Abstract
Objective:
To compare the rate of abnormal brain metabolism by FDG-PET/CT to other paraclinical findings and to describe brain metabolism patterns in autoimmune encephalitis (AE).
Methods:
A retrospective review of clinical data and initial dedicated brain FDG-PET/CT studies for neurology inpatients with AE, per consensus criteria, treated at a single tertiary center over 123 months. Z-score maps of FDG-PET/CT were made using 3-dimensional stereotactic surface projections with comparison to age group–matched controls. Brain region mean Z-scores with magnitudes ≥2.00 were interpreted as significant. Comparisons were made to rates of abnormal initial brain MRI, abnormal initial EEG, and presence of intrathecal inflammation.
Results:
Sixty-one patients with AE (32 seropositive) underwent brain FDG-PET/CT at median 4 weeks of symptoms (interquartile range [IQR] 9 weeks) and median 4 days from MRI (IQR 8.5 days). FDG-PET/CT was abnormal in 52 (85%) patients, with 42 (69%) demonstrating only hypometabolism. Isolated hypermetabolism was demonstrated in 2 (3%) patients. Both hypermetabolic and hypometabolic brain regions were noted in 8 (13%) patients. Nine (15%) patients had normal FDG-PET/CT studies. CSF inflammation was evident in 34/55 (62%) patients, whereas initial EEG (17/56, 30%) and MRI (23/57, 40%) were abnormal in fewer. Detection of 2 or more of these paraclinical findings was in weak agreement with abnormal brain FDG-PET/CT (κ = 0.16, p = 0.02).
Conclusions:
FDG-PET/CT was more often abnormal than initial EEG, MRI, and CSF studies in neurology inpatients with AE, with brain region hypometabolism the most frequently observed.
As early immunotherapy seems to contribute to improved outcomes in autoimmune encephalitis (AE), recent criteria have been proposed to facilitate early diagnosis.1 18F-fluorodeoxy-glucose PET (FDG-PET) is only included in criteria for definite autoimmune limbic encephalitis.1 However, FDG-PET has been recognized as a potentially useful biomarker in suspected AE.2–7 In autoimmune limbic encephalitis, hypermetabolism on FDG-PET in otherwise normal mesial temporal lobe structures by MRI suggests that FDG-PET may be more sensitive than MRI.2–4 Also, particular patterns of metabolism noted by FDG-PET have been identified in certain AE syndromes.8–11 The majority of prior studies of FDG-PET in AE have been limited to qualitative description of FDG-PET findings,2,3,5,6,10,12–16 used nondedicated brain FDG-PET studies,8 have been restricted to specific syndromes,9,11,17,18 or have made limited comparisons to other diagnostic results incorporated in the current clinical criteria.3–6
We sought to semiquantitatively describe dedicated brain FDG-PET/CT findings for neurology inpatients who met recent AE consensus criteria relative to a database of healthy controls, with comparisons between seronegative and seropositive patients with AE. We also sought to describe the rate of abnormal patterns of brain region metabolism relative to other paraclinical findings in these AE cases as well as prior case series.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Board of Johns Hopkins University.
Patients.
We identified admitted patients with AE who underwent FDG-PET/CT at Johns Hopkins Hospital through the course of admission using the diagnostic terms encephalitis and positron emission tomography (PET) to search the administrative database (December 1, 2005, to March 15, 2016). Patients were cross-referenced with the Johns Hopkins Hospital PET/CT Center database.
Included patients underwent a brain FDG-PET/CT study and had possible or definite AE, including definite limbic encephalitis, per consensus criteria.1 Diagnostic findings consistent with AE included MRI and EEG abnormalities and the presence of intrathecal inflammation on routine testing.1 Seropositive patients had a paraneoplastic or cell surface antibody detected in either the serum or the CSF using commercially available assays (Athena Diagnostics, Worcester, MA; Mayo Clinic Laboratories, Rochester, MN).
The electronic medical record was reviewed; data collected were demographic information, clinical history and presentation, diagnostic results, and whether corticosteroids or sedatives were administered within 24 hours preceding FDG-PET/CT study.19,20
Brain FDG-PET/CT review.
Blinded review of brain FDG-PET/CT was performed by 2 board-certified nuclear medicine radiologists (L.S. and M.S.J.). Dedicated 10-minute 3D brain FDG-PET/CT acquisitions were performed as per the institutional clinical protocol following whole-body acquisition and did not require additional radiopharmaceutical dose administration. All brain FDG-PET/CT images were acquired using a Discovery DRX or LX (GE Healthcare, Waukesha, WI) or Biograph mCT (Siemens, Knoxville, TN) in 3D mode for 10 minutes with in-line CT for attenuation correction. Filtered back projection and ordered subset expectation maximization methods were used to reconstruct images, with respective reconstructions used in the blinded review. The reconstructed data sets were fused and projected to predefined surface pixels (3-dimensional stereotactic surface projections) after anatomic standardization.21 Qualitative and quantitative PET image analysis was performed using a commercially available database of over 250 age-stratified healthy controls, CortexID (GE Healthcare).21,22 Z-scores were calculated for standard brain regions, and these regions were also scored as normal, hypometabolic, or hypermetabolic by the 2 board-certified nuclear medicine radiologists. Patients younger than 30 years were compared with the lowest age group of controls for Z-score calculations. The following standard CortexID brain regions were used as they could be reliably validated by radiologists' visual inspection: caudate, cerebellum, frontal lobe, occipital lobe, parietal lobe, and temporal lobe. FDG-PET/CT with regions demonstrating an average Z-score magnitude greater than 2.00 (i.e., greater than 2 SDs abnormal relative to the CortexID database) was recorded as quantitatively abnormal. Using these methods, by chance a healthy control would have a 26% probability of an abnormal study (i.e., hyper- or hypometabolism in at least 1 of the 6 brain regions evaluated). Brain FDG-PET/CT figures were generated using CortexID or the GE Advanced Workstation software package (GE Healthcare).
Review of brain MRI.
Blinded review of brain MRI was performed by 2 fellowship trained neuroradiologists (L.S. and E.Z.). Clinical MRIs were performed as per the institutional protocol at either 1.5- or 3-T on a Philips (Best, Netherlands), GE Healthcare or Siemens (Erlangen, Germany) scanner. For purposes of this study, T2/fluid-attenuated inversion recovery (FLAIR) signal, diffusion-weighted imaging and apparent diffusion coefficient, and T1 pre- and post-administration of gadolinium sequences were reviewed and rated by each reviewer as consistent or inconsistent with AE, with differences in rating reconciled by discussion between the reviewers.
Statistical methods.
The Mann-Whitney U test was used for comparisons of continuous variables. Categorical variables were compared using the χ2 test or Fisher exact test, as appropriate. Kappa measurement of agreement was performed to assess intermodality agreement of MRI, EEG, and CSF inflammatory markers with brain FDG-PET/CT metabolic patterns. Kappa measurement of agreement was performed for brain FDG-PET/CT metabolic patterns with detection of only 1 or at least 2 diagnostic findings consistent with AE. p < 0.05 was considered significant.
A Friedman test was performed to compare median Z-scores across brain regions for all patients. Serial Wilcoxon rank-sum tests were performed to compare Z-scores between brain regions for all patients with p < 0.008 considered significant after Bonferroni correction. Split-plot analyses of variance with significance of p < 0.008 after Bonferroni correction were performed to compare patterns of FDG-avidity across 6 FDG-PET/CT brain regions within patients and between the seropositive and seronegative AE groups, definite and possible AE groups, as well as for those treated with corticosteroids and those treated with sedatives within 24 hours of brain FDG-PET/CT and those not treated. Comparisons included Z-scores for both left and right hemisphere regions for all patients.
Review of the literature.
A PubMed search was performed using positron emission tomography and encephalitis as search terms, updated up to October 27, 2016. Included studies and case series reported brain FDG-PET findings (hypermetabolism and/or hypometabolism) of at least 5 patients with AE or paraneoplastic encephalitis. When provided, reports of abnormalities on brain MRI, EEG, and CSF assays were reviewed.
RESULTS
Clinical characteristics of patients with AE undergoing brain FDG-PET/CT.
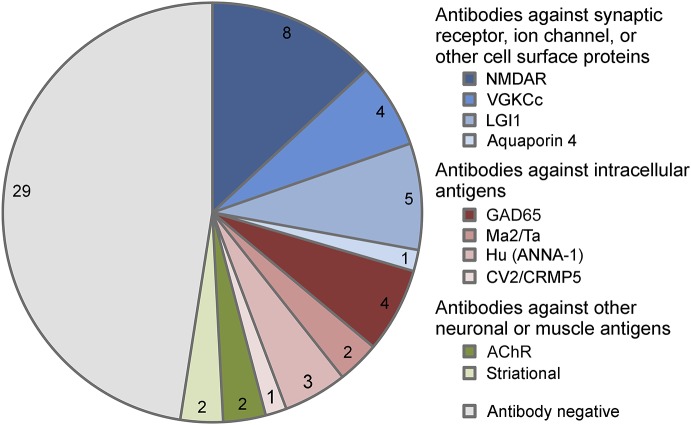
Of the 296 inpatients with the diagnosis of encephalitis, 61 patients met the consensus criteria for AE and underwent brain FDG-PET/CT with studies available for review (table 1). Thirty-two of the 61 (52%) patients had antibodies identified in the serum or CSF, 28 of whom with antibodies with known AE/paraneoplastic encephalitis significance, 24/61 (39%) with definite AE antibodies (figure 1). Of the other seropositive patients, 4 were anti–voltage-gated potassium channel-complex (VGKCc) seropositive for antibodies different from anti-LGI1 and anti-CASPR2 (3 with supportive CSF, EEG, and/or MRI); 2 were anti-α3 AChR seropositive (1 with supportive EEG); and 2 were anti–striational antibody seropositive (1 with supportive CSF and MRI). Two of the 4 anti–GAD65-seropositive patients had reviewable antibody levels (9,500 and 53,650 U/mL), whereas all had supportive CSF, EEG, and/or MRI.
Table 1.
Clinical characteristics of patients with AE
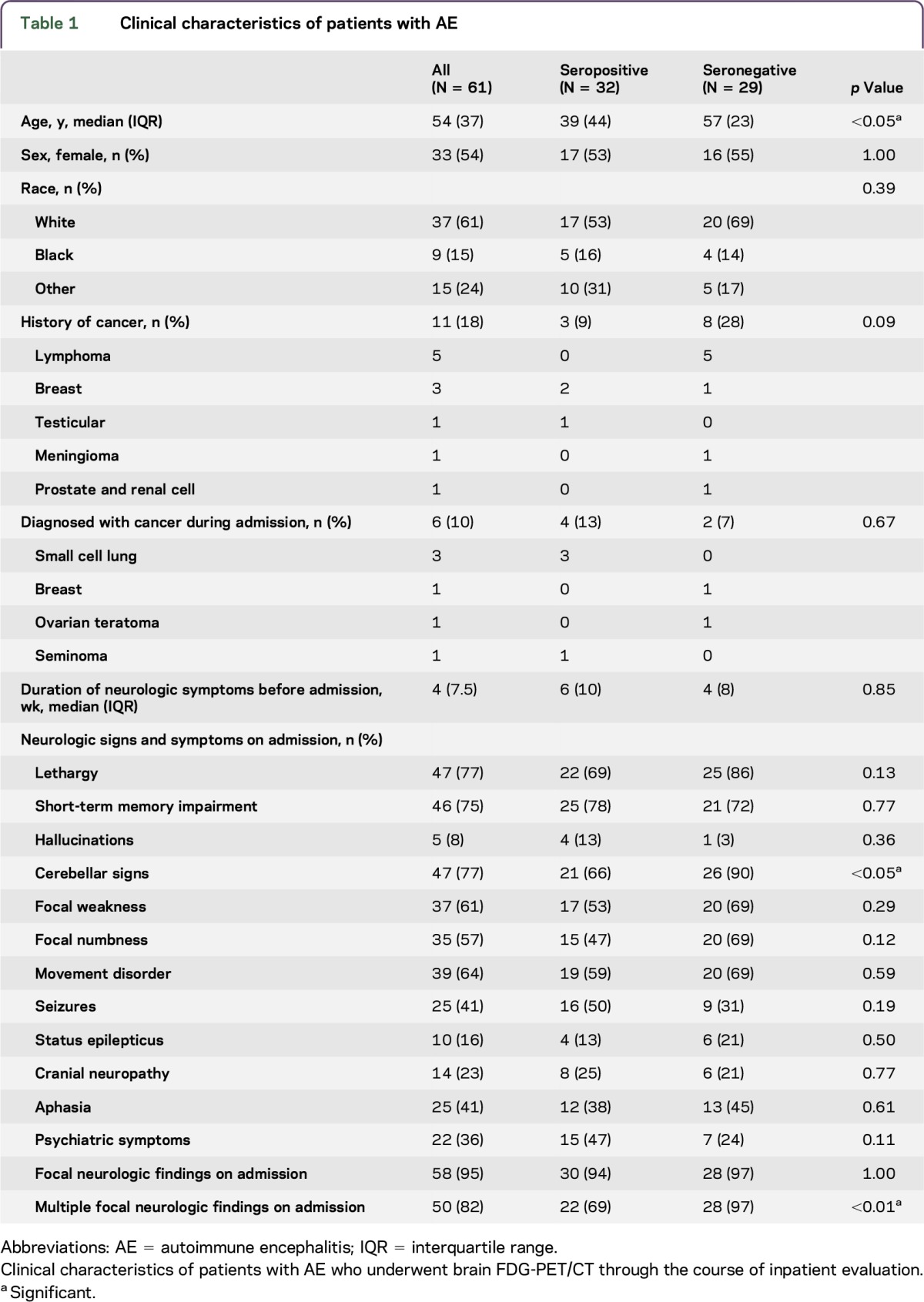
Figure 1. Antibody status of patients with AE.
Antibody status of patients with AE who underwent dedicated brain FDG-PET/CT (N = 61). AE = autoimmune encephalitis; ANNA-1 = anti–neuronal nuclear antibody 1; CRMP5 = collapsin response mediator protein 5; GAD65 = 65 kDa glutamic acid decarboxylase enzyme; VGKCc = voltage-gated potassium channel-complex antibodies different from leucine-rich inactivated 1 protein (LGI1) and contactin-associated protein-2 (CASPR2); AChR = acetylcholine receptor antibody.
Seropositive patients were younger than the seronegative patients (median 39 vs 57 years, p = 0.01). Durations of symptoms before admission were similar for both groups (median 6 vs 4 weeks, p = 0.85). Twelve of the 13 patients were younger than 30 years (5 anti-NMDA receptors [NMDARs]). Fourteen (23%) patients' CSF were tested for antibodies, with the anti–NMDAR antibody detected in 3 patients, all of whom were negative in the serum. No other antibodies were detected in tested CSF samples. Of the 17 patients with a history or subsequent diagnosis of cancer, 7 were found to be seropositive: small cell lung cancer (1 anti-GAD65, 1 anti-Hu, and 1 anti-CV2), breast (1 anti-NMDAR and 1 anti-LGI1), seminoma (1 anti-Ma2), and testicular cancer (1 anti-Ma2).
Other paraclinical findings.
Routine CSF studies were consistent with intrathecal inflammation in 34/55 patients (62%, figure e-1 at Neurology.org/nn). Initial EEG for 17/56 (30%) patients demonstrated temporal area slowing, epileptiform discharges, or seizures consistent with the diagnosis of AE. This was more frequently observed among patients younger than 30 years (8/13, 62%) than others (9/43, 21%, p = 0.01, table e-1). Brain MRI studies for 57 patients were available for blinded review, and 23 (40%) were consistent with the diagnosis of AE. No differences were observed across antibody status, antibody class, or AE classification (table e-1).
Fifty-one of the 61 patients (84%) had at least 1 paraclinical finding consistent with AE on routine CSF analysis, brain MRI, or EEG. Thirty-one patients (51%) had only 1 paraclinical finding; 17 patients (28%) had 2 findings; and 3 patients (5%) had 3 findings consistent with AE. Ten patients were included based on clinical criteria, 4 of whom with definite AE based on detected antibodies, and 3 seropositive for other antibodies (table e-2).
Brain FDG-PET/CT findings.
Brain FDG-PET/CT was performed a median of 4 weeks after symptom onset (interquartile range [IQR] 9 weeks) and a median of 4 days (IQR 8.5 days) from brain MRI. Brain FDG-PET/CT was abnormal in 52/61 patients (85%, figure e-1) when compared with the healthy control database. FDG-PET/CT demonstrated brain region hypometabolism alone in 42/61 (69%), hypermetabolism alone in 2/61 (3%) patients, and regions of abnormal hypometabolism and abnormal hypermetabolism in 8/61 (13%) of patients (figure e-1, table e-3). No differences were observed across age group, antibody status, antibody class, or AE classification (table e-1). No difference in proportion with abnormal metabolism was noted between those evaluated by FDG-PET/CT within 4 weeks of symptoms (27/31 [87%]) and those evaluated later (25/30 [83%], p = 0.73).
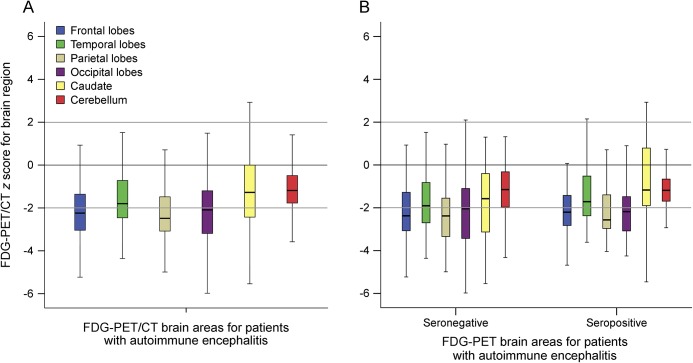
Across brain regions in patients with AE, metabolism was greater for the caudate (−1.28, IQR 2.43) relative to the frontal (−2.24, IQR 2.69, p < 0.005), temporal (−1.80, IQR 1.77, p = 0.002), parietal (−2.49, IQR 1.61, p < 0.005), and occipital (−2.09, IQR 2, p < 0.005) brain regions (p < 0.005, figure 2A). Brain region metabolism patterns did not vary between seropositive and seronegative AE patient groups (F(1,120) = 3.18, p = 0.08, ηp2 0.03, figure 2B) nor definite AE and possible AE (F(1,120) = 2.69, p = 0.10, ηp2 0.02). Similarly, brain region metabolism patterns did not vary between those treated with corticosteroids (F(1,120) = 0.200, p = 0.656, ηp2 0.002) nor those treated with sedatives (F(1,120) = 1.95, p = 0.165, ηp2 0.016) within 24 hours of brain FDG-PET/CT and those not treated.
Figure 2. Metabolism across brain regions in AE.
Boxplots of Z-scores for FDG-avidity for brain areas on dedicated FDG-PET/CT for (A) patients meeting consensus criteria for AE, (B) seronegative and seropositive patients meeting the consensus criteria for AE. Z-scores varied across brain regions for patients with AE (p < 0.005), with values for the caudate being greater than those for frontal (p < 0.005), temporal (p = 0.002), parietal (p < 0.005), and occipital (p < 0.005) lobes. No difference was noted between seronegative and seropositive patient groups (p = 0.08). AE = autoimmune encephalitis.
Concordance between FDG-PET/CT and other paraclinical findings.
The finding of an abnormal metabolic pattern on brain FDG-PET/CT was not in agreement with the presence of CSF inflammation on routine assessment (table e-4). By contrast, brain MRI findings consistent with AE were in weak agreement with the finding of abnormal metabolism on brain FDG-PET/CT (κ = 0.17, p < 0.05), most notably with hypometabolism (κ = 0.25, p < 0.05; table e-4, figure 3). In general, the presence of any FDG-PET/CT abnormality was not in agreement with the presence of EEG findings consistent with AE (table e-4). However, detection of EEG findings consistent with the diagnosis of AE was in weak agreement with detection of brain region hypermetabolism (κ = 0.16, p < 0.05) and in fair agreement with having regions of both hypermetabolism and hypometabolism in the same FDG-PET/CT study (κ = 0.26, p < 0.05, table e-4).
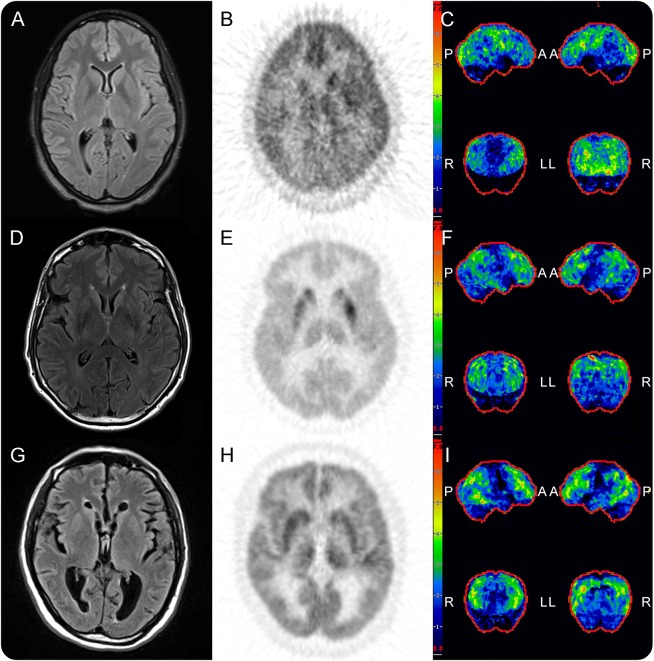
Figure 3. Brain MRI, brain FDG-PET/CT and hypometabolic 3D-SSP maps for 3 patients with AE.
Brain MRI, brain FDG-PET/CT, and hypometabolic 3D-SSP maps, respectively, for patients with anti-NMDAR encephalitis (A–C), anti-LGI1 encephalitis (D–F), and seronegative AE (G–I). For the anti-NMDAR encephalitis patient, note normal T2/FLAIR MRI (A) with right basal ganglia, right frontotemporoparietal, left frontal, and bilateral posterior cortical hypometabolism centered on the middle occipital lobe on FDG-PET/CT and 3D-SSP maps (B and C). For the anti-LGI1 patient, note normal T2/FLAIR MRI (D) with relatively normal basal ganglia metabolism (E) in setting of diffuse frontotemporoparietal hypometabolism on FDG-PET/CT and 3D-SSP maps (E and F). For the seronegative AE patient, again note normal T2/FLAIR MRI (G) with diffuse frontotemporoparietal hypometabolism on FDG-PET/CT and 3D-SSP maps (H and I). A = anterior; AE = autoimmune encephalitis; L = left; LGI1 = leucine-rich inactivated 1 protein; NMDAR = NMDA receptor; P = posterior; R = right; and 3D-SSP, 3-dimensional stereotactic surface projection.
Detection of at least 1 paraclinical finding consistent with AE was not in agreement with detection of abnormal metabolism by FDG-PET/CT (table e-4). Detection of 2 or more consistent findings was in weak agreement with detection of abnormal brain metabolism by FDG-PET/CT (κ = 0.16, p = 0.02; table e-4).
Literature review.
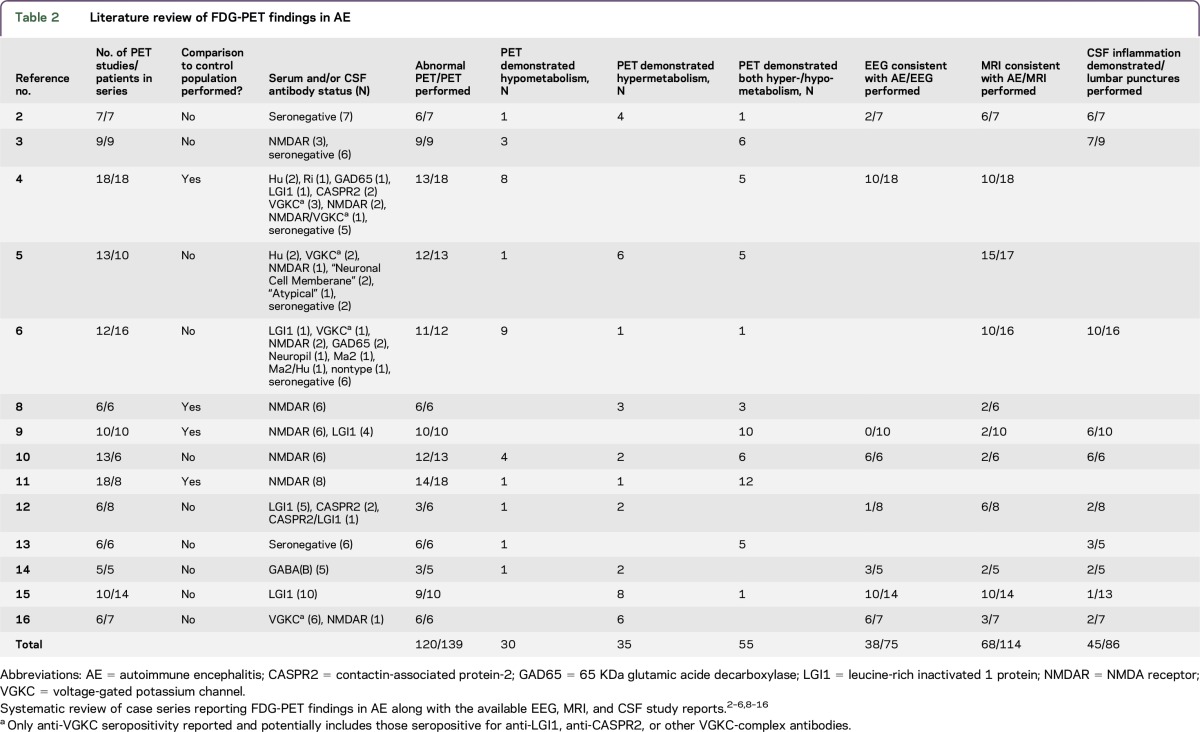
Fourteen studies were identified which met the inclusion criteria (table 2). Of the 139 FDG-PET studies reported, 120 (86%) were abnormal, with 55 (40%) demonstrating both hypo- and hypermetabolism, 30 (22%) demonstrating only hypometabolism, and 35 (25%) demonstrating only hypermetabolism. This is compared with the sum report of 38/75 (51%) EEGs, 68/114 (60%) brain MRI, and 45/86 (52%) routine CSF studies consistent with the diagnosis of possible AE.
Table 2.
Literature review of FDG-PET findings in AE
DISCUSSION
Here, we describe dedicated semiquantitative brain FDG-PET/CT findings among patients meeting the consensus AE criteria. Dedicated brain FDG-PET/CT was abnormal in 85% of patients with AE, and FDG-PET abnormalities were more sensitive for AE compared with EEG, MRI, or routine CSF findings. Although brain region hypometabolism was most commonly noted, some studies demonstrated areas of both hyper- and hypometabolism and a minority demonstrated hypermetabolism alone. The combination of abnormalities in at least 2 of the 3 other paraclinical tests (routine CSF studies, brain MRI, and EEG) was in fair agreement with abnormal findings on dedicated brain FDG-PET/CT. Our results suggest that brain FDG-PET/CT may be helpful in supporting evidence of brain dysfunction in suspected patients with AE.
Brain region hypometabolism in multiple regions likely reflects widespread impairment of neuronal activity in AE.23 Whether such hypometabolism results from functional changes, structural changes, or a combination of both is not yet clear. Many of the areas of regional hypometabolism did not have correlates on MRI, suggesting the possibility of neuronal dysfunction in the absence of structural disturbance. Longitudinal studies will be needed to clarify whether the observed hypometabolism in AE is reversible. Moreover, although widespread regional hypometabolism was observed across various AE syndromes, there are likely syndrome-specific patterns of brain region metabolism.8,9,15 Previous series primarily report hypermetabolism in AE. These series contain larger proportions of patients with anti-NMDAR (36/130 reported patients) or patients with anti-LGI1, anti-CASPR2, or anti-VGKCc antibodies (39/130) than our cohort, potentially limiting their generalizability to other seropositive and seronegative AE.4–6 We observed brain region hypermetabolism in a subset of patients, many of whom had anti-NMDAR or anti-VGKCc encephalitis, compatible with previous literature.5,8–12,15
The current AE consensus criteria only include FDG-PET findings in the criteria for definite autoimmune limbic encephalitis. Bilateral FLAIR/T2 abnormalities of the medial temporal lobes are required, and in the absence of such findings, FDG-PET hypermetabolism in the medial temporal lobes may meet this requirement. Observations provided here suggest that AE may lead to broader metabolic abnormalities detectable by FDG-PET outside the confines of the medial temporal lobes and these may inform future FDG-PET AE criteria.
Concerns raised regarding the incorporation of FDG-PET in the evaluation of patients with AE include availability of FDG-PET imaging modalities in urgent clinical situations. Moreover, as a newer modality, further work is needed to validate it as a method in the diagnosis of AE.24 Worldwide, FDG-PET/CT represents one of the medical imaging modalities with the largest growth in terms of number of scanners.25 In addition, FDG-PET/CT has been found to be diagnostically superior to other conventional imaging modalities in other clinical settings, and it has demonstrated cost-effectiveness in settings such as non–small lung cancer staging.25 FDG-PET also plays an important role in screening for occult malignancy in paraneoplastic syndromes, including encephalitis.26 Thus, FDG-PET is likely to become an increasingly used modality in the evaluation of patients with suspected AE beyond occult malignancy screening. Many institutions use a “vertex to toe” field of view for their whole-body protocols. The addition of a 10-minute dedicated 3D PET acquisition of the brain requires no extra radiopharmaceutical administration, is easily incorporated in conventional clinical workflows, provides increased statistical quality in comparison with “vertex to toe” imaging, and allows for higher-resolution images with more robust quantitation. As the utility of FDG-PET is evaluated further, collaborative evaluation by neurologists and radiologists will be necessary for careful characterization and correlation of syndromes with associated imaging findings, with comparisons to healthy and other neurologic patient populations.
A major limitation of this study is that it is retrospective, involving all patients meeting the criteria for AE who underwent FDG-PET/CT at a single tertiary medical center with associated selection bias. Although performed at a single center, it benefits from the consensus inclusion criteria for AE and uniformity of PET equipment, protocols, and analyses. Also, the observations reported here were limited to those patients admitted to the hospital for onset of symptoms of 3 months or less and do not include findings for those with longer duration of symptoms. Future prospective studies involving serial FDG-PET studies may help clarify the specificity and evolution of patterns of metabolism through the phases of encephalitis, as has been suggested in cases series of specific encephalitides such as anti-NMDAR encephalitis.11 Our study included the initial FDG-PET studies for patients regardless of antibody status, and future larger prospective studies of specific antibody syndromes may further clarify patterns of abnormality, pattern associations with clinical status, and pattern changes in the setting of immune therapy as has been observed in cases of autoimmune dementia.27 Not all patients underwent CSF antibody testing, which may be more sensitive, and thus we may underestimate the number of seropositive patients. However, there was no difference noted in brain region metabolism between seropositive and seronegative groups. Also, 4 patients were anti-VGKCc seropositive without further specification, and although 3 had other findings supportive of AE, the clinical value of such antibodies is unknown and cautious interpretation is advised. One-third of patients studied here were treated with either corticosteroids or sedatives within 24 hours of FDG-PET/CT. Although both corticosteroids and sedatives have been reported to decrease cortical metabolism,19,20 no differences in brain region metabolism were noted between patients exposed and unexposed to these medications before FDG-PET/CT. In addition, FDG-PET/CT metabolism patterns for patients with AE were not compared with other patients with neurologic diseases (such as infectious encephalitis); psychiatric diseases; intoxications; or other syndromes which may also have abnormal FDG-PET findings.21,22,28–31 It will be important for future prospective studies to incorporate patients with other neurologic, psychiatric, and medical diseases to assess the specificity of metabolic findings by FDG-PET described here. Finally, the CortexID control population used for comparison ranges from 30 to 85 years. The 13 patients younger than 30 years studied had a similar rate of abnormal brain region metabolism compared with those older. Ideally, a concurrent age- and sex-matched control population could be used for direct comparison, although such data collection is limited by the radiation exposure to otherwise normal patients.
Here, brain FDG-PET/CT was commonly abnormal in AE, most often demonstrating brain region hypometabolism. The frequency of metabolic abnormalities was greater than that of diagnostic studies currently included in consensus criteria for the diagnosis of AE. Overall, FDG-PET/CT may represent a sensitive and early biomarker for AE and could play a complementary role to currently proposed tests in the diagnosis of AE. Future prospective studies may further clarify the role FDG-PET may play in the diagnosis and monitoring of AE in general and specific antibody syndromes in particular.
Supplementary Material
GLOSSARY
- AE
autoimmune encephalitis
- FDG-PET
18F-fluorodeoxy-glucose PET
- FLAIR
fluid-attenuated inversion recovery
- IQR
interquartile range
- NMDAR
NMDA receptor
- VGKCc
voltage-gated potassium channel-complex
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Dr. Probasco: design and conceptualization of the study, analysis and interpretation of the data, and drafting and revising of the manuscript. Dr. Solnes: design and conceptualization of the study, analysis and interpretation of the data, and revising of the manuscript. Mr. Nalluri: analysis and interpretation of data. Mr. Cohen, Dr. Jones, and Dr. Zan: analysis and interpretation of the data and revising of the manuscript. Dr. Javadi and Dr. Venkatesan: design and conceptualization of the study, analysis and interpretation of the data, and revising of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
J.C. Probasco serves on the editorial board for The Neurohospitalist, is an associate editor for The Neurohospitalist, and is editor-in-chief for NEJM Journal Watch Neurology. L. Solnes, A. Nalluri, J. Cohen, K.M. Jones, E. Zan, and M.S. Javadi report no disclosures. A. Venkatesan received speaker honoraria from Almirall, served as a medical expert for U.S. Government Vaccine Injury Compensation Program, received research support from NIH, and served as medical expert for Carnival Cruise Lines. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Graus F, Titulaer MJ, Balu R, et al. . A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ances BM, Vitaliani R, Taylor RA, et al. . Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain 2005;128:1764–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher RE, Patel NR, Lai EC, Schulz PE. Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med 2012;37:e213–e218. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 2013;260:2744–2753. [DOI] [PubMed] [Google Scholar]
- 5.Masangkay N, Basu S, Moghbel M, Kwee T, Alavi A. Brain 18F-FDG-PET characteristics in patients with paraneoplastic neurological syndrome and its correlation with clinical and MRI findings. Nucl Med Commun 2014;35:1038–1046. [DOI] [PubMed] [Google Scholar]
- 6.Aupy J, Collongues N, Blanc F, Tranchant C, Hirsch E, De Seze J. Autoimmune encephalitis, clinical, radiological and immunological data [in French]. Rev Neurol [Paris] 2013;169:142–153. [DOI] [PubMed] [Google Scholar]
- 7.Morbelli S, Djekidel M, Hesse S, Pagani M, Barthel H. Role of (18)F-FDG-PET imaging in the diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:1009–1010. [DOI] [PubMed] [Google Scholar]
- 8.Leypoldt F, Buchert R, Kleiter I, et al. . Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry 2012;83:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegner F, Wilke F, Raab P, et al. . Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol 2014;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagarde S, Lepine A, Caietta E, et al. . Cerebral (18)FluoroDeoxy-Glucose Positron Emission Tomography in paediatric anti N-methyl-d-aspartate receptor encephalitis: a case series. Brain Dev 2016;38:461–470. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, Guan H, Zhou X, et al. . Changing brain metabolism patterns in patients with ANMDARE: serial 18F-FDG PET/CT findings. Clin Nucl Med 2016;41:366–370. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Xing XW, Zhang JT, et al. . Autoimmune encephalitis mimicking sporadic Creutzfeldt-Jakob disease: a retrospective study. J Neuroimmunol 2016;295-296:1–8. [DOI] [PubMed] [Google Scholar]
- 13.Lee BY, Newberg AB, Liebeskind DS, Kung J, Alavi A. FDG-PET findings in patients with suspected encephalitis. Clin Nucl Med 2004;29:620–625. [DOI] [PubMed] [Google Scholar]
- 14.Kim TJ, Lee ST, Shin JW, et al. . Clinical manifestations and outcomes of the treatment of patients with GABAB encephalitis. J Neuroimmunol 2014;270:45–50. [DOI] [PubMed] [Google Scholar]
- 15.Shin YW, Lee ST, Shin JW, et al. . VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013;265:75–81. [DOI] [PubMed] [Google Scholar]
- 16.Newey CR, Sarwal A, Hantus S. [(18)F]-Fluoro-deoxy-glucose positron emission tomography scan should be obtained early in cases of autoimmune encephalitis. Autoimmune Dis 2016;2016:9450452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EM, Kang JK, Oh JS, Kim JS, Shin YW, Kim CY. 18F-Fluorodeoxyglucose positron-emission tomography findings with anti-N-methyl-d-aspartate receptor encephalitis that showed variable degrees of catatonia: three cases report. J Epilepsy Res 2014;4:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novy J, Allenbach G, Bien CG, Guedj E, Prior JO, Rossetti AO. FDG-PET hyperactivity pattern in anti-NMDAr encephalitis. J Neuroimmunol 2016;297:156–158. [DOI] [PubMed] [Google Scholar]
- 19.Fulham MJ, Brunetti A, Aloj L, Raman R, Dwyer AJ, Di Chiro G. Decreased cerebral glucose metabolism in patients with brain tumors: an effect of corticosteroids. J Neurosurg 1995;83:657–664. [DOI] [PubMed] [Google Scholar]
- 20.Matheja P, Weckesser M, Debus O, et al. . Drug-induced changes in cerebral glucose consumption in bifrontal epilepsy. Epilepsia 2000;41:588–593. [DOI] [PubMed] [Google Scholar]
- 21.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer's disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238–1248. [PubMed] [Google Scholar]
- 22.Josephs KA, Duffy JR, Strand EA, et al. . Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135:1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graus F, Dalmau J. Role of (18)F-FDG-PET imaging in the diagnosis of autoimmune encephalitis - Authors' reply. Lancet Neurol 2016;15:1010. [DOI] [PubMed] [Google Scholar]
- 25.Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, Schreyogg J. Economic evaluation of PET and PET/CT in oncology: evidence and methodologic approaches. J Nucl Med Technol 2010;38:6–17. [DOI] [PubMed] [Google Scholar]
- 26.McKeon A, Apiwattanakul M, Lachance DH, et al. . Positron emission tomography-computed tomography in paraneoplastic neurologic disorders: systematic analysis and review. Arch Neurol 2010;67:322–329. [DOI] [PubMed] [Google Scholar]
- 27.Flanagan EP, McKeon A, Lennon VA, et al. . Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clinic Proc 2010;85:881–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster NL, Heidebrink JL, Clark CM, et al. . FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain 2007;130:2616–2635. [DOI] [PubMed] [Google Scholar]
- 29.Wong KK, Tolia B, Bohnen N. Chronic sequelae of herpes simplex encephalitis demonstrated on interictal F-18 FDG PET/CT. Clin Nucl Med 2008;33:443–444. [DOI] [PubMed] [Google Scholar]
- 30.Hubele F, Bilger K, Kremer S, Imperiale A, Lioure B, Namer IJ. Sequential FDG PET and MRI findings in a case of human herpes virus 6 limbic encephalitis. Clin Nucl Med 2012;37:716–717. [DOI] [PubMed] [Google Scholar]
- 31.Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 2010;11:642–651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.