Abstract
Background
Chloroquine, a bitter tastant, inhibits Ca2+ signaling, resulting in suppression of B cell activation; however, the inhibitory mechanism remains unclear.
Results
In this study, thapsigargin (TG), but not caffeine, induced sustained intracellular Ca2+ increases in mouse splenic primary B lymphocytes, which were markedly inhibited by chloroquine. Under Ca2+-free conditions, TG elicited transient Ca2+ increases, which additionally elevated upon the restoration of 2 mM Ca2+. The former were from release of intracellular Ca2+ store and the latter from Ca2+ influx. TG-induced release was inhibited by 2-APB (an inhibitor of inositol-3-phosphate receptors, IP3Rs) and chloroquine, and TG-caused influx was inhibited by pyrazole (Pyr3, an inhibitor of transient receptor potential C3 (TRPC3) and stromal interaction molecule (STIM)/Orai channels) and chloroquine. Moreover, chloroquine also blocked Ca2+ increases induced by the engagement of B cell receptor (BCR) with anti-IgM.
Conclusions
These results indicate that chloroquine inhibits Ca2+ elevations in splenic B cells through inhibiting Ca2+ permeable IP3R and TRPC3 and/or STIM/Orai channels. These findings suggest that chloroquine would be a potent immunosuppressant.
Keywords: B cells, Ca2+, Chloroquine, IP3R, TRPC3 channels, STIM/Orai channels
Background
Chloroquine is a bitter tastant [1–4], which was used to treat malaria [5] and immune-related diseases such as rheumatic disease, systemic lupus erythematosus [6], early-stage AIDS [7] and chronic graft-versus-host disease [8]. Moreover, it inhibits Ia molecule biosynthesis [9] and CpG DNA-induced protection [10] in B cells. These results imply that chloroquine might be an immunosuppressant of B cell activation. Cytosolic Ca2+ increases play an important role in B cell development [9], survival [11], activation [12], and differentiation [13, 14], cytokine production [12] and cell death [15]. Ca2+ increases in B cells are induced by antigen or anti-B cell receptor (BCR) ligation. BCR antibodies bind to the BCR, resulting in the phosphorylation of tyrosine in phospholnositide-specific phospholipase C (PLC). The phosphorylated PLC catalyzes phosphatidylinositol-4, 5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3). IP3 binds to IP3Rs located on the surface of the endoplasmic reticulum (ER). The activated IP3Rs then mediate Ca2+ release from the ER, leading to increases in intracellular Ca2+ [15, 16]. In addition, Ca2+ increases can also be induced by thapsigargin (TG) [17]. However, whether and how bitter tastant chloroquine inhibits Ca2+ increases in B cells remains unclear.
In this study, we found that chloroquine inhibited Ca2+ increases induced by TG and BCR engagement with anti-IgM through inhibiting Ca2+ permeable ion channels.
Results
Chloroquine decreases TG-induced increases of intracellular Ca2+
In this study, we sought to investigate the effect of chloroquine on intracellular Ca2+ in primary B lymphocytes from mouse spleens. As shown in Fig. 1a and b, the increases of Ca2+ were induced by TG, an inhibitor of the ER Ca2+ ATPase, which were inhibited by chloroquine. The dose-relationship of inhibition is shown in Fig. 1c. The IC50 was 9.3 ± 0.7 mM (Fig. 1c). These results indicate that chloroquine inhibits TG-induced elevations of cytosolic Ca2+.
Fig. 1.
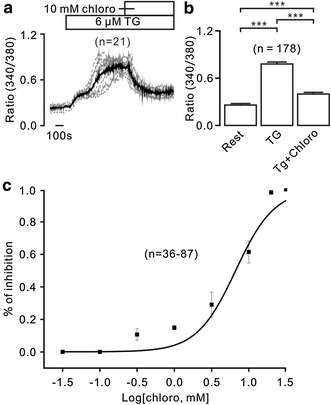
Chloroquine (chloro) blocks increases of Ca2+. a TG induced steady increases of Ca2+ in splenic primary B lymphocytes, which were blocked by chloro. The bold line represents the average values. b The average Ca2+ levels from 178 cells. c Dose-dependent inhibition of chloro on TG-induced Ca2+ increases. ***p < 0.001. These results indicate that chloro attenuates TG-induced elevations of Ca2+
Mechanism of chloroquine-caused inhibition on TG-induced Ca2+ increases
To study whether chloroquine inhibits extracellular Ca2+ entry, we performed the following experiments. Intracellular Ca2+ store was first depleted by TG under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA), which resulted in transient increases of Ca2+. Ca2+ (2 mM) was then restored in the extracellular solutions, which induced additional increases and were markedly blocked by chloroquine (Fig. 2a). The Ca2+ levels in 155 cells were summarized (Fig. 2b). These data indicate that chloroquine inhibits Ca2+ influx.
Fig. 2.
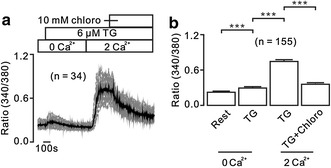
Chloro inhibits extracellular Ca2+ influx. a TG induced small transient increases of Ca2+ under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA). Following the restoration of 2 mM Ca2+, large sustained elevations occurred and were declined by chloro. b The average Ca2+ levels from 155 cells. ***p < 0.001. These results indicate that chloro inhibits extracellular Ca2+ influx
We next investigated whether chloroquine inhibits TG-induced intracellular Ca2+ release. Under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA), the incubation of chloroquine abolished TG-induced transient Ca2+ increases and the restoration of 2 mM Ca2+-caused additional elevations (Fig. 3a). This phenomenon was observed in 241 cells. These results indicate that chloroquine blocks Ca2+ release from the ER.
Fig. 3.
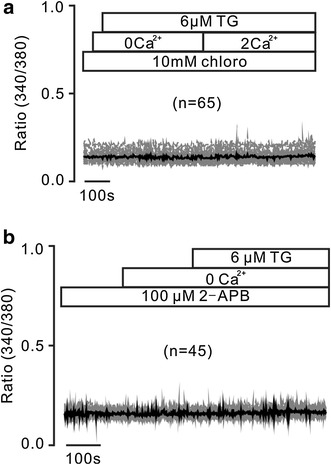
Chloro blocks TG-induced Ca2+ release by inhibiting IP3Rs on the ER membrane. a After cells were incubated with chloro, neither TG nor the addition of 2 mM Ca2+ induced increases of Ca2+. These experiments were performed in 241 cells. These data suggest that chloro inhibits TG-induced Ca2+ release. b Following cells were incubated with 2-APB, an IP3R blocker, TG failed to induce increases of Ca2+ under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA). These experiments were conducted in 129 cells. These data suggest that 2-APB inhibits TG-induced Ca2+ release from intracellular Ca2+ stores by inhibiting IP3Rs on the ER membrane
We then studied which Ca2+ release pathway was inhibited by chloroquine. It has known that Ca2+ release is mainly mediated by IP3Rs and RyRs. 2-APB is a blocker of IP3R [20]. As shown in Fig. 3b, 2-APB abrogated TG-induced increases of Ca2+ under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA). These inhibitions were observed in 129 cells. These results indicate that chloroquine inhibits IP3R-mediated Ca2+ release from the ER.
Next, we investigate the role of RyRs, since which mediate Ca2+ release from the ER [21]. Cells were stimulated with caffeine, a selective activator of RyRs, which failed to increase Ca2+ (Fig. 4). These results indicate that these cells have no functional RyRs, suggesting that RyRs do not contribute to TG-induced Ca2+ release.
Fig. 4.

Caffeine fails to trigger increases of Ca2+. Caffeine did not trigger increases of Ca2+ under 2 mM Ca2+ conditions. These were observed in 227 cells. These results suggest that these cells do not have functional RyRs
Chloroquine inhibits Ca2+ influx as shown in Fig. 2, we then studied the underlying mechanism. In previous studies, it has been found that chloroquine blocked TRPC3 and/or STIM/Orai channels, resulting in decreases of Ca2+ in airway smooth muscle cells [3, 18] and in murine CD4+ thymocytes [4]. As shown in Fig. 5, TG-induced increases were declined by Pyr3, an inhibitor of TRPC3 and/or STIM/Orai channels, suggesting that chloroquine inhibits TRPC3 and/or STIM/Orai channels-mediated Ca2+ influx.
Fig. 5.
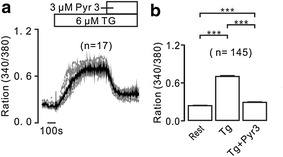
Pyr3 attenuates TG-induced Ca2+ elevations. a TG-induced sustained increases of Ca2+ were reduced by Pyr3, a selective inhibitor of TRPC3 and STIM/Orai channels. b The average values from 145 cells. ***p < 0.001. These results suggest that TRPC3 and/or STIM/Orai channel mediate TG-induced Ca2+ increases
Chloroquine inhibits BCR engagement-induced Ca2+ elevations
We finally observed the effect of chloroquine on BCR engagement-induced Ca2+ increases. Our previous results indicate that the engagement of BCR with anti-IgM induces Ca2+ elevations in B cells [16]. As shown in Fig. 6, anti-IgM induced Ca2+ increases. However, such increases were potently inhibited by the incubation of chloroquine, although that the statistical results show that anti-IgM still induced a significant elevation. These results indicate that chloroquine inhibits BCR engagement-induced Ca2+ elevations.
Fig. 6.
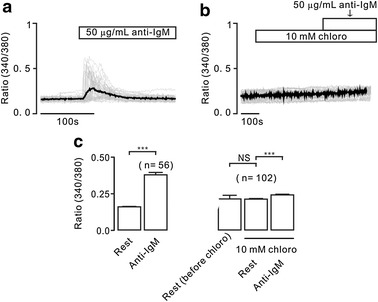
Chloro inhibits anti-IgM-induced Ca2+ increases. a Anti-IgM induced Ca2+ increases. b Chloro failed to affect the level of Ca2+, however, inhibited anti-IgM-induced increases were inhibited by chloro. c The summary results. NS: p > 0.05; ***p < 0.001. These results indicate that chloro blocks BCR engagement-induced Ca2+ increases
Discussion
In the present study, our results indicate that chloroquine depresses Ca2+ increases induced by TG and anti-IgM via inhibiting the intracellular Ca2+ release mediated by IP3R channels and inhibiting the extracellular Ca2+ influx mediated by TRPC3 and/or STIM/Orai channels.
The aim of this study was to investigate whether chloroquine inhibits increases of Ca2+ and the underlying mechanism. We found that chloroquine inhibited increases of Ca2+ induced by TG (Fig. 1). To define the inhibitory mechanism, we investigated the pathways that mediated TG-induced Ca2+ elevations. Intracellular Ca2+ increases will be mediated by Ca2+ permeation channels on the plasma and the ER membrane [22]. These have been demonstrated by the results that under Ca2+-free conditions, TG induced transient increases and followed by additional elevations upon the restoration of 2 mM Ca2+ (Fig. 2), since the former resulted from release and the latter from influx. Moreover, the release was mediated by IP3R in the ER membrane (Fig. 3a, b), because that 2-APB blocked release-induced Ca2+ elevations. IP3Rs have three isoforms (IP3R1, IP3R2, and IP3R3), which can be inhibited by 2-APB [20]. While, the influx was mediated by TRPC3 and/or STIM/Orai channels in the plasma membrane. Because that Pyr3 (Fig. 5) blocked TG-induced sustained increases, which will be mediated by influx based on the results shown in Fig. 2. These data indicate that TG-induced intracellular Ca2+ elevations were mediated by above described ion channels, and which will be inhibited by chloroquine and then resulting in decreases. These results are consistent with previous findings that chloroquine blocks TRPC3 and/or STIM/Orai channels resulting in decreases in Ca2+ levels in smooth muscle cells [1, 3] and in murine CD4+ thymocytes [4]. In addition, BCR engagement frequently occurs in vivo, which then results in immunological responses. Therefore, we observed whether the engagement can induce Ca2+ increases and are blocked by chloroquine. The results show that chloroquine inhibited BCR engagement-induced Ca2+ increases (Fig. 6).
Ca2+ is a crucial second messenger that modulates many cellular processes in splenic B cells, such as cell differentiation and activation [14, 23]. Therefore, our data would indicate that chloroquine might be a potent inhibitor for B cells-mediated immunological responses and inflammations.
Conclusions
Chloroquine inhibits Ca2+-permeable ion channels in the plasma and the ER membranes, resulting in decreases of Ca2+. These findings suggest that chloroquine would be an immunosuppressant.
Methods
Animals
6- to 8-week-old BALB/c male mice were purchased from the Hubei Provincial Center for Disease Control and Prevention, Wuhan, China. The mice were housed under controlled temperature (21–23 °C) and light (lights on between 08:00 and 20:00) conditions and were provided adequate water and food. All housing and experiments were performed in according with the Guide for the Institutional Animal Care and Use Committee of the South-Central University for Nationalities.
Reagents
Fura-2 AM was purchased from Invitrogen (Eugene, OR, USA). Pyrazole-3 (Pyr3) and 2-Aminoethoxydiphenyl borate (2-APB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Thapsigargin (TG) was purchased from Cayman (Tallinn, Estonia). RPMI 1640 medium and fetal bovine serum (FBS) were purchased from Gibco (Rockville, MD, USA). Anti-B220-PE and anti-IgM antibodies were purchased from BD Pharmingen (San Diego, CA, USA). All of the other chemicals were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China).
Isolation of B cells
B lymphocytes were isolated from mouse spleens as previously described [16]. Briefly, after the animals were killed, the spleens were removed and placed in RPMI 1640 medium containing 10% FBS and 1 mM l-glutamine. The spleens were then gently teased apart with two G27 syringe needles. The non-cellular tissues were removed by filtering this preparation through a 70-μM nylon mesh. The cells were maintained at room temperature.
Measurement of intracellular Ca2+
Intracellular Ca2+ was measured using fura-2 AM as previously described [18, 19]. The cells were loaded with 2.5 μM fura-2 AM. Paired 340/380 fluorescence images were acquired using a TILL imaging system (FEI Munich GmbH, Munich, Germany), and the fluorescence ratios represent the intracellular Ca2+ levels. The B cells were identified by anti-B220-PE.
Data analysis and statistics
All of the data are presented as the mean ± SEM. The n values represent the number of cells. Unpaired Student’s t tests were performed to identify significant differences between the means. Differences with p < 0.05 were considered statistically significant.
Authors’ contributions
YFW, PZ, XL, JCX, QZ and MRX did experiments and analyzed data; JHS, YBP, LX, MFY, WWC and LQM analyzed data; PZ and QHL designed experiments, analyzed data and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Hai-Xia Cheng (College of Life Sciences, South-Central University for Nationalities) for technical assistance. This work is supported by the National Natural Science Foundation of China (31571200, 31140087, and 30971514 to Q-H Liu; 31070744 to P Zhao) and the Fundamental Research Funds for the Central Universities, South-Central University for Nationalities (CZY17009 to P Zhao; CZW15025 and CZW15012 to Q-H Liu).
Competing interests
The authors declare that they have no competing interests.
Compliance with ethical guidelines
All animal housing and experiments were performed in according with the Guide for the Institutional Animal Care and Use Committee of the South-Central University for Nationalities.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- TG
thapsigargin
- IP3Rs
inositol-3-phosphate receptors
- TRPC3
transient receptor potential C3
- STIM
stromal interaction molecule
- BCR
B cell receptor
- PLC
phospholnositide-specific phospholipase C
- PIP2
phosphatidylinositol-4, 5-bisphosphate
- DAG
diacylglycerol
- IP3
inositol-1,4,5-trisphosphate
- ER
endoplasmic reticulum
- 2-APB
2-aminoethoxydiphenyl borate
- Pyr3
pyrazole-3
- FBS
fetal bovine serum
- RyRs
ryanodine receptors
Footnotes
Yi-Fan Wu, Ping Zhao and Xi Luo contributed equally to this work
Contributor Information
Yi-Fan Wu, Email: 396792617@qq.com.
Ping Zhao, Email: zping0124@163.com.
Xi Luo, Email: 639032960@qq.com.
Jin-Chao Xu, Email: 806424416@qq.com.
Lu Xue, Email: sparkler830305@hotmail.com.
Qi Zhou, Email: 609312675@qq.com.
Mingrui Xiong, Email: 443070413@qq.com.
Jinhua Shen, Email: shenjinhua2013@163.com.
Yong-Bo Peng, Email: pyb1980@hotmail.com.
Meng-Fei Yu, Email: 65556248@qq.com.
Weiwei Chen, Email: chenww0116@hotmail.com.
Liqun Ma, Email: ma.liqun@hotmail.com.
Qing-Hua Liu, Email: liu258q@yahoo.com, Email: qinghualiu@mail.scuec.edu.cn.
References
- 1.Sai WB, Yu MF, Wei MY, Lu Z, Zheng YM, Wang YX, Qin G, Guo D, Ji G, Shen J, Liu QH. Bitter tastants induce relaxation of rat thoracic aorta precontracted with high K(+) Clin Exp Pharmacol Physiol. 2014;41:301–308. doi: 10.1111/1440-1681.12217. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Luo XJ, Sai WB, Yu MF, Li WE, Ma YF, Chen W, Zhai K, Qin G, Guo D, et al. Non-selective cation channels mediate chloroquine-induced relaxation in precontracted mouse airway smooth muscle. PLoS ONE. 2014;9:e101578. doi: 10.1371/journal.pone.0101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu JC, Peng YB, Wei MY, Wu YF, Guo D, Qin G, Ji G, Shen J, Liu QH. Chloroquine inhibits Ca(2 +) signaling in murine CD4(+) thymocytes. Cell Physiol Biochem. 2015;36:133–140. doi: 10.1159/000374058. [DOI] [PubMed] [Google Scholar]
- 5.Gostner JM, Schrocksnadel S, Becker K, Jenny M, Schennach H, Uberall F, Fuchs D. Antimalarial drug chloroquine counteracts activation of indoleamine (2,3)-dioxygenase activity in human PBMC. FEBS Open Bio. 2012;2:241–245. doi: 10.1016/j.fob.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus. 2006;15:268–275. doi: 10.1191/0961203306lu2299oa. [DOI] [PubMed] [Google Scholar]
- 7.Paton NI, Goodall RL, Dunn DT, Franzen S, Collaco-Moraes Y, Gazzard BG, Williams IG, Fisher MJ, Winston A, Fox J, et al. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilman AL, Chan KW, Mogul A, Morris C, Goldman FD, Boyer M, Cirenza E, Mazumder A, Gehan E, Cahill R, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:327–334. doi: 10.1016/S1083-8791(00)70058-9. [DOI] [PubMed] [Google Scholar]
- 9.Nowell J, Quaranta V. Chloroquine affects biosynthesis of Ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha-beta dimers in B cells. J Exp Med. 1985;162:1371–1376. doi: 10.1084/jem.162.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi AK, Peckham DW, Ashman RF, Krieg AM. CpG DNA rescues B cells from apoptosis by activating NFkappaB and preventing mitochondrial membrane potential disruption via a chloroquine-sensitive pathway. Int Immunol. 1999;11:2015–2024. doi: 10.1093/intimm/11.12.2015. [DOI] [PubMed] [Google Scholar]
- 11.Dugas B, Calenda A, Delfraissy JF, Vazquez A, Bach JF, Galanaud P. The cytosolic free calcium in anti-mu-stimulated human B cells is derived partly from extracellular medium and partly from intracellular stores. Eur J Immunol. 1987;17:1323–1328. doi: 10.1002/eji.1830170916. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 13.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 14.Healy JI, Dolmetsch RE, Lewis RS, Goodnow CC. Quantitative and qualitative control of antigen receptor signalling in tolerant B lymphocytes. Novartis Found Symp. 1998;215:137–144. doi: 10.1002/9780470515525.ch10. [DOI] [PubMed] [Google Scholar]
- 15.Braun J, Sha’afi RI, Unanue ER. Crosslinking by ligands to surface immunoglobulin triggers mobilization of intracellular 45Ca2+ in B lymphocytes. J Cell Biol. 1979;82:755–766. doi: 10.1083/jcb.82.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu QH, Liu X, Wen Z, Hondowicz B, King L, Monroe J, Freedman BD. Distinct calcium channels regulate responses of primary B lymphocytes to B cell receptor engagement and mechanical stimuli. J Immunol. 2005;174:68–79. doi: 10.4049/jimmunol.174.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto Y, Furuno T, Hamano T, Nakanishi M. Confocal fluorescence microscopy for studying thapsigargin-induced bivalent-cation entry into B cells. Biochem J. 1995;305(Pt 3):1011–1015. doi: 10.1042/bj3051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badou A, Jha MK, Matza D, Flavell RA. Emerging roles of L-type voltage-gated and other calcium channels in T lymphocytes. Front Immunol. 2013;4:243. doi: 10.3389/fimmu.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 21.Johenning FW, Theis AK, Pannasch U, Ruckl M, Rudiger S, Schmitz D. Ryanodine receptor activation induces long-term plasticity of spine calcium dynamics. PLoS Biol. 2015;13:e1002181. doi: 10.1371/journal.pbio.1002181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim Biophys Acta. 2015;1853:1980–1991. doi: 10.1016/j.bbamcr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Baba Y, Matsumoto M, Kurosaki T. Calcium signaling in B cells: regulation of cytosolic Ca2+ increase and its sensor molecules, STIM1 and STIM2. Mol Immunol. 2014;62:339–343. doi: 10.1016/j.molimm.2013.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


