ABSTRACT
Toxoplasma gondii and Neospora caninum (both Apicomplexa) are closely related cyst-forming coccidian parasites that differ significantly in their host ranges and ability to cause disease. Unlike eutherian mammals, Australian marsupials (metatherian mammals) have long been thought to be highly susceptible to toxoplasmosis and neosporosis because of their historical isolation from the parasites. In this study, the carnivorous fat-tailed dunnart (Sminthopsis crassicaudata) was used as a disease model to investigate the immune response and susceptibility to infection of an Australian marsupial to T. gondii and N. caninum. The disease outcome was more severe in N. caninum-infected dunnarts than in T. gondii-infected dunnarts, as shown by the severity of clinical and histopathological features of disease and higher tissue parasite burdens in the tissues evaluated. Transcriptome sequencing (RNA-seq) of spleens from infected dunnarts and mitogen-stimulated dunnart splenocytes was used to define the cytokine repertoires. Changes in mRNA expression during the time course of infection were measured using quantitative reverse transcription-PCR (qRT-PCR) for key Th1 (gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α]), Th2 (interleukin 4 [IL-4] and IL-6), and Th17 (IL-17A) cytokines. The results show qualitative differences in cytokine responses by the fat-tailed dunnart to infection with N. caninum and T. gondii. Dunnarts infected with T. gondii were capable of mounting a more effective Th1 immune response than those infected with N. caninum, indicating the role of the immune response in the outcome scenarios of parasite infection in this marsupial mammal.
KEYWORDS: apicomplexan parasites, cytokines, real-time PCR, transcriptomics
INTRODUCTION
Neospora caninum and Toxoplasma gondii (Apicomplexa: Coccidia) are tissue cyst-forming parasites with a worldwide distribution. Although these closely related parasites share some similar morphological and biological features, they exhibit key differences in host range and pathogenicity (1, 2). Toxoplasma gondii is regarded as one of the most successful parasites due to its capacity to infect and cause disease in essentially any mammalian or avian species (3). In humans, it is also considered a pathogen of particular significance for pregnant and immunocompromised individuals (3, 4). In contrast, N. caninum is capable of infecting many different species but is not zoonotic and mainly causes disease in cattle and dogs (2, 5). In most immunocompetent species, N. caninum and T. gondii infections are subclinical and severe disease is uncommon (3, 5).
There is increasing interest in defining the role of the host immune response in T. gondii and N. caninum infection outcomes and in identifying the immune factors that influence control of infection and disease development (2, 3, 6–9). Numerous studies in eutherian models, particularly in mice, show the host immune response is a key determinant of disease outcome following infection with N. caninum and T. gondii (2, 3, 7, 9–12). Specifically, an efficient cell-mediated Th1 immune response, effected predominately by the proinflammatory cytokine gamma interferon (IFN-γ) and to a lesser extent by tumor necrosis factor alpha (TNF-α), is critical for controlling disease by restricting parasite replication and inducing chronic latent infections through parasite stage conversion and the formation of tissue cysts (11, 13–15). In contrast, a shift in bias toward a humoral Th2 immune response, characterized by increased expression of the anti-inflammatory cytokine interleukin 4 (IL-4), is associated with recrudescence of infection, uncontrolled parasite replication and pathological sequelae due to inhibitory effects of Th2 cytokines on Th1 immunity (11, 13, 16–19). More recently, IL-17, the signature cytokine of the Th17 immune response, which is promoted by the proinflammatory cytokine IL-6, has been implicated in protection against T. gondii and N. caninum infection and in immunopathology associated with disease (20–23). While there are many advantages to using laboratory mice for T. gondii and N. caninum investigations, common laboratory mouse strains are highly susceptible to toxoplasmosis and generally resistant to neosporosis (7, 24). Currently, nothing is known about the marsupial host immune response to N. caninum and T. gondii infection.
For millennia, Australian native marsupials evolved in geographic isolation without exposure to T. gondii and N. caninum, until their definitive hosts (cats for T. gondii and dogs and dingoes for N. caninum) were introduced to the continent (3, 25, 26). Current dogma asserts that Australian marsupials are particularly sensitive to developing severe, often fatal toxoplasmosis (3). However, the majority of debilitating marsupial toxoplasmosis cases are described for captive animals, and there are many reports of T. gondii infection in asymptomatic free-ranging fauna (3, 27–30). Importantly, few experimental investigations to study T. gondii infection have been conducted in marsupials (31–33). Even less is known about the effect of N. caninum infection in native marsupials. Seroprevalence surveys in Australian cattle and dogs have established that N. caninum infection may be common in these species, and yet, prevalence studies in native wildlife are lacking (34–40). As a result, the significance of N. caninum as a disease threat for wild populations of marsupials is unknown.
The fat-tailed dunnart (Sminthopsis crassicaudata) is a shrew-sized, arid-zone, carnivorous dasyurid marsupial that is closely related to the endangered iconic Tasmanian devil and is one of the few available laboratory-bred marsupial models (41). Recent experimental infections in the fat-tailed dunnart have demonstrated that this species is highly susceptible to N. caninum and that the resultant disease is associated with severe clinical signs, overwhelming systemic infection, and the production of numerous tissue cysts (25). This outcome was unexpected, given that most immunocompetent animals are largely resistant to neosporosis and tissue cysts are rarely found in infected animals. Accordingly, the fat-tailed dunnart was identified as a valuable experimental model for Neospora research, especially for the discovery of the mechanisms contributing to the rapid onset of disease in an immunocompetent species.
Defining the immune response to T. gondii and N. caninum is critical to understanding the pathogenesis of neosporosis and toxoplasmosis in marsupial species. The purpose of this study was to investigate the role of the host immune response in the development of disease subsequent to infection with N. caninum and T. gondii. The unique animal model, the fat-tailed dunnart, was used as a laboratory host due to its known susceptibility to N. caninum. The aims of the present study were to characterize the histopathological changes, parasite dissemination, and cytokine expression profiles for IFN-γ, TNF-α, IL-4, IL-17A, and IL-6 at early time points of N. caninum and T. gondii infection (Fig. 1). This study presents the first investigation of the marsupial host immune response to T. gondii and N. caninum, providing valuable information about the potential impact of these parasites on native host species and furthering our knowledge about host-parasite interactions in marsupials.
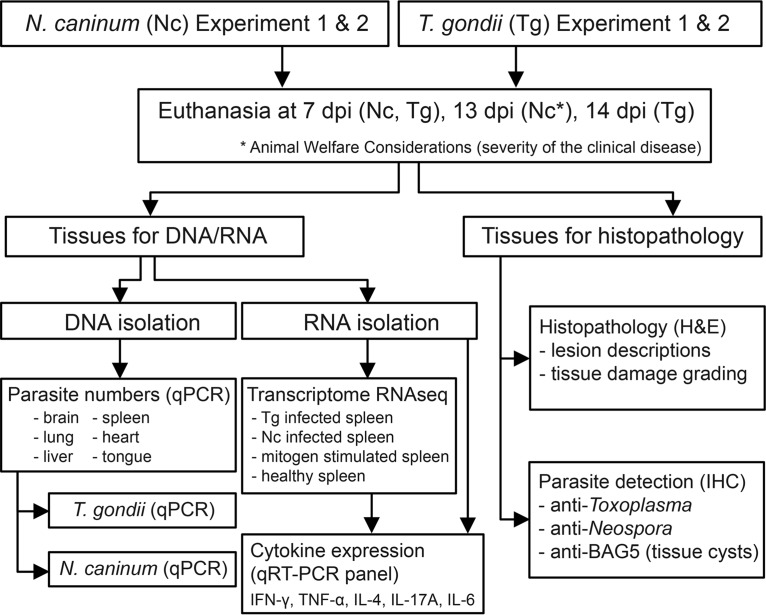
FIG 1.
Experimental overview. A total of eight dunnarts were infected with either Toxoplasma gondii or Neospora caninum and were euthanized at 7 days postinfection (dpi) or 13 or 14 dpi. Two independent experiments were conducted for both N. caninum and T. gondii investigations; each experiment included four parasite-infected animals and one uninfected negative control. For the histopathological analysis, multiple tissues were evaluated by light microscopy, and lesion severity was assessed in the brain, tongue, lung, heart, liver, and spleen from each infected and uninfected animal. To identify parasites and parasite tissue cysts by light microscopy, N. caninum- and T. gondii-specific immunohistochemistry and BAG5 bradyzoite-specific immunohistochemistry were used on sections from parasite-infected tissue. For the detection of N. caninum and T. gondii, DNA isolated from the brain, tongue, lung, heart, liver, and spleen of each parasite-infected animal was subjected to qPCR targeting the Nc5 (N. caninum) and SAG1 (T. gondii) genes. For RNA-seq analysis, RNA was extracted from the following samples to generate four separate dunnart transcriptomes: (i) uninfected dunnart, (ii) T. gondii-infected dunnart at 14 dpi, (iii) N. caninum-infected dunnart at 13 dpi, and (iv) mitogen-stimulated splenocytes from an uninfected healthy dunnart. Transcript sequences for IFN-γ, TNF-α, IL-4, IL-17A, and IL-6 identified in dunnart transcriptomes were used to design qRT-PCR assays in order to investigate splenic cytokine expression profiles in each uninfected or infected animal.
RESULTS
In contrast to N. caninum strain NC-Nowra-infected dunnarts, T. gondii strain TgAuDg1-infected dunnarts exhibited only mild disease.
N. caninum-infected dunnarts demonstrated a significantly higher mean morbidity score than those infected with T. gondii (P < 0.0001). From 11 to 12 days postinfection (p.i.), N. caninum-infected dunnarts presented with rapidly progressive clinical signs of disease that necessitated euthanasia at 13 days p.i. The signs included ruffled dull pelage, hunched appearance, aimless diurnal wandering (abnormal for this nocturnal species), reduced awareness of surroundings, obtunded behavior, hindlimb to generalized paresis, and urinary incontinence. These signs were consistent with those previously reported for dunnarts with neosporosis (25) and were not observed in negative-control animals or T. gondii-infected animals, which remained clinically normal throughout the course of the experiment. In N. caninum-infected dunnarts, daily activity, as measured by distance run and time spent in the exercise wheel, was reduced from day 6 to 7 onward (see Fig. S1C, D, G, and H in the supplemental material) and reduced daily food intake was observed from 5 to 7 days p.i. (see Fig. S1K and L). In contrast, T. gondii-infected dunnarts showed less pronounced reductions in daily activity or had activity levels similar to their individual baseline levels and control animal activity levels (see Fig. S1A, B, E, and F), and food consumption was not noticeably different from that of controls (see Fig. S1I and J).
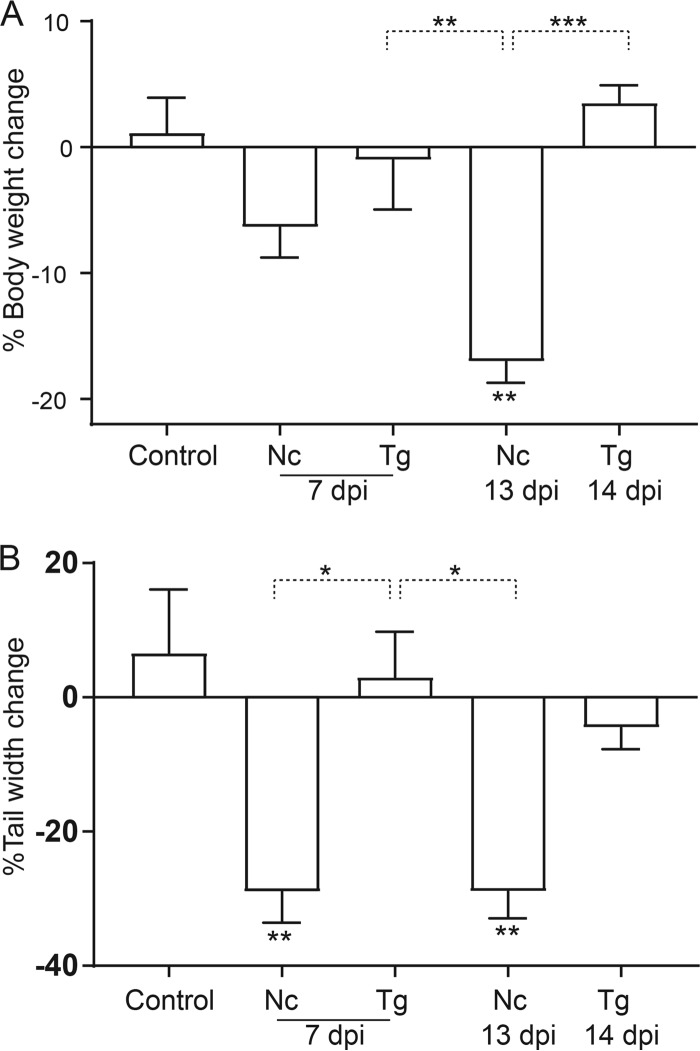
Body mass was significantly reduced for N. caninum-infected dunnarts at 13 days p.i. compared to that of controls (P < 0.01) and T. gondii-infected animals at 7 and 14 days p.i. (P < 0.01 and P < 0.001, respectively). Body mass was statistically similar between other experimental groups (Fig. 2A). N. caninum-infected animals at 7 and 13 days p.i. had significantly thinner tails than controls or T. gondii-infected animals at 7 days p.i. (P < 0.01 and P < 0.05, respectively) but not at 14 days p.i. (Fig. 2B).
FIG 2.
Percent changes in body weight and tail width for Toxoplasma gondii- and Neospora caninum-infected fat-tailed dunnarts at 7 and 13 or 14 dpi. The control group is the uninfected animals. Percentages of body weight (A) and tail width (B) change were calculated for each animal based on initial and postmortem measurements. Each bar and error bar represents the mean value ± standard error of the mean (SEM) from the four biological replicates contained in each experimental group. One-way ANOVA was used to compare changes in body weight and tail width between experimental groups, followed by the Tukey-Kramer post hoc adjustment. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data shown are representative of two independent T. gondii experiments and two independent N. caninum experiments. Nc, N. caninum; Tg, T. gondii; dpi, days postinfection.
Pathological lesions were more widespread and severe in dunnarts with neosporosis than in dunnarts with toxoplasmosis.
At necropsy, macroscopic lesions were not detected in dunnarts at 7 days p.i. In contrast, at 13 to 14 days p.i., all infected animals had some degree of pulmonary consolidation and congestion, mesenteric lymphadenomegaly, and splenomegaly. T. gondii-infected dunnarts were in good body condition with no appreciable difference in adipose tissue stores and muscle mass compared to those in uninfected animals, while N. caninum-infected dunnarts were in poor body condition with severe atrophy of subcutaneous, visceral, and tail adipose tissue and moderate to marked atrophy of skeletal muscle. In three of four N. caninum-infected dunnarts, the liver was mildly enlarged and friable, with multifocal, small, poorly delineated red foci scattered throughout the parenchyma. The histopathological changes in T. gondii-infected dunnarts (7 and 14 days p.i.) and N. caninum-infected dunnarts (7 and 13 days p.i.) are detailed in Text S2 in the supplemental material.
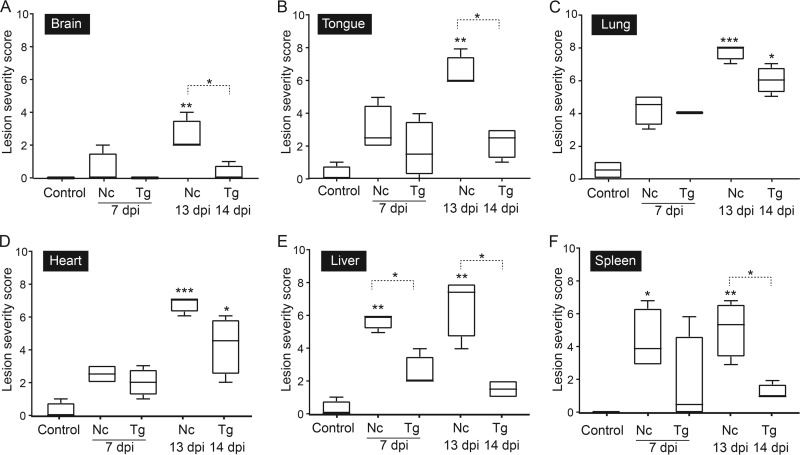
To better understand disease development in early T. gondii and N. caninum infection, the severity of histopathological lesions in the brain, tongue, lung, heart, liver, and spleen was semiquantitatively evaluated and ranked, with scores reflecting the extent of inflammation and necrosis in different tissues (see Table S4 in the supplemental material). When the histopathological scores of lesion severity in the tissues analyzed were compared between the control group and infected groups, dunnarts with toxoplasmosis had significantly more severe lesions only in the heart and lung at 14 days p.i. (P < 0.05) (Fig. 3C and D). In contrast, significantly more severe lesions were found in tissues examined in N. caninum-infected animals than in the control group at 13 days p.i. (P < 0.001 for lung and heart; P < 0.01 for brain, tongue, liver, and spleen) and in the spleen at 7 days p.i. (P < 0.05) (Fig. 3A to F). When infected dunnarts were compared at similar time points, significantly more severe lesions were observed in N. caninum-infected dunnarts at 13 days p.i. than in T. gondii-infected dunnarts at 14 days p.i. in the brain, tongue, liver, and spleen (P < 0.05), and the differences in lesion severity approached statistical significance in the lung and heart (P = 0.0571) (Fig. 3A to F). Additionally, hepatic lesions were significantly more severe in dunnarts infected with N. caninum than in dunnarts infected with T. gondii at 7 days p.i. (P < 0.05) (Fig. 3E).
FIG 3.
Summary of lesion severity scores observed in brain, tongue, lung, heart, liver, and spleen of Neospora caninum- and Toxoplasma gondii-infected fat-tailed dunnarts. The control group consists of uninfected dunnarts and is representative of background lesions normally found in this species. For the sectional area of a given tissue evaluated, the extents of inflammation and necrosis were scored separately, and scoring ranged from no observation (score of 0) to minimal (<5% affected; score of 1), mild (5 to 10% affected; score of 2), moderate (11 to 30% affected; score of 3), or severe (>30% affected; score of 4). The final lesion severity scores for brain (A), tongue (B), lung (C), heart (D), liver (E), and spleen (F) are based on the sum of scores assigned for inflammation and necrosis for each tissue. Boxes represent the 25th to 75th percentiles of the four biological replicates contained in each experimental group, middle lines show the median values, and whiskers are the minimum-to-maximum values. Kruskal-Wallis one-way ANOVA was used to compare parasite-infected groups to controls, followed by Dunn's post hoc adjustment, and the Mann-Whitney test was used to compare N. caninum- and T. gondii-infected animals at 7 dpi and 13 or 14 dpi, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data shown are representative of two independent T. gondii experiments and two independent N. caninum experiments. Nc, N. caninum; Tg, T. gondii; dpi, days postinfection.
Increased parasite burdens were observed in tissues from N. caninum NC-Nowra-infected dunnarts compared to those in T. gondii TgAuDg1-infected dunnarts.
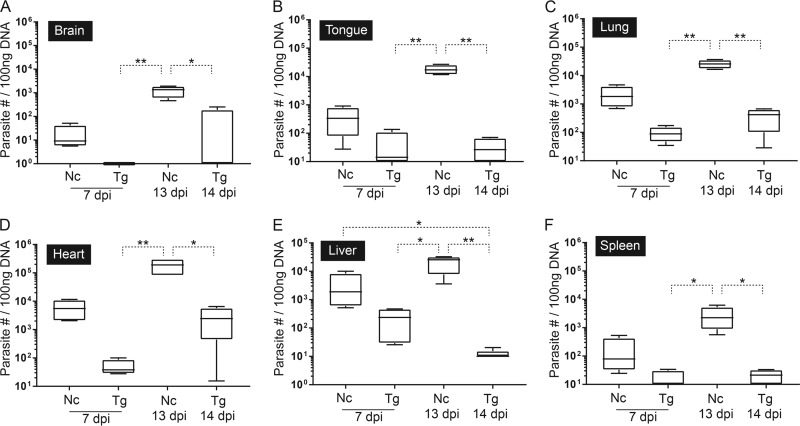
The parasite tissue loads in the brain, tongue, lung, heart, liver, and spleen were quantified by quantitative PCR (qPCR), and this approach was more sensitive than histopathology and immunohistochemistry in detecting parasites (see Tables S4 and S5 in the supplemental material). In T. gondii-infected dunnarts, parasites were consistently detected in the liver, lung, and heart at 7 days p.i., and the liver showed the highest parasite load (see Table S5). The tongue and spleen were positive for T. gondii in two animals, and parasites were undetectable in the brain of all animals at 7 days p.i. (see Table S5). With the exception of the liver and brain, the median parasite burdens continued to increase in vivo in the tissues evaluated, as demonstrated by 1.9- to 64.4-fold increases at 14 days p.i., with the highest and lowest increases seen in the heart and tongue, respectively (see Table S5). At 14 days p.i., the heart and lung were consistently infected and contained the highest numbers of parasites (see Table S5). The heart was the only tissue with a difference in median parasite number of more than 1 log unit between 7 and 14 days p.i. Parasites were also detected in low numbers in T. gondii-infected dunnarts at 14 days p.i. in the spleen of three animals, tongue of two animals, and the brain and liver of a single animal (see Table S5). In contrast, parasites were consistently detected in all tissues analyzed in N. caninum-infected dunnarts (see Table S5). The heart contained the highest number of parasites at both time points, followed by the liver and lung (see Table S5). The parasite burdens increased in all tissues more dramatically than was seen in T. gondii infection, with all tissues showing a minimum 1 to 2 log unit increase in parasite numbers between 7 and 13 days p.i. The brain had the lowest parasite loads at 7 and 13 days p.i. (see Table S5). The brain and tongue showed the highest (148.4- and 50.7-fold) increases in median parasite numbers over time in N. caninum-infected dunnarts, and the liver had the smallest (13.4-fold) increase (see Table S5).
The median tissue parasite burden was consistently higher in dunnarts with neosporosis than in those with toxoplasmosis (see Table S5 in the supplemental material). At 7 days p.i., N. caninum-infected dunnarts showed 8- to 146-fold differences in median parasite loads compared to those infected with T. gondii, with the lowest and highest differences in the spleen and heart, respectively. At 13 days p.i., N. caninum-infected dunnarts showed 61- to 25,400-fold differences in median parasite loads compared to T. gondii-infected dunnarts at 14 days p.i., with the lowest and highest differences in the lung and liver, respectively (see Table S5). Significant increases in tissue parasite loads were observed in N. caninum-infected animals at 13 days p.i. compared with T. gondii-infected animals for the brain (P < 0.01 and P < 0.05 for 7 and 14 days p.i., respectively) (Fig. 4A), tongue (P < 0.01) (Fig. 4B), lung (P < 0.01) (Fig. 4C), heart (P < 0.01 and P < 0.05 for 7 and 14 days p.i., respectively) (Fig. 4D), liver (P < 0.05 and P < 0.01 for 7 and 14 days p.i., respectively) (Fig. 4E), and spleen (P < 0.05) (Fig. 4F). The liver parasite load was also significantly increased in N. caninum-infected animals at 7 days p.i. compared with that in T. gondii-infected animals at 14 days p.i. (P < 0.05) (Fig. 4E).
FIG 4.
Parasite tissue loads in fat-tailed dunnarts experimentally infected with Toxoplasma gondii and Neospora caninum at 7 and 13 or 14 dpi determined by qPCR assays targeting the SAG1 gene of T. gondii and Nc5 gene of N. caninum. Results are expressed as total number of parasites in 100 ng DNA isolated from the brain (A), tongue (B), lung (C), heart (D), liver (E), and spleen (F). Boxes represent the 25th to 75th percentiles of the four biological replicates contained in each experimental group, middle lines are the median values, and whiskers are the minimum-to-maximum values. Parasite loads for each tissue were compared between different groups by Kruskal-Wallis one-way ANOVA, followed by Dunn's post hoc adjustment. *, P < 0.05; **, P < 0.01. Data shown are representative of two independent T. gondii experiments and two independent N. caninum experiments. Nc, N. caninum; Tg, T. gondii; dpi, days postinfection.
Parasite tissue loads were positively correlated with lesion severity in most tissues in N. caninum NC-Nowra-infected dunnarts but not in T. gondii TgAuDg1-infected dunnarts.
There was a strong positive correlation between parasite tissue load and overall lesion severity in N. caninum-infected dunnarts for the brain (r = 0.9129, P < 0.01), tongue (r = 0.9452, P < 0.01), lung (r = 0.8961, P < 0.01), heart (r = 0.9636, P < 0.01), and liver (r = 0.9636, P < 0.05) but not the spleen (r = 0.6177, P > 0.05). No significant correlations between tissue parasite loads and overall lesion severity in the brain, tongue, lung, heart, liver, and spleen were found in T. gondii-infected dunnarts. T. gondii- and N. caninum-infected dunnarts showed evidence of seroconversion by 14 and 13 days p.i., respectively (see Table S1 in the supplemental material).
Transcriptome sequencing (RNA-seq) revealed that transcriptional changes in cytokine genes occur in response to parasite infection in the dunnart spleen.
In order to obtain dunnart-specific cytokine sequences to use for assay development, four transcriptome sequences were generated using RNA purified from uninfected and infected dunnart spleens and mitogen-stimulated dunnart immune cells. Different immune gene transcripts (n = 28) were identified (Table 1). The cytokine repertoires of a T. gondii- and an N. caninum-infected dunnart suggested differences in the expression of major cytokines compared to their expression in an uninfected control (Table 1).
TABLE 1.
TMMa-normalized expression values of immune gene transcripts identified in fat-tailed dunnart (Sminthopsis crassicaudata) transcriptomesb
| Trinity transcript ID | Gene productc | Normalized expression value in: |
|||
|---|---|---|---|---|---|
| Uninfected dunnart (JS1633) | T. gondii infected dunnart (JS2097) | N. caninum infected dunnart (JS2095) | Mitogen-stimulated dunnart splenocytes (JS2093) | ||
| TRINITY_DN82803_c0_g1_i1 | IL-1α | 0 | 0.022 | 0.074 | 11.775 |
| TRINITY_DN84816_c0_g1_i1 | IL-1β | 3.214 | 12.775 | 15.454 | 1364.71 |
| TRINITY_DN153847_c0_g1_i1 | IL-2 | 0 | 0.15 | 0.143 | 467.473 |
| TRINITY_DN5397_c0_g1_i1 | IL-4 | 0 | 0 | 0.083 | 7.088 |
| TRINITY_DN53045_c0_g1_i1 | IL-5 | 0.039 | 0.08 | 0.254 | 3.159 |
| TRINITY_DN45695_c0_g1_i2 | IL-6 | 0 | 0.236 | 1.302 | 31.46 |
| TRINITY_DN82539_c0_g1_i2 | IL-10 | 0.055 | 0.762 | 3.472 | 10.282 |
| TRINITY_DN72236_c0_g1_i4 | IL-11 | 0.105 | 7.661 | 31.564 | 0.675 |
| TRINITY_DN101701_c0_g1_i1 | IL-12a | 0 | 0 | 0.147 | 0.778 |
| TRINITY_DN115371_c0_g1_i1 | IL-12b | 0.042 | 0.086 | 0.082 | 4.29 |
| TRINITY_DN80613_c0_g1_i1 | IL-13 | 0.671 | 0.308 | 0.439 | 36.154 |
| TRINITY_DN85376_c0_g1_i1 | IL-15 | 0.115 | 1.013 | 1.73 | 1.245 |
| TRINITY_DN86202_c3_g2_i2 | IL-16 | 6.979 | 22.923 | 17.215 | 5.7 |
| TRINITY_DN53989_c1_g1_i1 | IL-17A | 0.06 | 0.083 | 0 | 406.244 |
| TRINITY_DN92343_c0_g1_i1 | IL-17C | 0 | 0.037 | 0.07 | 1.228 |
| TRINITY_DN50692_c0_g1_i2 | IL-17D | 0.47 | 0.215 | 2.033 | 0.1 |
| TRINITY_DN54141_c0_g1_i1 | IL-17F | 0.084 | 0 | 0.055 | 292.502 |
| TRINITY_DN88373_c2_g2_i1 | IL-21 | 0.079 | 0.776 | 0.528 | 31.338 |
| TRINITY_DN48114_c0_g1_i1 | IL-22 | 0 | 0 | 0.051 | 36.596 |
| TRINITY_DN55354_c0_g1_i1 | IL-23α | 0.085 | 0.177 | 0.643 | 75.463 |
| TRINITY_DN138057_c0_g1_i1 | IL-25 (IL-17E) | 0 | 0 | 0 | 1.547 |
| TRINITY_DN86798_c3_g1_i9 | IL-27 | 1.353 | 1.183 | 1.181 | 0.93 |
| TRINITY_DN60643_c0_g1_i2 | IL-33 | 0 | 0.449 | 0.227 | 0 |
| TRINITY_DN29707_c0_g1_i1 | IFN-γ | 0.041 | 4.094 | 0.483 | 195.057 |
| TRINITY_DN89327_c9_g1_i1 | TNF-α | 2.774 | 6.005 | 3.313 | 251.596 |
| TRINITY_DN66548_c0_g2_i2 | LTα | 1.504 | 1.461 | 0.185 | 14.014 |
| TRINITY_DN91456_c2_g2_i1 | LTβ | 53.669 | 56.419 | 8.468 | 39.825 |
| TRINITY_DN5167_c0_g1_i1 | TSLP | 0.432 | 0.908 | 1.156 | 1.126 |
TMM, trimmed mean of M values.
Transcriptomes included in this table are (i) uninfected-dunnart spleen (animal JS1633), (ii) T. gondii-infected-dunnart spleen at 14 days p.i. (animal JS2097), (iii) N. caninum-infected-dunnart spleen at 13 days p.i. (animal JS2095), and (iv) mitogen-stimulated dunnart splenocytes (50 ng/ml phorbol myristate acetate [PMA], 1 μg/ml ionomycin) (animal JS2093).
IL, interleukin; IFN, interferon; TSLP, thymic stromal lymphopoietin; LT, lymphotoxin; TNF, tumor necrosis factor.
Splenic cytokine gene mRNA expression in T. gondii TgAuDg1-infected dunnarts differed from that in N. caninum NC-Nowra-infected dunnarts.
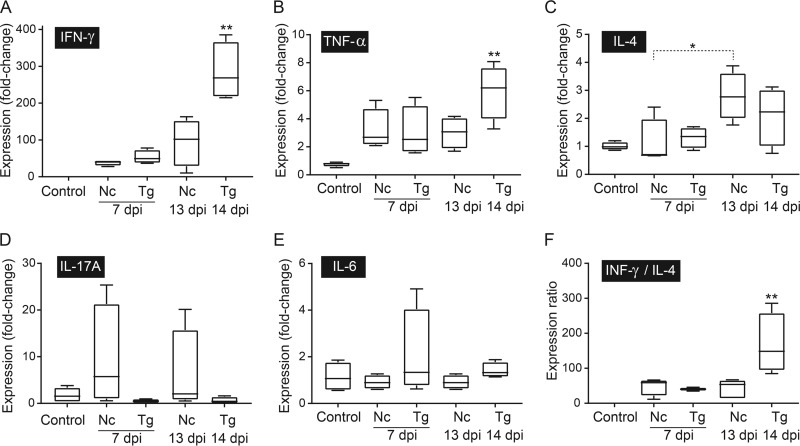
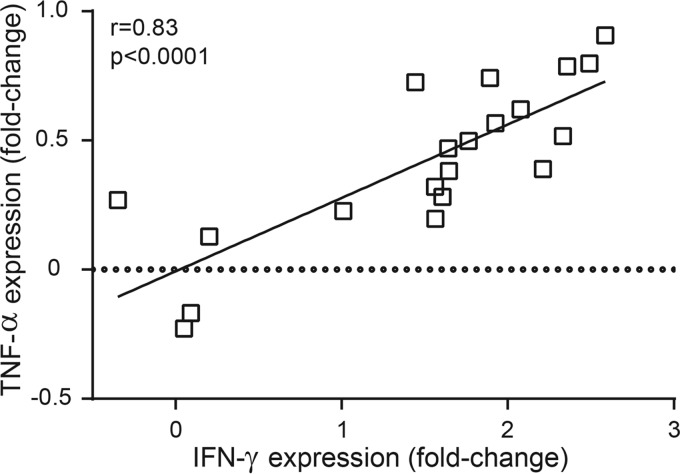
To characterize the immune response following infection with T. gondii or N. caninum, the splenic mRNA expression levels of IFN-γ, TNF-α, IL-4, IL-6, and IL-17A were measured by quantitative reverse transcription PCR (qRT-PCR). Of the cytokines evaluated, IFN-γ was the most strongly upregulated in dunnarts infected with T. gondii and N. caninum (median increases of 40- to 284-fold compared to the levels in the control group) (see Table S6 in the supplemental material). The IFN-γ and TNF-α mRNA expression levels were significantly higher in asymptomatic T. gondii-infected dunnarts at 14 days p.i. than in the uninfected control group (P < 0.01) (Fig. 5A and B). A nonsignificant trend of higher median expression levels of Th1 cytokines, with respective increases of 2.8- and 2-fold for IFN-γ and TNF-α, was observed in asymptomatic T. gondii-infected dunnarts at 14 days p.i. compared with the levels in clinically diseased N. caninum-infected dunnarts at 13 days p.i. (Fig. 5A and B). The correlation between TNF-α expression and IFN-γ expression was significant (r = 0.83, P < 0.0001) (Fig. 6). The median fold change for IL-4 expression was higher (1.2- to 4-fold increase) in N. caninum-infected animals at 13 days p.i. than for the other experimental groups, but a significant difference was only identified in N. caninum-infected animals at 7 days p.i. (P < 0.05), when three of four animals had downregulated IL-4 mRNA expression compared with that in uninfected controls (Fig. 5C). The IFN-γ/IL-4 gene expression ratio was calculated for each animal based on individual relative fold cytokine expression. The median IFN-γ/IL-4 gene expression ratios were 2.5- to 118-fold higher in asymptomatic T. gondii-infected dunnarts at 14 days p.i. than in other experimental groups; however, this difference was only significant in the comparison with uninfected controls (P < 0.01) (Fig. 5F).
FIG 5.
Splenic cytokine mRNA expression profiles as determined by RT-qPCR in fat-tailed dunnarts experimentally infected with Toxoplasma gondii and Neospora caninum at 7 and 13 or 14 dpi. The control group represents the uninfected animals. The results are expressed as normalized relative fold changes compared to the expression of reference GAPDH and 28S genes and were scaled to the results for control animals. Relative expression levels of IFN-γ (A), TNF-α (B), IL-4 (C), IL-17A (D), and IL-6 (E) and IFN-γ/IL-4 ratios (F) are shown. Boxes represent the 25th to 75th percentiles of the four biological replicates contained in each experimental group, middle lines are the median values, and whiskers are the minimum-to-maximum values. Kruskal-Wallis one-way ANOVA was used to compare cytokine expression between experimental groups, followed by Dunn's post hoc adjustment. *, P < 0.05; **, P < 0.01. Data shown are representative of two independent T. gondii experiments and two independent N. caninum experiments. Nc, N. caninum; Tg, T. gondii; dpi, days postinfection.
FIG 6.
Correlation between IFN-γ and TNF-α mRNA expression in fat-tailed dunnarts infected with Toxoplasma gondii and Neospora caninum. Each point represents one animal (uninfected, n = 4; T. gondii-infected, n = 4 each for 7 and 14 days p.i.; N. caninum-infected, n = 4 each for 7 and 13 days p.i.) The correlation graph shows a fit line with a confidence curve. r and P values are shown from Spearman rank correlation analysis. r, correlation coefficient; P, significance level.
IL-17A mRNA did not amplify in two T. gondii-infected animals at both 7 and 14 days p.i. or in one uninfected control animal. Of the T. gondii-infected animals with detectable IL-17A mRNA levels, three of four had downregulated IL-17A mRNA expression compared to that in uninfected controls. No significant differences in IL-17A and IL-6 mRNA expression levels were found between infected and uninfected groups (Fig. 5D and E). There was no correlation between the expression of the cytokines evaluated and the presence of parasite tissue cysts in infected dunnarts (data not shown).
DISCUSSION
Studies in mice and cattle (eutherian models) show that the host immune response to infection with T. gondii and N. caninum is a key determinant in infection outcome; however, studies in marsupials (metatherian model) are lacking. In our study, adult outbred fat-tailed dunnarts were infected with the Australian isolates N. caninum NC-Nowra and T. gondii TgAuDg1 (type II). Both of these strains are considered to be low-virulence strains and produce little to no disease in murine models (42–44). Despite similarities in the parasite inoculum doses and virulence, the disease outcome was more severe in dunnarts infected with N. caninum than in those infected with T. gondii. Evidence of differential regulation of Th-related cytokines supports the hypothesis that the immune response plays a role in the outcome of parasite infection in this species.
The development of a cell-mediated Th1 immune response, characterized by increased expression of proinflammatory cytokines IFN-γ and TNF-α and decreased expression of the anti-inflammatory Th2 cytokine IL-4, has long been recognized as a critical component of host protection against intracellular pathogens, including T. gondii and N. caninum (7, 11, 13, 15). Therefore, it was hypothesized that Th1 cytokines would be expressed at higher levels in clinically normal dunnarts. Consistent with a Th1-polarized immune response, T. gondii infection resulted in significantly upregulated splenic IFN-γ and TNF-α mRNA expression and higher IFN-γ/IL-4 gene expression ratio by 2 weeks p.i. compared to the results for uninfected controls, while N. caninum infection did not. These findings suggest that dunnarts with toxoplasmosis were capable of mounting a more effective Th1-type immune response than dunnarts with neosporosis by 2 weeks p.i. (45, 46), which may account for the milder disease and lower parasite burden observed in T. gondii infection.
Of the Th1 cytokines, IFN-γ is generally recognized as the most crucial element of protective immunity in N. caninum and T. gondii infections (10, 47). IFN-γ is important for restricting intracellular replication due to its role in activating macrophage-mediated mechanisms to kill intracellular pathogens, particularly in the early stages of infection (13–15, 47). TNF-α can work synergistically by activating IFN-γ-primed macrophages to restrict intracellular parasite replication and promote clearance of parasites (47). An inadequate Th1-type immune response and imbalance of Th1/Th2 cytokines may have contributed to disease pathogenesis in dunnarts with neosporosis. The modest elevation in IFN-γ and TNF-α expression induced by N. caninum infection was unable to control parasite replication in the dunnart, as seen by early, widespread parasite dissemination and a markedly high tissue parasite load. In mice, there is some evidence that females produce lower levels of IFN-γ and TNF-α than their male counterparts during the initial stages of T. gondii infection, which may enhance their susceptibility to disease (48). Although the present N. caninum investigations were female biased, severe fatal neosporosis was previously shown in male dunnarts infected with N. caninum. Those observations are similar to the disease seen in the females in this current study (25). Therefore, any possible sex biases in the dunnart immune response are unlikely to influence susceptibility to N. caninum infection.
Studies of neosporosis in murine models have shown that resistant mouse strains have a mixed immune response characterized by a high IFN-γ/IL-4 ratio, while a low IFN-γ/IL-4 ratio is associated with greater disease susceptibility (49). A trend toward higher IL-4 mRNA expression and a lower IFN-γ/IL-4 ratio was observed in N. caninum-infected dunnarts compared to the results for those infected with T. gondii at 2 weeks p.i. Despite the absence of statistical significance, these findings suggest that the correct balance of Th1 and Th2 cytokines may play a critical role in limiting disease severity subsequent to infection with T. gondii and N. caninum in fat-tailed dunnarts (49, 50). While a Th2-biased immune response is generally associated with a detrimental disease outcome, Th2-type cytokines can mitigate immunopathology during acute toxoplasmosis and neosporosis by limiting excessive Th1 cytokine secretion and subsequent pathological effects (11, 17–19, 45, 49–51).
Although significant differences in IL-17A and IL-6 mRNA expression were not identified in this study, a general trend was noted for IL-17A, suggestive of some difference between the infected dunnarts. IL-17A was consistently detected in all N. caninum-infected animals, and the median mRNA expression levels were elevated compared to the levels in other groups. In contrast, IL-17A transcripts were downregulated or undetectable in T. gondii-infected animals. Studies in T. gondii-infected IL-17R-deficient mice have shown that while IL-17 plays a role in reducing parasite burden (22), an exacerbated Th17 response is generally associated with increased tissue damage and greater mortality (21). In koalas, IL-17A is considered a marker for chlamydial pathogenesis, with higher levels of expression detected in animals with more severe disease (52).
The parasite burden in host tissue is an important element of disease development in eutherian models (3, 10, 53, 54). Our findings suggest that in the dunnart model, N. caninum NC-Nowra disseminates better, with a higher capacity for growth and/or better evasion of the host immune response than T. gondii TgAuDg1. Both heart and lung were the tissues in which parasites were consistently identified, and these tissues should therefore be considered targets for disease surveillance in marsupials in order to increase the likelihood of parasite detection in early infection (55).
The severity of acute clinical neosporosis and associated pathological lesions observed in dunnarts in the present study mirrors the findings in a previous report of N. caninum infection in this species (25). We also found necrosis to be a characteristic feature and the muscle, liver, pancreas, and lungs to be severely affected. Involvement of mesometrium and reproductive tissues was seen in N. caninum-infected dunnarts and may indicate a predilection of N. caninum for these tissues. Extensive tissue cyst production appears to be a unique feature of neosporosis in the dunnart model (25).
Australian marsupials are generally considered to be highly susceptible to toxoplasmosis (3). The lack of apparent clinical disease in T. gondii-infected dunnarts in this study was unexpected, although not inconsistent with previous reports in marsupials. Infected marsupials can succumb to fulminant disease and die peracutely without evidence of premonitory clinical signs (56). Experimental infections in Tammar wallabies (Macropus eugenii) (33) and eastern barred bandicoots (Perameles gunnii) (31) given T. gondii oocysts, per os, of the low virulence strains ME49 (type II) and VEG (type III), respectively, found that infection was fatal within 9 to 16 days and that the animals displayed no clinical signs or exhibited only mild behavioral changes prior to death.
A unique finding in our study was the rarity of central nervous system (CNS) lesions in T. gondii-infected dunnarts. Necrosis, inflammation, and tissue cysts in the CNS tissues are common features of toxoplasmosis in marsupials (57). This is particularly true for dasyurid species, where the brain and spinal cord are reported to be the most likely tissues to contain lesions and parasites in infected animals (56, 57). Experimental infections in mice have also shown that the brain is parasitized early in infection, irrespective of the method of inoculation (53), and that encephalitis and tissues cysts commonly appear by the second week of infection (58). It is possible that there is a diminished capacity of the TgAuDg1 strain to cross the blood-brain barrier in dunnarts or that there was irregular distribution of parasites in the brain, such that sampling was not accurately reflective of infection rates. It is also possible that brain infection occurs later in disease in T. gondii-infected dunnarts than is reported for murine models.
In summary, the data presented herein show that under similar experimental conditions, fat-tailed dunnarts infected with T. gondii TgAuDg1 are capable of mounting a stronger Th1-type immune response by 2 weeks p.i. and have reduced morbidity, pathological lesions, and tissue parasite burdens compared to dunnarts infected with N. caninum NC-Nowra. This work illustrates the utility of the fat-tailed dunnart as an experimental model for Neospora and Toxoplasma research. The suite of optimized and validated cytokine assays designed for this study can be used to evaluate the Th immune response in this species. These assays are useful and reproducible for the evaluation of systemic immune responses in dunnart immune tissues and will serve as valuable tools to elucidate host pathogen interactions and be beneficial to future research into additional diseases and the efficacy of therapeutic treatments and vaccination in the fat-tailed dunnart.
MATERIALS AND METHODS
Ethics statements and animals.
Animal experiments were approved by the University of Sydney Animal Ethics Committee (project number 551) and complied with the New South Wales Animal Welfare Act (59) and the NHMRC code of practice (2013) (60). Sexually mature outbred fat-tailed dunnarts (Sminthopsis crassicaudata), 1 to 2 years of age, weighing 13.2 to 18.9 g, were sourced from a breeding colony at the University of Sydney. All animals were housed as outlined in Text S1 in the supplemental material.
Preparation and inoculation of N. caninum and T. gondii tachyzoites.
The NC-Nowra isolate of N. caninum and TgAuDg1 isolate of T. gondii were used throughout the study (42, 43). Tachyzoites were propagated by serial passages in Vero cells as outlined in Text S1 in the supplemental material. Each experimental infection included batches of five animals: four were inoculated with the parasite dose, and one was inoculated as a negative control (Fig. 1). Two independent experiments were done for each parasite species, using the previously established minimum dose of tachyzoites required for the development of neosporosis in dunnarts (25). Animals were inoculated intraperitoneally with 105 viable tachyzoites (T. gondii or N. caninum) suspended in 0.3 ml of sterile phosphate-buffered saline (PBS) (pH 7.2) or the same volume of PBS alone. Animals were euthanized by CO2 inhalation at 7 days postinfection (p.i.) or at 13 or 14 days p.i. for N. caninum- and T. gondii-infected animals, respectively (Fig. 1; see also Table S1 in the supplemental material). Animal monitoring, clinical evaluation, sample collection, histopathology, and immunohistochemistry are detailed in Fig. 1 and also in Text S1.
Quantification of tissue parasite loads from N. caninum- and T. gondii-infected dunnarts.
Genomic DNA was extracted from a variety of tissues (Fig. 1), and parasite tissue burdens were assessed by quantitative PCR (qPCR) in a SYBR green-based assay using T. gondii primers targeting a 128-bp fragment of the single-copy SAG1 gene (61) or N. caninum primers targeting a 76-bp fragment of the multicopy Nc5 gene (62). Primer sequences and in-house assay optimization conditions are reported in Tables S2 and S3 in the supplemental material. All samples were tested with triplicate qPCRs using SsoAdvanced universal SYBR green supermix (Bio-Rad, Australia) in the CFX96 Touch real-time PCR detection system and the corresponding CFX Manager 3.1 software (Bio-Rad). Standard curves were generated using 10-fold serial dilutions (range, 3 to 3 × 105 T. gondii or 6 to 6 × 105 N. caninum parasites). Data handling and calculations for qPCR data were carried out in qBasePlus version 3.0 (Biogazelle, Ghent, Belgium). Parasite numbers are expressed as log10 parasites per 100 ng of total DNA. The limits of detection for N. caninum and T. gondii were <1 parasite and 2 parasites in 100 ng DNA, respectively (see Table S3 in the supplemental material). DNA extraction and qPCR assay protocols are provided in Text S1.
Quantification of anti-Neospora and anti-Toxoplasma antibodies.
A competitive ELISA for N. caninum (VMRD, Pullman, WA, USA) was used to detect N. caninum antibodies, following the manufacturer's protocol, using undiluted sera and a cutoff of ≥30%. The Toxo-Screen DA modified agglutination test (MAT) kit (bioMérieux, Marcy l'Etoile, France) was used to detect T. gondii antibodies using sera diluted 1:40 following the manufacturer's instructions. These serological assays have previously been shown to be suitable for use in Australian marsupial species (25, 40).
RNA-seq and analysis.
Transcriptomes were generated using total RNA isolated from 1 × 106 mitogen-stimulated splenocytes from an uninfected dunnart (JS2093; see Text S1 in the supplemental material), a spleen from an uninfected dunnart (JS1633), a spleen from an N. caninum-infected dunnart at 2 weeks p.i. (JS2095), and a spleen from a T. gondii-infected dunnart at 2 weeks p.i. (JS2097). RNA was isolated using an Isolate II RNA minikit (Bioline), and RNA integrity was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies Australia); each had an RNA integrity number (RIN) of >8.0 (Agilent Technologies, Waldbronn, Germany). Purified RNA samples were transferred into tubes containing RNAstable (Biomatrica, San Diego, USA). Illumina HiSeq 2000 RNA sequencing was carried out at Macrogen (Seoul, South Korea). See Text S1 in the supplemental material for de novo assembly and bioinformatics analysis.
Quantitative reverse transcription-PCR (qRT-PCR) assays to evaluate cytokine expression.
Cytokine transcript sequences for IFN-γ, TNF-α, IL-4, IL-17A, and IL-6 obtained from the dunnart spleen transcriptome were used to design specific primers, using Primer3Plus (63) and recommended parameters for designing SYBR green primers (64). All assays were designed within exons, and the amplicon lengths varied between 91 and 178 bp (see Table S2 in the supplemental material). qPCR assays were carried out in the CFX96 Touch real-time PCR detection system (Bio-Rad). Triplicate technical replicates were run for each cDNA sample with SsoAdvanced universal SYBR green supermix (Bio-Rad, Australia). Each run included no-template negative controls and no-RT controls. Assay optimization values are reported in Table S3.
RNA quantity and quality were evaluated by spectrophotometry and the Agilent 2100 Expert chip system on an Agilent 2100 Bioanalyzer with associated 2100 Expert software (Agilent Technologies Australia), and RNA extraction was repeated for any sample with an RIN of <7.5. RNA samples were subjected to an additional DNase treatment using the Turbo DNA-free kit (ThermoFisher Scientific, Australia). cDNA was synthesized from 2 μg total RNA in a final volume of 20 μl using the RevertAid first-strand cDNA synthesis kit (ThermoFisher Scientific, Australia). Raw quantification cycle (Cq) scores, determined by baseline settings in the CFX Manager 3.1 software (Bio-Rad, Australia), were uploaded to qBasePlus software, where the geometric mean values for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 28S genes were used to normalize the expression level of each target transcript in each sample, and “target specific amplification efficiency” and “scale to control” parameters were selected to generate the calibrated normalized relative quantity (CNRQ) value of cytokine expression for each sample (65). Details of RNA extraction and qRT-PCR assay protocols are provided in Text S1 in the supplemental material.
Statistical analysis.
CNRQ values for gene expression and tissue parasite load were calculated in qBasePlus and log transformed to normalize data for statistical analysis. Statistical tests were carried out using GraphPad Prism 7 software (GraphPad, LaJolla, CA, USA). A Shapiro-Wilk test was used to determine the distribution of data, and parametric (one-way analysis of variance [ANOVA]) or nonparametric (Kruskal-Wallis test or Mann-Whitney U) tests were used to compare differences between groups, followed by post hoc adjustment for multiple comparisons using Tukey-Kramer and Dunn's correction as appropriate (66). Spearman rank correlation tests were used for all correlation tests. Statistical significance for all analyses was established using a P value of <0.05. Results are expressed as the mean values ± standard deviations for parametric data and median values ± interquartile ranges for nonparametric data; confidence intervals are provided when appropriate. Experimental qPCR practice and reporting were performed in accordance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines (67).
Accession number(s).
The Transcriptome Shotgun Assembly project has been deposited at DDBJ/EMBL/GenBank under accession number GFCN00000000. The version described in this paper is the first version, GFCN01000000.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elaine Chew and Huy Tran (Veterinary Pathology Diagnostic Services), as well as Mike Johnson (University of Technology Sydney) for excellent technical assistance. We also acknowledge Emma Hoolihan and Ryan O'Handley for assistance with T. gondii serological analysis, Damien Higgins for helpful discussion on qPCR assay design and supplying reference gene qPCR primers, Iona Maher for guidance on qPCR assay optimization and mitogen stimulation assays, and Katrina Gilchrist for manuscript review.
This work was supported by an ARC Discovery Project (grant number DP130101589) to B.M.M. and The Whitehead Bequest (University of Sydney). S.L.D. is supported by an International Postgraduate Research Scholarship (IPRS) and an Australian Postgraduate Award (APA) tenable at the University of Sydney.
The authors declare they have no competing interests.
S.L.D., D.N.P., M.M.M., J.E., and J.S. conceived and designed the experiments. S.L.D. and M.M.M. performed the experiments. S.L.D., D.N.P., D.O., M.M.M., and J.S. analyzed the data. B.M.M., D.O., M.M.M., J.E., and J.S. contributed reagents, materials, and analysis tools. S.L.D., D.N.P., and J.S. wrote the manuscript. S.L.D., D.N.P., B.M.M., D.O., M.M.M., J.E., and J.S. reviewed manuscript drafts.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00173-17.
REFERENCES
- 1.Dubey JP, Barr BC, Barta JR, Bjerkas I, Bjorkman C, Blagburn BL, Bowman DD, Buxton D, Ellis JT, Gottstein B, Hemphill A, Hill DE, Howe DK, Jenkins MC, Kobayashi Y, Koudela B, Marsh AE, Mattsson JG, McAllister MM, Modry D, Omata Y, Sibley LD, Speer CA, Trees AJ, Uggla A, Upton SJ, Williams DJ, Lindsay DS. 2002. Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol 32:929–946. doi: 10.1016/S0020-7519(02)00094-2. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Schares G. 2011. Neosporosis in animals—the last five years. Vet Parasitol 180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Dubey JP. 2010. Toxoplasmosis of animals and humans, 2nd ed CRC Press, Boca Raton, FL. [Google Scholar]
- 4.Dubey JP, Jones JL. 2008. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 38:1257–1278. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Donahoe SL, Lindsay SA, Krockenberger M, Phalen D, Slapeta J. 2015. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int J Parasitol Parasites Wildl 4:216–238. doi: 10.1016/j.ijppaw.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemphill A, Vonlaufen N, Naguleswaran A. 2006. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology 133:261–278. doi: 10.1017/S0031182006000485. [DOI] [PubMed] [Google Scholar]
- 7.Innes EA. 1997. Toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis 20:131–138. doi: 10.1016/S0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 8.Khan IA, Schwartzman JD, Fonseka S, Kasper LH. 1997. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol 85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 9.Munoz M, Liesenfeld O, Heimesaat MM. 2011. Immunology of Toxoplasma gondii. Immunol Rev 240:269–285. doi: 10.1111/j.1600-065X.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 10.Dubey JP, Lindsay DS. 1996. A review of Neospora caninum and neosporosis. Vet Parasitol 67:1–59. doi: 10.1016/S0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 11.Innes EA, Wright S, Bartley P, Maley S, Macaldowie C, Esteban-Redondo I, Buxton D. 2005. The host-parasite relationship in bovine neosporosis. Vet Immunol Immunopathol 108:29–36. doi: 10.1016/j.vetimm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Monney T, Hemphill A. 2014. Vaccines against neosporosis: what can we learn from the past studies? Exp Parasitol 140:52–70. doi: 10.1016/j.exppara.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Baszler TV, Long MT, McElwain TF, Mathison BA. 1999. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int J Parasitol 29:1635–1646. doi: 10.1016/S0020-7519(99)00141-1. [DOI] [PubMed] [Google Scholar]
- 14.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153:2533–2543. [PubMed] [Google Scholar]
- 15.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 16.Baszler TV, McElwain TF, Mathison BA. 2000. Immunization of BALB/c mice with killed Neospora caninum tachyzoite antigen induces a type 2 immune response and exacerbates encephalitis and neurological disease. Clin Diagn Lab Immunol 7:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Oswald IP, James SL, Sher A. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol 148:1792–1796. [PubMed] [Google Scholar]
- 18.Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. 1996. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun 64:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thouvenin M, Candolfi E, Villard O, Klein JP, Kien T. 1997. Immune response in a murine model of congenital toxoplasmosis: increased susceptibility of pregnant mice and transplacental passage of Toxoplasma gondii are type 2-dependent. Parassitologia 39:279–283. [PubMed] [Google Scholar]
- 20.Flynn RJ, Marshall ES. 2011. Parasite limiting macrophages promote IL-17 secretion in naive bovine CD4(+) T-cells during Neospora caninum infection. Vet Immunol Immunopathol 144:423–429. doi: 10.1016/j.vetimm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Guiton R, Vasseur V, Charron S, Arias MT, Van Langendonck N, Buzoni-Gatel D, Ryffel B, Dimier-Poisson I. 2010. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis 202:427–435. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 22.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. 2005. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun 73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peckham RK, Brill R, Foster DS, Bowen AL, Leigh JA, Coffey TJ, Flynn RJ. 2014. Two distinct populations of bovine IL-17(+) T-cells can be induced and WC1(+)IL-17(+)gammadelta T-cells are effective killers of protozoan parasites. Sci Rep 4:5431. doi: 10.1038/srep05431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay DS, Dubey JP. 1989. Neospora caninum (Protozoa: apicomplexa) infections in mice. J Parasitol 75:772–779. doi: 10.2307/3283063. [DOI] [PubMed] [Google Scholar]
- 25.King JS, McAllan B, Spielman DS, Lindsay SA, Hurkova-Hofmannova L, Hartigan A, Al-Qassab SE, Ellis JT, Slapeta J. 2011. Extensive production of Neospora caninum tissue cysts in a carnivorous marsupial succumbing to experimental neosporosis. Vet Res 42:75. doi: 10.1186/1297-9716-42-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch K, Algar D, Searle JB, Pfenninger M, Schwenk K. 2015. A voyage to Terra Australis: human-mediated dispersal of cats. BMC Evol Biol 15:262. doi: 10.1186/s12862-015-0542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donahoe SL, Slapeta J, Knowles G, Obendorf D, Peck S, Phalen DN. 2015. Clinical and pathological features of toxoplasmosis in free-ranging common wombats (Vombatus ursinus) with multilocus genotyping of Toxoplasma gondii type II-like strains. Parasitol Int 64:148–153. doi: 10.1016/j.parint.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Obendorf DL, Statham P, Driessen M. 1996. Detection of agglutinating antibodies to Toxoplasma gondii in sera from free-ranging eastern barred bandicoots (Perameles gunnii). J Wildl Dis 32:623–626. doi: 10.7589/0090-3558-32.4.623. [DOI] [PubMed] [Google Scholar]
- 29.Parameswaran N, Thompson RC, Sundar N, Pan S, Johnson M, Smith NC, Grigg ME. 2010. Non-archetypal type II-like and atypical strains of Toxoplasma gondii infecting marsupials of Australia. Int J Parasitol 40:635–640. doi: 10.1016/j.ijpara.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RC, Lymbery AJ, Smith A. 2010. Parasites, emerging disease and wildlife conservation. Int J Parasitol 40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Bettiol SS, Obendorf DL, Nowarkowski M, Goldsmid JM. 2000. Pathology of experimental toxoplasmosis in eastern barred bandicoots in Tasmania. J Wildl Dis 36:141–144. doi: 10.7589/0090-3558-36.1.141. [DOI] [PubMed] [Google Scholar]
- 32.Lynch MJ, Obendorf DL, Statham P, Reddacliff GL. 1993. An evaluation of a live Toxoplasma gondii vaccine in Tammar wallabies (Macropus eugenii). Aust Vet J 70:352–353. doi: 10.1111/j.1751-0813.1993.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 33.Reddacliff GL, Hartley WJ, Dubey JP, Cooper DW. 1993. Pathology of experimentally-induced, acute toxoplasmosis in macropods. Aust Vet J 70:4–6. doi: 10.1111/j.1751-0813.1993.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 34.Boulton JG, Gill PA, Cook RW, Fraser GC, Harper PA, Dubey JP. 1995. Bovine Neospora abortion in north-eastern New South Wales. Aust Vet J 72:119–120. doi: 10.1111/j.1751-0813.1995.tb15026.x. [DOI] [PubMed] [Google Scholar]
- 35.Davison HC, Otter A, Trees AJ. 1999. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int J Parasitol 29:1683–1689. doi: 10.1016/S0020-7519(99)00129-0. [DOI] [PubMed] [Google Scholar]
- 36.Fordyce G, Holroyd RG, Taylor J, Kirkland PD. 2013. Neospora caninum and reproductive wastage in extensively managed Queensland beef herds. Aust Vet J 91:385–390. doi: 10.1111/avj.12097. [DOI] [PubMed] [Google Scholar]
- 37.King JS, Brown GK, Jenkins DJ, Ellis JT, Fleming PJ, Windsor PA, Slapeta J. 2012. Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia. Vet Parasitol 187:85–92. doi: 10.1016/j.vetpar.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Reichel MP. 2000. Neospora caninum infections in Australia and New Zealand. Aust Vet J 78:258–261. doi: 10.1111/j.1751-0813.2000.tb11751.x. [DOI] [PubMed] [Google Scholar]
- 39.Eymann J, Herbert CA, Cooper DW, Dubey JP. 2006. Serologic survey for Toxoplasma gondii and Neospora caninum in the common brushtail possum (Trichosurus vulpecula) from urban Sydney, Australia. J Parasitol 92:267–272. doi: 10.1645/GE-709R.1. [DOI] [PubMed] [Google Scholar]
- 40.Parameswaran N, O'Handley RM, Grigg ME, Fenwick SG, Thompson RC. 2009. Seroprevalence of Toxoplasma gondii in wild kangaroos using an ELISA. Parasitol Int 58:161–165. doi: 10.1016/j.parint.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyndale-Biscoe CH. 2005. Life of marsupials. CSIRO Publishing, Collingwood, Australia. [Google Scholar]
- 42.Al-Qassab S, Reichel MP, Su C, Jenkins D, Hall C, Windsor PA, Dubey JP, Ellis J. 2009. Isolation of Toxoplasma gondii from the brain of a dog in Australia and its biological and molecular characterization. Vet Parasitol 164:335–339. doi: 10.1016/j.vetpar.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Miller CM, Quinn HE, Windsor PA, Ellis JT. 2002. Characterisation of the first Australian isolate of Neospora caninum from cattle. Aust Vet J 80:620–625. doi: 10.1111/j.1751-0813.2002.tb10967.x. [DOI] [PubMed] [Google Scholar]
- 44.Miller C, Quinn H, Ryce C, Reichel MP, Ellis JT. 2005. Reduction in transplacental transmission of Neospora caninum in outbred mice by vaccination. Int J Parasitol 35:821–828. doi: 10.1016/j.ijpara.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. 2011. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol 127:701–21.e1–70. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 46.Commins SP, Borish L, Steinke JW. 2010. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol 125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Miller CM, Boulter NR, Ikin RJ, Smith NC. 2009. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol 39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Roberts CW, Cruickshank SM, Alexander J. 1995. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun 63:2549–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long MT, Baszler TV, Mathison BA. 1998. Comparison of intracerebral parasite load, lesion development, and systemic cytokines in mouse strains infected with Neospora caninum. J Parasitol 84:316–320. doi: 10.2307/3284489. [DOI] [PubMed] [Google Scholar]
- 50.Nishikawa Y, Inoue N, Makala L, Nagasawa H. 2003. A role for balance of interferon-gamma and interleukin-4 production in protective immunity against Neospora caninum infection. Vet Parasitol 116:175–184. doi: 10.1016/j.vetpar.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Dimier-Poisson I, Aline F, Mevelec MN, Beauvillain C, Buzoni-Gatel D, Bout D. 2003. Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells. Infect Immun 71:5254–5265. doi: 10.1128/IAI.71.9.5254-5265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathew M, Waugh C, Beagley KW, Timms P, Polkinghorne A. 2014. Interleukin 17A is an immune marker for chlamydial disease severity and pathogenesis in the koala (Phascolarctos cinereus). Dev Comp Immunol 46:423–429. doi: 10.1016/j.dci.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Djurkovic-Djakovic O, Djokic V, Vujanic M, Zivkovic T, Bobic B, Nikolic A, Slavic K, Klun I, Ivovic V. 2012. Kinetics of parasite burdens in blood and tissues during murine toxoplasmosis. Exp Parasitol 131:372–376. doi: 10.1016/j.exppara.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Collantes-Fernandez E, Alvarez-Garcia G, Perez-Perez V, Pereira-Bueno J, Ortega-Mora LM. 2004. Characterization of pathology and parasite load in outbred and inbred mouse models of chronic Neospora caninum infection. J Parasitol 90:579–583. doi: 10.1645/GE-3290. [DOI] [PubMed] [Google Scholar]
- 55.Elmore SA, Huyvaert KP, Bailey LL, Iqbal A, Su C, Dixon BR, Alisauskas RT, Gajadhar AA, Jenkins EJ. 2016. Multi-scale occupancy approach to estimate Toxoplasma gondii prevalence and detection probability in tissues: an application and guide for field sampling. Int J Parasitol 46:563–570. doi: 10.1016/j.ijpara.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Attwood HD, Woolley PA, Rickard MD. 1975. Toxoplasmosis in dasyurid marsupials. J Wildl Dis 11:543–551. doi: 10.7589/0090-3558-11.4.543. [DOI] [PubMed] [Google Scholar]
- 57.Canfield PJ, Hartley WJ, Dubey JP. 1990. Lesions of toxoplasmosis in Australian marsupials. J Comp Pathol 103:159–167. doi: 10.1016/S0021-9975(08)80172-7. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson DJ, Graham DI, Hutchison WM. 1991. Pathological changes in the brains of mice infected with Toxoplasma gondii: a histological, immunocytochemical and ultrastructural study. Int J Exp Pathol 72:463–474. [PMC free article] [PubMed] [Google Scholar]
- 59.New South Wales Government. Prevention of Cruelty to Animals Act 1979 and Prevention of Cruelty to Animals Regulation 2012. New South Wales Government, Sydney, New South Wales, Australia: http://www.legislation.nsw.gov.au. [Google Scholar]
- 60.National Health and Medical Research Council. 2013. Australian code for the care and use of animals for scientific purposes, 8th ed National Health and Medical Research Council, Australian Government, Canberra, Australian Capital Territory, Australia: https://www.nhmrc.gov.au. [Google Scholar]
- 61.Yu H, Huang B, Zhuo X, Chen X, Du A. 2013. Evaluation of a real-time PCR assay based on the single-copy SAG1 gene for the detection of Toxoplasma gondii. Vet Parasitol 197:670–673. doi: 10.1016/j.vetpar.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Collantes-Fernandez E, Zaballos A, Alvarez-Garcia G, Ortega-Mora LM. 2002. Quantitative detection of Neospora caninum in bovine aborted fetuses and experimentally infected mice by real-time PCR. J Clin Microbiol 40:1194–1198. doi: 10.1128/JCM.40.4.1194-1198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. 2007. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornton B, Basu C. 2011. Real-time PCR (qPCR) primer design using free online software. Biochem Mol Biol Educ 39:145–154. doi: 10.1002/bmb.20461. [DOI] [PubMed] [Google Scholar]
- 65.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benjamini Y, Krieger AM, Yekutieli D. 2006. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93:491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 67.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.