-ptx region, AY548383 for the phoBR genes, and AY548384 for the 16S rRNA gene of A. faecalis.
RESULTS
Isolation of A. faecalis WM2072, a new hypophosphite- and phosphate-oxidizing bacterium.
A water sample from the waste lagoon at the University of Illinois Swine Research Facility was diluted and plated onto agar-solidified 20 mM succinate-MOPS medium containing hypophosphite as the sole P source. Colonies that grew after several days of incubation at 30°C were purified by streaking on the same medium. One organism, identified by 16S rRNA gene sequence analysis as a strain of the β-proteobacterial species A. faecalis (designated strain WM2072), was chosen for further characterization.
A. faecalis uses both hypophosphite and phosphite as alternative P sources. This organism grows with doubling times of 1.5 ± 0.2 and 3.0 ± 0.1 h in succinate media containing hypophosphite and phosphite, respectively, as the sole P sources. A significant growth lag was observed after transfer from rich media into the various minimal media. This lag period was longer and more variable, ranging from 106 to 130 h, when phosphite was used as the sole P source, whereas the lag time was reproducibly ca. 60 h with hypophosphite as the P source. By comparison, the organism grew with a doubling time of 0.61 ± 0.02 h with phosphate as the sole P source, with a lag period of only 12.5 h. Because phosphate is required for growth of all organisms, the ability to utilize hypophosphite and phosphite as sole P sources indicates that A. faecalis encodes the enzymes and transport systems needed for conversion of these reduced P substrates to phosphate.
Two phenotypic tests were used to examine whether phosphite oxidation in A. faecalis might be mediated by the phn- or phoA-dependent pathways described above. The phn operon also allows the use of alkyl-phosphonates, such as methylphosphonate, as sole phosphorus sources. A. faecalis was unable to utilize methylphosphonate as a sole P source; thus, it is unlikely that C-P lyase-encoding genes are present in this organism. A. faecalis does, however, possess a phosphate starvation-inducible phosphatase activity, as shown by the hydrolysis of the chromogenic indicator XP when it was grown in media with limiting levels of phosphate (hydrolysis was not observed during growth on phosphate-replete medium).
Identification of the genes involved in oxidation of reduced P compounds by transposon mutagenesis.
To identify the genes required for hypophosphite and phosphite oxidation, we screened ca. 38,000 transposon-induced mutants for the ability to utilize hypophosphite and phosphite as sole P sources. The mutants were also screened for loss of phosphatase activity by plating them on low-phosphate medium with XP. The transposon insertions and flanking DNA of selected mutants were subsequently cloned, sequenced, and used as query sequences in BLAST searches against the sequences in the GenBank database to identify the genes responsible for each phenotype.
Two mutants were found that were unable to use hypophosphite or phosphite as a sole phosphorus source and that were also unable to produce the phosphate starvation-inducible phosphatase. Both mutants had insertions into a homolog of the E. coli phoB gene. Additional sequencing of the surrounding region showed that this gene forms a putative operon with a homolog of the E. coli phoR gene. The E. coli phoBR operon encodes a transcriptional regulatory system that controls the expression of genes involved in the acquisition of P from alternate sources in response to phosphate starvation (known collectively as the Pho regulon) (20). Thus, it is likely that the pleiotropic phenotype of the A. faecalis phoB mutants is caused by a failure of the organisms to induce expression of required genes in response to Pi starvation.
Ten mutants were obtained that were incapable of using either phosphite or hypophosphite as a sole P source but that retained the ability to produce the phosphate starvation-inducible phosphatase activity. Each of these mutants had transposon insertions into homologs of the P. stutzeri htx operon; two had mutations in a gene encoding a putative hypophosphite:2-oxogluarate dioxygenase (htxA), while eight had insertions in genes encoding a putative binding protein-dependent hypophosphite transporter (three had insertions in htxB, four had insertions in htxC, and one had insertions in htxD). These data are consistent with the hypothesis that hypophosphite is oxidized by a hypophosphite:2-oxogluarate dioxygenase after uptake by a specific transporter. Interestingly, P. stutzeri htx mutants retain the ability to utilize phosphite as a sole phosphorus source (15), whereas the A. faecalis mutants had lost this ability. Sequencing of the region surrounding the htx insertions suggested why the phenotype of the A. faecalis mutants differed from that of similar P. stutzeri mutants. The A. faecalis htxA, htxB, htxC, and htxD genes appear to comprise an operon, which, in addition, contains homologs of the P. stutzeri ptxD and ptxE genes (Fig. 1). The htxA, htxB, htxC, and htxD genes are present in the same order and are extraordinarily similar to their P. stutzeri counterparts (Table 3). Thus, the transposon insertions into htx probably exert a polar effect on the downstream ptxDE region.
FIG. 1.
Structures of the htx and ptx regions of A. faecalis WM2072 (A) and P. stutzeri WM88 (15) (B). The sequence of the A. faecalis region was determined by sequencing out from the transposon in both directions, compiling the sequences, and extending the resulting sequence by primer walking. The flags indicate locations of transposon insertions. The P. stutzeri htx region is located approximately 15 kb downstream of the ptx region (15).
TABLE 3.
Comparison of htx and ptx genes of A. faecalis WM2072 and P. stutzeri WM88
| Gene | % Nucleotide identity | % Amino acid identity | No. of residues in A. faecalis/no. of residues in P. stutzeri |
|---|---|---|---|
| htxA | 100 | 100 | 287/287 |
| htxB | 97 | 98 | 298/298 |
| htxC | 99 | 99 | 282/282 |
| htxDa | 99 | 99 | 266/341 |
| ptxD | 57 | 52 | 333/336 |
| ptxE | 41 | 34 | 289/289 |
Calculated for the region of homolog. Note that the A. faecalis gene is significantly shorter than the P. stutzeri gene.
Sequence analysis of the A. faecalis htx-ptx operon.
Remarkably, the nucleotide sequences of the P. stutzeri and A. faecalis htxA genes are 100% identical, while the sequences of the regions upstream of the htx genes, htxB, htxC, and most of htxD are ca. 98% identical at the nucleotide level. The P. stutzeri htxD gene is longer than the A. faecalis htxD gene; however, the genes are nearly identical (∼98% nucleotide identity) in the shared region. Given that the htxA product of P. stutzeri has been biochemically proven to be a hypophosphite:2-oxogluarate dioxygenase (23), there can be no doubt that the identical A. faecalis HtxA protein also possesses this property. HtxBCD probably form a phosphate-hypophosphite transporter (see below). Although A. faecalis does not encode two membrane subunits, this a common difference among binding protein-dependent transporters (10). Thus, an A. faecalis HtxD homodimer probably plays the same role as an HtxDE heterodimer in P. stutzeri. The observation that the upstream regions of the htx operons of A. faecalis and P. stutzeri are identical is fully consistent with the known role of phoBR in P. stutzeri and the phenotype of the A. faecalis phoBR mutants described above.
Interestingly, the extraordinary DNA homology between the htx operons of P. stutzeri and A. faecalis ends abruptly with the end of the htxD gene. Following the htx genes in A. faecalis there are homologs of ptxD and ptxE, whereas in P. stutzeri there are genes encoding components of a C-P lyase. In P. stutzeri, ptxD encodes an NAD:phosphite oxidoreductase, which is required for growth on both hypophosphite and phosphite. The ptxE gene encodes a putative lysR type transcriptional regulator; however, no role has been found for this gene. Unlike their htx counterparts, the ptxD and ptxE genes of A. faecalis are significantly different from their homologs in P. stutzeri. Thus, the PtxD proteins of the two organisms are only 52% identical at the amino acid level, and the PtxE proteins are only 34% identical (Table 3).
Activity of PtxD from A. faecalis WM2072.
To confirm that the divergent PtxD from A. faecalis has the same function as its counterpart from P. stutzeri, the proteins from both organisms were overexpressed as MBP-PtxD fusions in E. coli, purified, and biochemically characterized. The apparent Km and Vmax for phosphite and the effects of known substrate analog inhibitors on the activity of each enzyme were virtually identical (Table 4). Thus, the data suggest that A. faecalis oxidizes phosphite to phosphate via a new member of the phosphite dehydrogenase enzyme family.
TABLE 4.
Properties of MBP-PtxD from A. faecalis WM2072 and MBP-PtxD from P. stutzeri WM88
| Property | A. faecalis MBP-PtxD | P. stutzeri MBP-PtxD |
|---|---|---|
| Apparent Km for Pt (μM)a | 89.18 ± 3.26 | 130.16 ± 1.78 |
| Vmax (U/mg) | 1.19 ± 0.02 | 2.87 ± 0.01 |
| Effects of substrate analog inhibitors (% activity)b | ||
| None | 100 | 100 |
| Sulfite | <0.01 | <0.01 |
| Arsenite | 69 | 70 |
| Nitrate | 25 | 21 |
The concentration of NAD was kept constant at 1 mM, while the phosphite concentration was varied. The kinetic analysis was done by using a modified version (17) of the program of Cleland (4).
Substrate analogs were added at a concentration of 4 mM. The reaction mixtures also contained 50 μM phosphite and 1.0 mM NAD.
Construction and phenotypic characterization of deletion mutants with mutations in genes of the htx-ptx region.
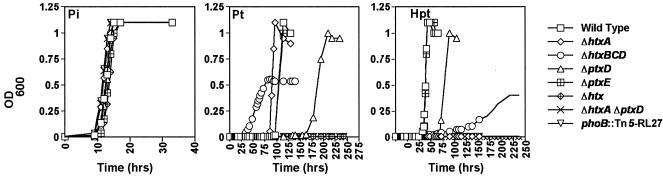
To confirm the predicted in vivo functions of the genes in the htx region, in-frame deletions of the following genes were constructed: htxA, ptxD, htxBCD, ptxE, the entire htx region, and a double mutant with mutations in both htxA and ptxD. The growth of these mutants was compared to that of the wild-type strain in 20 mM succinate-MOPS minimal media with no P source or with phosphate, phosphate, or hypophosphite as the sole P source (Fig. 2). All of the strains displayed similar growth characteristics with phosphate as the sole P source, and none grew in the absence of P. The htxA deletion mutant grew with phosphite but not with hypophosphite, supporting the hypothesis that this gene plays a role in hypophosphite oxidation. The htxBCD deletion mutant displayed a shorter lag period than the wild-type strain when the organisms were grown on phosphite; however, it also grew more slowly and only reached about one-half the maximum optical density that the wild-type strain reached. Similar results were obtained with growth on hypophosphite. Thus, although it was clear that the organism could take up hypophosphite and phosphite in the absence of HtxBCD, it was very likely that these proteins comprised the principal transporter for these reduced P compounds. Surprisingly, the ptxD deletion mutant remained capable of growth on both hypophosphite and phosphite, although very pronounced lag periods were observed prior to the onset of growth. These data not only indicate that the organism possessed an alternate mechanism for phosphite oxidation but also suggest that PtxD primarily played this role. The inabilities of the htxA ptxD double deletion mutant, the htx region deletion mutant, and the phoB::Tn5-RL27 mutant to grow with hypophosphite or phosphite as the sole P source suggest that the alternate phosphate-oxidizing enzyme may be HtxA. The ptxE deletion mutant had no detectable phenotype on any of the media examined.
FIG. 2.
Growth of A. faecalis WM2072 mutants in 20 mM succinate-MOPS media with phosphate (A), phosphite (B), and hypophosphite (C) as the sole phosphorus sources. The cultures were grown at 37°C. Growth was monitored by measuring the optical density at 600 nm (OD600) of the cultures. Control cultures containing no phosphorus source were monitored as well, and none of these cultures grew.
DISCUSSION
Until recently, little was known about the ability of microorganisms to oxidize the reduced phosphorus compounds hypophosphite and phosphite. Although many organisms that could use these compounds as sources of phosphorus had been isolated, very little was known about the pathways involved. However, one metabolic pathway for oxidation of these compounds has been genetically and biochemically characterized: the htx-ptx pathway of P. stutzeri WM88 (15). In this report, we describe A. faecalis WM2072, an organism that is also capable of oxidizing the reduced phosphorus compounds hypophosphite and phosphite. Although our data suggest that metabolism of hypophosphite and phosphite is similar in A. faecalis and P. stutzeri, analysis of the A. faecalis genes involved in this pathway produced numerous surprises.
The genetic studies presented here confirmed that A. faecalis oxidizes reduced P compounds via the same enzymatic steps used in P. stutzeri. Thus, hypophosphite is oxidized to phosphite via the product of the htxA gene, 2-oxoglutarate:hypophosphite dioxygenase. Phosphite produced in the HtxA reaction or phosphite supplied exogenously is then oxidized by the product of the ptxD gene, NAD:phosphite oxidoreductase, which provides the phosphate needed for growth. Accordingly, htxA mutants of both organisms fail to utilize hypophosphite as a sole P source. We were surprised to find, however, that A. faecalis ptxD mutants remained capable of growth on both phosphite and hypophosphite, although they grew significantly less well than wild-type strains, while P. stutzeri ptxABCDE deletion mutants cannot grow on either of these P substrates. These data indicate that there is an alternate phosphate-oxidizing activity in A. faecalis, which, based on the lack of growth by ptxD htxA double mutants, can be attributed to the HtxA protein. Consistent with this result, HtxA from P. stutzeri, which is identical to A. faecalis HtxA, has been shown to be capable of poorly oxidizing phosphite in vitro (23). The apparent contradiction in the P. stutzeri in vivo and in vitro data (i.e., HtxA catalyzes phosphite oxidation in vitro, but ptxABCDE deletion mutants cannot perform this reaction in vivo even though they are htxA+) may be explained by differences in the uptake systems for reduced P compounds in the two organisms. Thus, it may be that the ptxABCDE deletion mutants of P. stutzeri cannot transport phosphite, whereas A. faecalis can. Our finding that A. faecalis HtxBCD mutants remain capable of growth on phosphite clearly indicates that multiple phosphite transport systems are present in this organism.
Perhaps the most surprising result obtained in this study is the near identity of a large segment of the htx-ptx operon and upstream region with the corresponding DNA from P. stutzeri. Although P. stutzeri (a γ-proteobacterium) and A. faecalis (a β-proteobacterium) are distantly related, only 22 nucleotide changes were observed in a single contiguous stretch of more than 4.2 kbp (99.5% nucleotide identity). These data clearly indicate that this locus has moved by horizontal gene transfer. While this is the first evidence for horizontal transfer involving the htx genes, we have previously observed evidence for similar horizontal gene transfer of the ptxABCDE operon (although it was restricted to members of the γ-proteobacteria) (L. Thompson and W. W. Metcalf, unpublished data). With this in mind, it is puzzling that the ptxDE genes of A. faecalis, found in the same operon with nearly identical htxABCD genes, are only distantly related to their P. stutzeri counterparts. Thus, the evolutionary history of ptxDE is much more complex. Our biochemical data clearly indicate that both PtxD proteins are phosphite dehydrogenases. The large divergence in their primary amino acid sequences indicates that the two genes evolved separately, in contrast to HtxABCD. Therefore, PtxD has an ancient evolutionary origin in this family of NAD-dependent dehydrogenases (8).
The htx gene arrangements in the two organisms are also strikingly different and support the notion of horizontal gene transfer. In P. stutzeri, two discrete operons, htxABCDEFGHIJKLMNP and ptxABCDE, separated by approximately 15 kb, are required for oxidation of hypophosphite and phosphite (15). In P. stutzeri, the genes downstream of htxABCDE encode a C-P lyase, which allows the use of phosphonic acids (also reduced P compounds) as sole P sources (24). In contrast, in A. faecalis parts of the htx and ptx operons are fused into a single htxABCD-ptxDE operon. Thus, in both organisms the htxABCD genes are physically linked to other genes involved in reduced P metabolism but with different substrates. A precise reconstruction of the evolutionary history of these genes would be highly speculative and open to argument. Nevertheless, the observation that these genes are transferred horizontally among microorganisms and are subject to recombination with other genes involved in reduced P metabolism suggests that they are under strong selective pressure. The fact that P is often a limiting nutrient, coupled with the observation that these genes are transcribed in response to phosphate starvation (22), suggests the nature of this selective pressure. These data provide strong, albeit indirect, evidence for the prevalence and importance of reduced P compounds in nature.
Acknowledgments
We thank Rachael A. Larsen and Lisa Thompson for technical assistance.
This work was supported by grant GM59334 from the National Institute of General Medical Sciences. M.M.W. was supported in part by training grant PHS T32 GM07283 from the National Institutes of Health.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology, vol. 1 and 2. John Wiley & Sons, New York, N.Y.
- 3.Casida, L. E., Jr. 1960. Microbial oxidation and utilization of orthophosphite during growth. J. Bacteriol. 80:237-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleland, W. W. 1979. Statistical analysis of enzyme kinetic data. Methods Enzymol. 63:103-138. [DOI] [PubMed] [Google Scholar]
- 5.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costas, A. M. G., A. K. White, and W. W. Metcalf. 2001. Purification and characterization of a novel phosphorus oxidizing enzyme from Pseudomonas stutzeri WM88. J. Biol. Chem. 276:17429-17436. [DOI] [PubMed] [Google Scholar]
- 7.Foster, T. L., L. Winans, Jr., and S. J. Helms. 1978. Anaerobic utilization of phosphite and hypophosphite by Bacillus sp. Appl. Environ. Microbiol. 35:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant, G. A. 1989. A new family of 2-hydroxyacid dehydrogenases. Biochem. Biophys. Res. Commun. 165:1371-1374. [DOI] [PubMed] [Google Scholar]
- 9.Grant, S. G. N., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 11.Larsen, R. A., M. Wilson, A. Guss, and W. W. Metcalf. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178:193-201. [DOI] [PubMed] [Google Scholar]
- 12.Malacinski, G., and W. A. Konetzka. 1966. Bacterial oxidation of orthophosphite. J. Bacteriol. 91:578-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 14.Metcalf, W. W., and B. L. Wanner. 1991. Involvement of the Escherichia coli phn (psiD) gene cluster in assimilation of phosphorus in the form of phosphonates, phosphite, Pi esters, and Pi. J. Bacteriol. 173:587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metcalf, W. W., and R. S. Wolfe. 1998. Molecular genetic analysis of phosphite and hypophosphite oxidation by Pseudomonas stutzeri WM88. J. Bacteriol. 180:5547-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson, J. G. 1991. IntelliKinetics, version 1.01. Pennsylvania State University, State College.
- 18.Schink, B., and M. Friedrich. 2000. Phosphite oxidation by sulphate reduction. Nature 406:37. [DOI] [PubMed] [Google Scholar]
- 19.Wanner, B. L. 1986. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J. Mol. Biol. 191:39-58. [DOI] [PubMed] [Google Scholar]
- 20.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 21.Wanner, B. L., and J. A. Boline. 1990. Mapping and molecular cloning of the phn (psiD) locus for phosphonate utilization in Escherichia coli. J. Bacteriol. 172:1186-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White, A. K., and W. W. Metcalf. 2004. The htx and ptx operons of Pseudomonas stutzeri WM88 are new members of the Pho regulon. J. Bacteriol. 186:5876-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, A. K., and W. W. Metcalf. 2002. Isolation and biochemical characterization of hypophosphite/2-oxoglutarate dioxygenase: a novel phosphorus oxidizing enzyme from Pseudomonas stutzeri WM88. J. Biol. Chem. 277:38262-38271. [DOI] [PubMed] [Google Scholar]
- 24.White, A. K., and W. W. Metcalf. 2004. Two C-P lyase operons in Pseudomonas stutzeri and their roles in the oxidation of phosphonates, phosphate, and hypophosphite. J. Bacteriol. 186:4730-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, K., and W. W. Metcalf. 2004. A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase. Proc. Natl. Acad. Sci. USA 101:7919-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]