Abstract
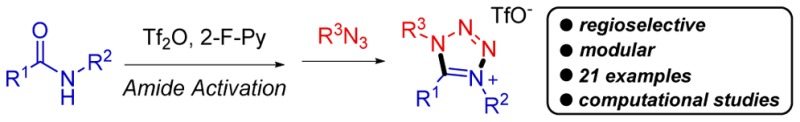
Tetrazolium salts are biologically active molecules that have found broad applications in biochemical assays. A regioselective synthesis of tetrazolium salts is described through a formal (3 + 2) cycloaddition. The possibility of employing simple amides and azides as starting material and the mild conditions allow a broad functional group tolerance.
The tetrazole core is a recurrent fragment in drugs and biologically active compounds.1 In particular, tetrazolium salts occupy a prominent role in clinical biochemistry and have found wide application in histochemical and cytochemical assays, in which they are metabolically transformed to their highly colored reduced form, formazans.2 Furthermore, applications of tetrazolium salts as energetic ionic liquids with a low toxicity have progressively gained more space in the field of materials chemistry.3
Tetrazolium salts were historically prepared by oxidation of the corresponding formazanes (Scheme 1 a).4 In order to increase the level of synthetic flexibility of that method, different approaches have been developed over the past decades. The most common, and obvious, of them is alkylation of preformed aminotetrazoles using suitable alkyl electrophiles (Scheme 1 b).5 However, this simple alkylation approach is plagued by regioselectivity issues, as it is not always possible to access a single regioisomer in a predictable manner. A different approach to obtain 1,4,5-substituted tetrazolium salts would be the synthesis of the tetrazolium core through (3 + 2) cycloaddition between azides and nitrilium ions. This approach was reported in 1976 by Quast and Bieber6 and in 1984 by Carboni7 (Scheme 1c). However, those reactions mandated the use of isolable nitrilium salts as reagents, which considerably narrowed the scope of the method.
Scheme 1. Literature Syntheses of Tetrazolium Salts (a–c) and Method Proposed Herein (d).
Secondary amides are a family of diverse and easily accessible substrates, known to be synthetic precursors of nitrilium ions. Notably, amide activation using triflic anhydride is now a powerful chemoselective synthetic tool allowing the generation of nitrilium ions, among other activated intermediates.8−10 Herein, we report a chemo- and regioselective synthesis of tetrazolium salts through formal (3 + 2) cycloaddition, using alkyl azides and secondary amides as starting materials, where the nitrilium ion is formed in situ through amide activation with triflic anhydride (Scheme 1d). The versatility of the method is showcased by a broad array of successful examples.
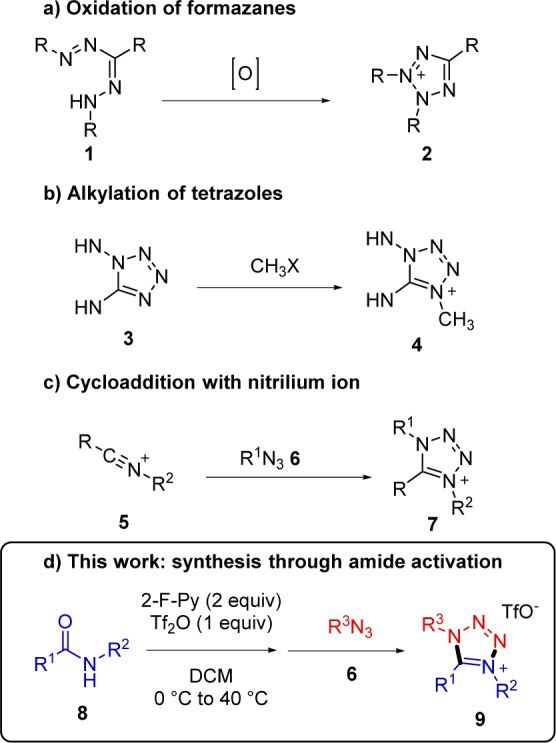
In our initial attempt, performed using the amide activation conditions previously reported by our group, we were pleased to observe that the amide 8a underwent regioselective formation of a single cycloaddition product 9a (Scheme 2). Single-crystal X-ray analysis unambiguously confirmed the proposed connectivity of the 1,4,5-trisubstituted tetrazolium product.
Scheme 2. Initial Attempt and X-ray Analysis.
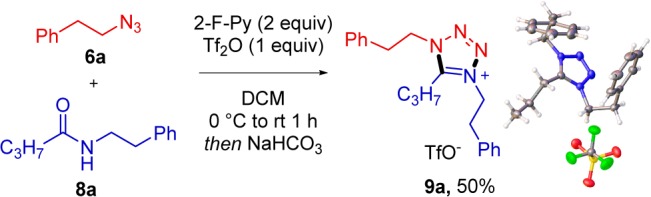
A brief optimization of reaction conditions showed that full conversion was reached when the reaction time was increased from 1 to 12 h, along a temperature increase to 40 °C (Table 1). Aqueous workup was found to be unnecessary, and the reaction mixture was simply purified through fast column chromatography with neutral alumina after evaporation of the solvent to deliver 9b in an excellent 90% yield.
Table 1. Optimization of the Direct Tetrazolium Salt Synthesis.
| entry | time (h) | temp (°C) | workup | yieldb (%) |
|---|---|---|---|---|
| 1 | 0.5 | rt | solvent evaporation | 25 |
| 2 | 1 | rt | solvent evaporation | 74 |
| 3 | 1 | rt | NaHCO3 1 h | 64 |
| 4 | 12 | rt | solvent evaporation | 97 |
| 5 | 12 | 40 | solvent evaporation | 100c |
To a mixture of 8a and 2-F-Py was added Tf2O at 0 °C. After 15 min, 6a was added, and the reaction was allowed to stir at the reported temperature.
NMR yield.
90% isolated yield.
With these optimized conditions, we studied the scope of the reaction (Scheme 3). The reactions were carried out on a 0.2 mmol scale; however, it was possible to perform the reaction on a gram scale without significantly affecting the yield (9b). As depicted, tetrazolium salts were formed in uniformly good to high yields starting from secondary amides carrying various aliphatic chains (9d,g,i,j). Aromatic substituents or unsaturations (9h,k,l) did not disrupt the reaction. Interestingly, other carbonyl functionalities, such as esters (9m), were tolerated, with selective activation at the amide being observed. The use of a large-ring secondary amide as substrate leads to a fused bicyclic tetrazolium as product (9n). Different substituents at nitrogen of the amide partner were also tolerated, as shown in examples 9o and 9p. Unfortunately, N-arylamides were found to give products in low yields.
Scheme 3. Scope of Secondary Amides for the Synthesis of Tetrazolium Salts.
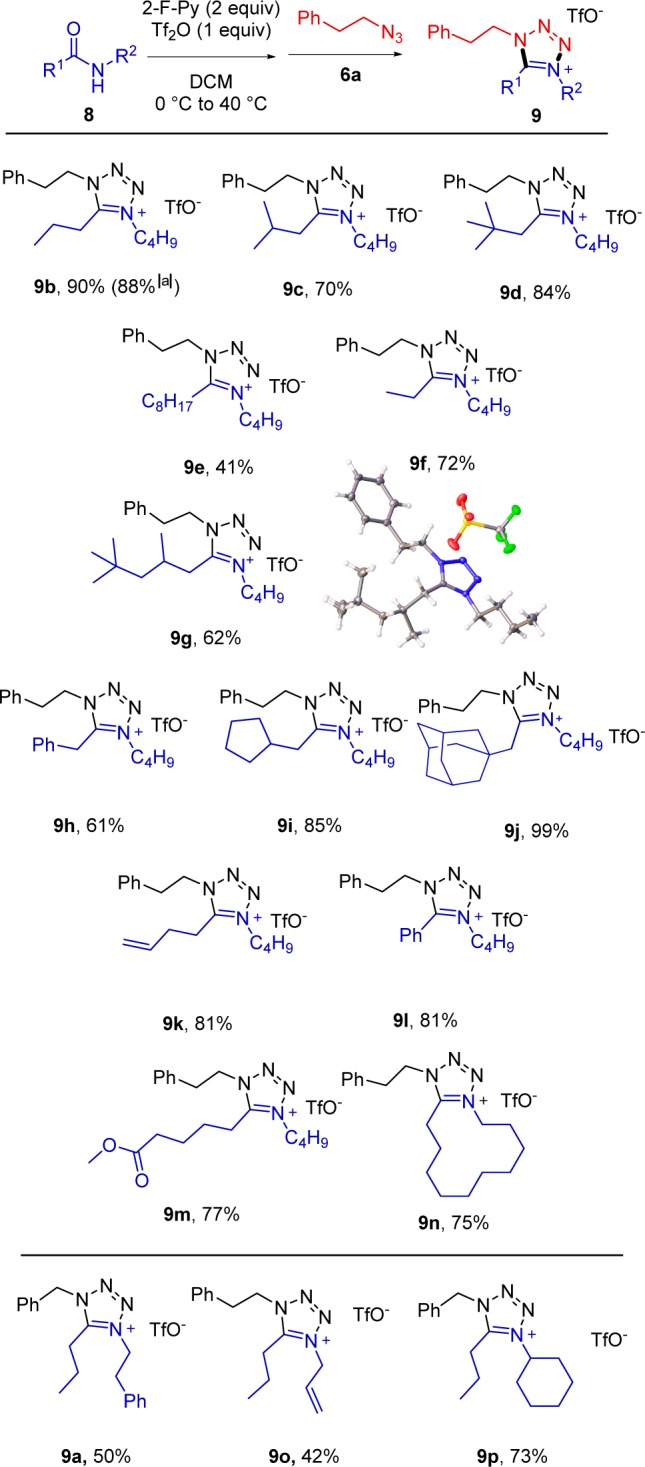
Reaction performed on a 7 mmol scale.
With these results in hand, we decided to examine the possibility of varying the azide partner (Scheme 4). Pleasingly, different functional groups were tolerated, and the reaction proceeded with high yields in the presence of a cyano moiety (9t) or substituted aromatic rings (9r).
Scheme 4. Scope of Azides in the Direct Tetrazolium Synthesis.
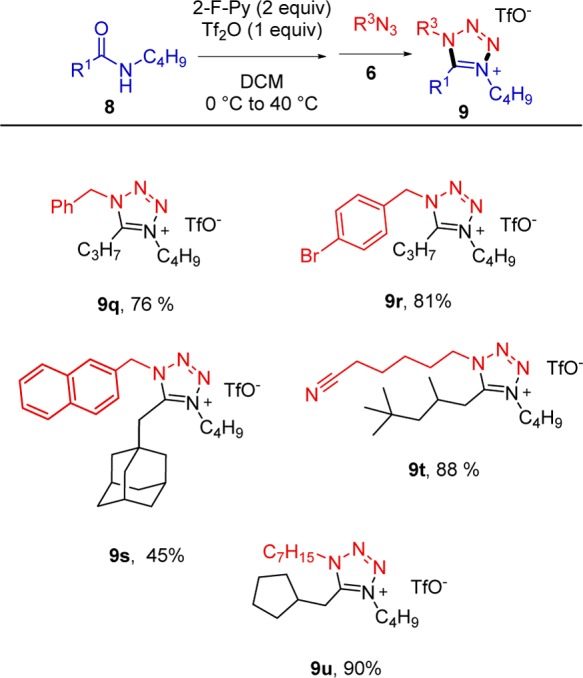
The reaction was also performed with a (naphthylmethyl) azide (9s) with more modest yield; however, again the same regioisomer as in previous examples was observed exclusively, in spite of the considerable steric congestion of product 9s.
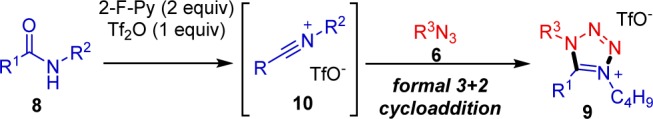
On the basis of prior literature,10 we postulated that amide 8, via formation of an iminium triflate and ensuing 2-F-Py-mediated deprotonation, would lead to a nitrilium ion 10. That intermediate could then react with the alkyl azide in a formal (3 + 2) cycloaddition (Scheme 5).
Scheme 5. Proposed Mechanism.
A concerted cycloaddition mechanism would conceivably allow the formation of two different regioisomers. Experimentally, however, a single regioisomer 9, where the substituents R3 and R1 (see Scheme 5) are located on the same side of the molecule, was consistently observed (cf. Schemes 2–4). Furthermore, the regioselectivity of the process is undisturbed even in cases where steric hindrance would appear to favor an alternative outcome (cf. Scheme 4, 9s). Why is 9 the only observed regioisomer? Is the mechanism of this cycloaddition step concerted? To address these (and other) questions, a quantum chemical study was performed (see the SI for computational details). Indeed, we found that the reaction proceeds via a stepwise and not a concerted mechanism. Figure 1 shows the calculated reaction profile for the model system with R1 = Et, R2 = R3 = Me. The first step is the formation of intermediate B with one newly built C–N bond. The second step is an annulation to the final product C. This mechanism explains the experimentally observed regioselectivity. It must be noted that several attempts to locate a concerted transition state did not lead to any alternative mechanisms.
Figure 1.
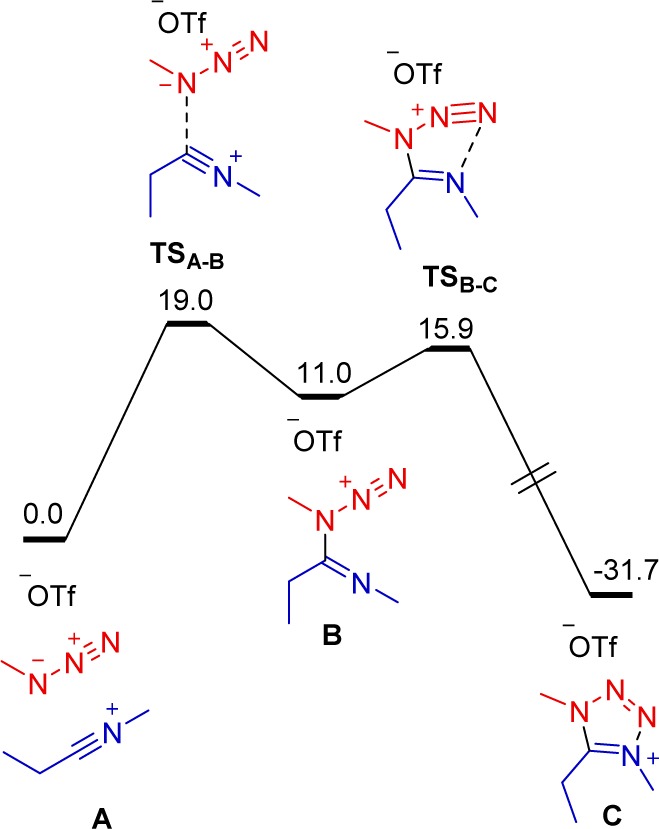
Computed reaction profile (ΔG298,DCM, kcal mol–1) for the direct synthesis of tetrazoliums from nitrilium ions and azides.
Figure 2 shows the computed structures of the reactant complex A (left) and the intermediate B (right). Calculated NBO (Natural Bond Orbital) charges of several atoms are also presented. The attractive electrostatic force between the N4 (the most negatively charged) and C5 (the most positively charged) atoms of the complex A allows the formation of intermediate B and predetermines the regioselectivity. In an analogous manner to the attractive interaction ultimately leading to the N4–C5 bond, there is an attraction between the atoms N2 and N1 in intermediate B which is conducive to the final annulation step.
Figure 2.
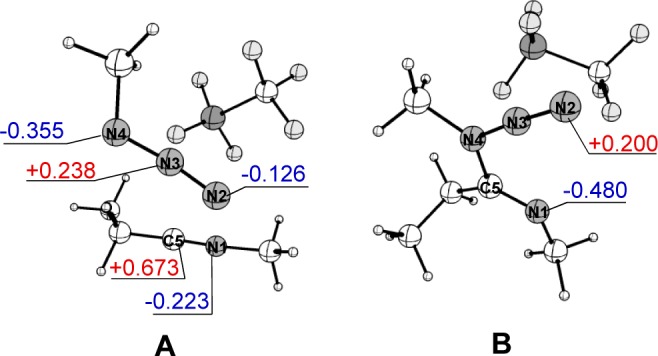
Computed structures of reactant complex A and intermediate B with NBO charges (au).
In conclusion, we report a practical regio- and chemoselective synthesis of tetrazolium salts using simple secondary amides as starting materials through a formal 1,3-dipolar cycloaddition. Our methodology possesses the advantage of forming the highly reactive nitrilium intermediate in situ through amide activation with triflic anhydride and a base under mild conditions, allowing a broad functional group tolerance. Quantum chemical calculations explain the observed regioselectivity and elucidate the stepwise, charge-controlled nature of the reaction mechanism.
Acknowledgments
Generous support of this research by the DFG, FWF, and ERC (StG FLATOUT and CoG VINCAT) is acknowledged. We are grateful to the University of Vienna for continued support of our research program. Calculations were partially performed at the Vienna Scientific Cluster (VSC). N.M. is the recipient of the Elisabeth-Lutz Preis of the Austrian Academy of Sciences. Generous support of this research by the DFG, FWF (P 27194), and ERC 153 (StG FLATOUT and CoG VINCAT) is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b01004.
Experimental procedures, computational details, and full characterization data for new compounds; X-ray data for compound 9a and 9g (PDF)
Author Contributions
† A.d.l.T. and B.M. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Wei C.-X.; Bian M.; Gong G.-H. Molecules 2015, 20, 5528–5553. 10.3390/molecules20045528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Şenöz H. Hacettepe J. Biol. Chem. 2012, 40, 293. [Google Scholar]; b Berridge M. V.; Herst M. P.. Biotech. Ann. Rev. 2005, Elsevier B. V.11, 127. [DOI] [PubMed] [Google Scholar]; c Berridge M. V.; Tan A. S.; McCoy R. D.; Wang R. Biochemica 1996, 4, 14. [Google Scholar]
- Jones C. B.; Haiges R.; Schroer T.; Christe K. O. Angew. Chem., Int. Ed. 2006, 45, 4981. 10.1002/anie.200600735. [DOI] [PubMed] [Google Scholar]
- a Nieham A. W.The Chemistry of Formazans and Tetrazolium Salts, Research Laboratories; May & Baker Ltd.: Essex, 1954. [Google Scholar]; b Buzykin B. I. Chem. Heterocycl. Compd. 2010, 46, 379–408. 10.1007/s10593-010-0523-0. [DOI] [Google Scholar]
- Klapötke T. M.; Mayer P.; Schulz A.; Weigand J. J. J. Am. Chem. Soc. 2005, 127, 2032–2033. 10.1021/ja042596m. [DOI] [PubMed] [Google Scholar]; b Klapötke T. M.; Sabaté C. M.; Rusan M.; Welch J. M. Eur. J. Inorg. Chem. 2009, 2009, 880–896. 10.1002/ejic.200800995. [DOI] [Google Scholar]; c Aridoss G.; Laali K. K. Eur. J. Org. Chem. 2011, 2011, 6343–6355. 10.1002/ejoc.201100957. [DOI] [Google Scholar]
- Quast H.; Bieber L. Tetrahedron Lett. 1976, 17, 1465. [Google Scholar]
- Carboni B.; Carrié R. Tetrahedron 1984, 40, 4115. 10.1016/0040-4020(84)85093-0. [DOI] [Google Scholar]
- For general reviews about amide activation, see:; Charette A. B.; Grenon M. Can. J. Chem. 2001, 79, 1694. 10.1139/v01-150. [DOI] [Google Scholar]; b Kaiser D.; Maulide N. J. Org. Chem. 2016, 81, 4421. 10.1021/acs.joc.6b00675. [DOI] [PubMed] [Google Scholar]
- For recent examples of amide activation, see:; a Movassaghi M.; Hill M. D. J. Am. Chem. Soc. 2006, 128, 4592–4593. 10.1021/ja060626a. [DOI] [PubMed] [Google Scholar]; b Movassaghi M.; Hill M. D.; Ahmad O. K. J. Am. Chem. Soc. 2007, 129, 10096–10097. 10.1021/ja073912a. [DOI] [PubMed] [Google Scholar]; c Barbe G.; Charette A. B. J. Am. Chem. Soc. 2008, 130, 18–19. 10.1021/ja077463q. [DOI] [PubMed] [Google Scholar]; d Pelletier G.; Bechara W. S.; Charette A. B. J. Am. Chem. Soc. 2010, 132, 12817–12819. 10.1021/ja105194s. [DOI] [PubMed] [Google Scholar]; e Bechara W. S.; Pelletier G.; Charette A. B. Nat. Chem. 2012, 4, 228–234. 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]; f Xiao K.-J.; Wang A.-E.; Huang P.-Q. Angew. Chem., Int. Ed. 2012, 51, 8314–8317. 10.1002/anie.201204098. [DOI] [PubMed] [Google Scholar]; g Peng B.; Geerdink D.; Maulide N. J. Am. Chem. Soc. 2013, 135, 14968–14971. 10.1021/ja408856p. [DOI] [PubMed] [Google Scholar]; h Huang P.-Q.; Huang Y.-H.; Xiao K.-J.; Wang Y.; Xia Y.-E. J. Org. Chem. 2015, 80, 2861–2868. 10.1021/jo502929x. [DOI] [PubMed] [Google Scholar]; i Lumbroso A.; Behra J.; Kolleth A.; Dakas P.-Y.; Karadeniz U.; Catak S.; Sulzer-Mossé S.; De Mesmaeker A. Tetrahedron Lett. 2015, 56, 6541–6545. 10.1016/j.tetlet.2015.09.103. [DOI] [Google Scholar]; j Kolleth A.; Lumbroso A.; Tanriver G.; Catak S.; Sulzer-Mossé S.; De Mesmaeker A. Tetrahedron Lett. 2016, 57, 2697–2702. 10.1016/j.tetlet.2016.04.092. [DOI] [Google Scholar]; k Huang P.-Q.; Huang Y.-H.; Xiao K.-J. J. Org. Chem. 2016, 81, 9020–9027. 10.1021/acs.joc.6b01647. [DOI] [PubMed] [Google Scholar]; l Kaiser D.; Maulide N. J. Org. Chem. 2016, 81, 4421–4428. 10.1021/acs.joc.6b00675. [DOI] [PubMed] [Google Scholar]; m Tona V.; de la Torre A.; Padmanaban M.; Ruider S.; González L.; Maulide N. J. Am. Chem. Soc. 2016, 138, 8348–8351. 10.1021/jacs.6b04061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of nitrilium ion generation from amides using triflic anhydride, see:; a Wang A.-E.; Chang Z.; Sun W.-T.; Huang P.-Q. Org. Lett. 2015, 17, 732–735. 10.1021/acs.orglett.5b00004. [DOI] [PubMed] [Google Scholar]; b Zheng J.-F.; Qian X.-Y.; Huang P.-Q. Org. Chem. Front. 2015, 2, 927–935. 10.1039/C5QO00146C. [DOI] [Google Scholar]; c Huang P.-Q.; Ou W.; Ye J.-L. Org. Chem. Front. 2015, 2, 1094. 10.1039/C5QO00191A. [DOI] [Google Scholar]; d Geng H.; Huang P.-Q. Tetrahedron 2015, 71, 3795–3801. 10.1016/j.tet.2015.03.094. [DOI] [Google Scholar]; e Huang P.-Q.; Lang Q.-W.; Wang A. E.; Zheng J.-F. Chem. Commun. 2015, 51, 1096–1099. 10.1039/C4CC08330J. [DOI] [PubMed] [Google Scholar]; f Huang P.-Q.; Huang Y.-H.; Geng H.; Ye J.-L. Sci. Rep. 2016, 6, 28801. 10.1038/srep28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


