ABSTRACT
Campylobacter jejuni is a zoonotic pathogen, and a hypervirulent clone, named clone SA, has recently emerged as the predominant cause of ovine abortion in the United States. To induce abortion, orally ingested Campylobacter must translocate across the intestinal epithelium, spread systemically in the circulation, and reach the fetoplacental tissue. Bacterial factors involved in these steps are not well understood. C. jejuni is known to produce capsular polysaccharide (CPS), but the specific role that CPS plays in systemic infection and particularly abortion in animals remains to be determined. In this study, we evaluated the role of CPS in bacteremia using a mouse model and in abortion using a pregnant guinea pig model following oral challenge. Compared with C. jejuni NCTC 11168 and 81-176, a clone SA isolate (IA3902) resulted in significantly higher bacterial counts and a significantly longer duration of bacteremia in mice. The loss of capsule production via gene-specific mutagenesis in IA3902 led to the complete abolishment of bacteremia in mice and abortion in pregnant guinea pigs, while complementation of capsule expression almost fully restored these phenotypes. The capsule mutant strain was also impaired for survival in guinea pig sera and sheep blood. Sequence-based analyses revealed that clone SA possesses a unique CPS locus with a mosaic structure, which has been stably maintained in all clone SA isolates derived from various hosts and times. These findings establish CPS as a key virulence factor for the induction of systemic infection and abortion in pregnant animals and provide a viable candidate for the development of vaccines against hypervirulent C. jejuni.
KEYWORDS: Campylobacter, capsule, systemic infection, abortion, sheep, bacteremia
INTRODUCTION
As a zoonotic pathogen, Campylobacter is a leading cause of bacterial foodborne gastroenteritis in humans (1). In addition, Campylobacter species have been recognized as one of the most common causes of ovine abortion in the United States and worldwide, with an overall abortion rate of 5 to 50% in affected flocks (2). As a consequence, Campylobacter poses a significant economic burden on sheep producers and greatly impacts sheep health and welfare. A national study, NAHMS Sheep 2001, conducted by the USDA/APHIS/Veterinary Services in collaboration with the American Sheep Industry Association revealed that Campylobacter species ranked first among all infectious causes of abortion within the last 3 years of the study, with 53.7% of the reported cases being confirmed by a veterinarian or diagnostic laboratory (3).
Historically, Campylobacter fetus subsp. fetus accounted for the majority of the Campylobacter spp. associated with ovine abortion; however, we recently discovered a remarkable shift in the etiology of the disease (4, 5). Specifically, a highly virulent Campylobacter jejuni clone (clone SA for sheep abortion) has replaced C. fetus subsp. fetus as the predominant cause of ovine abortion outbreaks in the United States. Considering the high genetic diversity of Campylobacter species and the strains responsible for sheep abortion in the United States prior to the predominance of the SA clone (5–8), this finding was unexpected and indicates that clone SA has evolved to possess novel virulence traits that may have been favored or selected by current agricultural practices in sheep production. In contrast, Campylobacter isolates from sheep abortions in Great Britain and New Zealand remain genetically diverse and comprise both species (predominantly C. fetus subsp. fetus) (5, 9). The hypervirulence of clone SA in the induction of abortion was confirmed in a pregnant guinea pig model, in which it showed a distinct abortifacient ability compared with other C. jejuni strains (10). Additionally, C. jejuni clone SA is zoonotic and has been implicated in a number of human foodborne gastroenteritis cases in the United States, most of which were linked to the consumption of raw milk (11).
Campylobacter species usually live as commensals in the intestines and gallbladder of healthy sheep without causing clinical disease (6–8). However, highly virulent C. jejuni strains, such as clone SA, can cause systemic infections. In susceptible pregnant ewes, initial ingestion of C. jejuni is believed to be followed by intestinal invasion and bacteremia with subsequent placentitis, fetal infection, and abortion (2). The ability of this organism to induce bacteremia is believed to be one of the key elements in the abortion process. To become bacteremic, C. jejuni must first penetrate the intestinal mucus layer, invade the mucosal epithelium, enter the vasculature, and surpass host immune responses in the blood long enough to survive and spread systemically. Clone SA is highly abortifacient in both sheep and the pregnant guinea pig model; therefore, it must possess distinctive virulence characteristics enabling it to invade the host intestine and bloodstream.
Despite the global importance of Campylobacter as a zoonotic pathogen and recent advancements in our understanding of its pathogenesis (1, 12–14), little is known about the molecular mechanisms responsible for Campylobacter-associated abortion. Previous research on Campylobacter pathogenesis primarily focused on factors involved in enteric infection (13, 15–17); however, systemic infection (such as bacteremia and abortion) requires virulence factors that function beyond intestinal colonization. Although the S-layer protein is a known virulence factor for C. fetus subsp. fetus-induced abortion (18), C. jejuni does not have the S-layer protein, and how it causes abortion is essentially unknown. To start to address these questions, we recently employed a multiomics approach in an effort to decipher the molecular mechanism underlying the hypervirulence of C. jejuni clone SA in systemic spread and the induction of sheep abortion (19). The genome of a representative clone SA isolate (IA3902) was found to be strikingly syntenic and homologous to that of C. jejuni NCTC 11168, which, despite being able to colonize the intestine, failed to induce abortion in pregnant guinea pigs (10). However, a closer examination indicated the presence of 12 variable regions (VRs) between the two genomes, with the capsular polysaccharide (CPS) biosynthesis locus being the most divergent region (14 of the 22 IA3902 genes have no homologs in NCTC 11168) (19). Interestingly, this divergent region in the CPS locus of IA3902 has very close homology to those of three other strains (C. jejuni subsp. doylei 269.97, C. jejuni G1, and C. jejuni strain X) that were associated with systemic infection, bacteremia, and severe bloody diarrhea (19, 20), suggesting the existence of a link between a particular capsular structure and the disease phenotype. This possibility was further substantiated by the observation that C. jejuni isolates belonging to clone SA had a homogenous CPS structure, as suggested by DNA microarray analysis of sheep abortion strains (5).
Surface polysaccharides represent the predominant structures on the outermost layer of the cell of many bacterial species, and they are often important in interactions between pathogens, their hosts, and the environment (21, 22). C. jejuni produces a phase-variable CPS, which is known to be highly variable and immunogenic and serves as the major determinant of Penner serotypes of this bacterium (21, 23–25). Currently, there are 47 recognized Penner or heat-stable serotypes of C. jejuni, with some forming related serotype complexes (26, 27). CPS is one of the few clearly defined virulence factors of C. jejuni and has been shown to be involved in diarrheal disease in ferrets, commensal colonization in chickens and mice, killing of larvae of wax moths, in vitro adherence to/invasion of human epithelial cells, modulation of host immune responses, and resistance to killing by serum/complement (22, 28–32). Indirect evidence for the role of capsule in gastrointestinal disease has been demonstrated in a vaccination study with nonhuman primates, in which a capsule conjugate vaccine was found to be highly protective against oral C. jejuni challenge in monkeys (33). Despite these advances, the exact role that CPS plays in the disease pathogenesis of C. jejuni still remains incompletely understood (22). In particular, little is known regarding the role of CPS in the development of bacteremia and systemic infection by C. jejuni. The availability of a highly pathogenic C. jejuni clone with a reliable animal model provided us with an unprecedented opportunity to address this question. In this study, the contribution of capsule to systemic infection caused by C. jejuni clone SA was evaluated by using a mouse model of bacteremia and a pregnant guinea pig model of fetal abortion.
RESULTS
C. jejuni IA3902 induces bacteremia in mice.
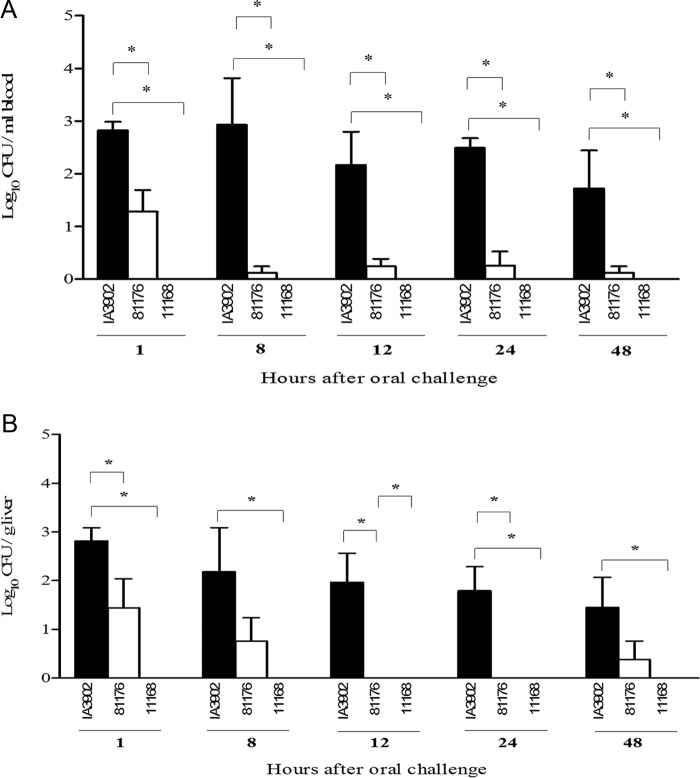
Separate groups of CD-1 mice (5/group) were orally challenged with 1 × 108 CFU of C. jejuni IA3902, NCTC 11168, and 81-176. One hour, 8 h, 12 h, 24 h, and 48 h after oral inoculation, cardiac blood and liver tissues were cultured for C. jejuni. As shown in Fig. 1, bacteremia and liver infection occurred quickly after oral inoculation, but the three C. jejuni strains showed differences in pathogenicity. IA3902 was the most bacteremic (P < 0.05), and NCTC 11168 was the least bacteremic, with no isolation of bacteria from either blood or liver. Strain 81-176 caused only transient infection, and the bacterial titers were much lower than those of IA3902 in both blood and liver (Fig. 1). For all three groups, the infected mice did not appear ill or show any clinical symptoms of infection. Animals challenged with IA3902 and 81-176 were bacteremic within 1 h after dosing. For IA3902, the bacterial burden persisted through 48 h, at which time the experiment was terminated. Quantitative cultures of IA3902 in the blood and livers of orally infected mice did not show any significant differences (P > 0.05) (Fig. 1). These results indicated that compared to 81-176 and NCTC 11168, clone SA possesses a remarkably increased ability to induce bacteremia in mice.
FIG 1.
Quantitation of systemic infection by IA3902, 81-176, and NCTC 11168 in CD-1 mice. Mice were orally challenged with 108 CFU of each strain. At each time point, 5 mice were sacrificed, and the numbers of C. jejuni bacteria in cardiac blood (A) and liver tissue (B) were determined. Each bar represents the log10 CFU per milliliter of blood or gram of liver (means ± SEM) for 5 mice. *, P < 0.05 (statistically significant). Data were collected in three different trials, which were performed under similar conditions.
Capsule is necessary for bacteremia in mice.
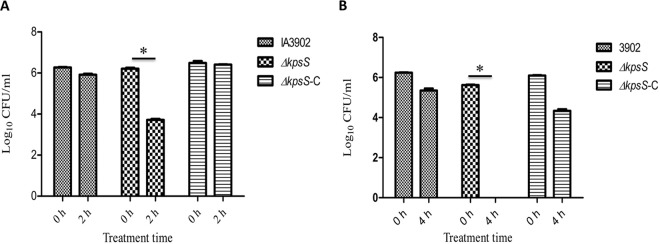
We hypothesized that capsule is important for systemic infection and that the loss of capsule would make C. jejuni clone SA unable to induce bacteremia. To test this hypothesis, we generated a mutant strain (ΔkpsS) that was deficient in capsule production (Fig. 2) and evaluated this mutant strain along with its parent strain (IA3902) and the genetic complement (ΔkpsS-C) in the mouse model. As shown in Fig. 2, the ΔkpsS strain lost capsule, while the ΔkpsS-C strain restored the production of capsule to the wild-type level. Three groups of CD-1 mice (n = 8/group) were orally challenged with the ΔkpsS, ΔkpsS-C, and IA3902 strains, respectively. At 1, 8, and 12 h postinoculation (p.i.), cardiac blood and liver tissues were collected for bacterial culture and CFU counts. The ΔkpsS mutant completely lost its ability to induce bacteremia and liver infection, while the complemented strain (ΔkpsS-C) restored virulence to near-wild-type levels (Fig. 3). This result indicates that capsule is required for the induction of bacteremia.
FIG 2.
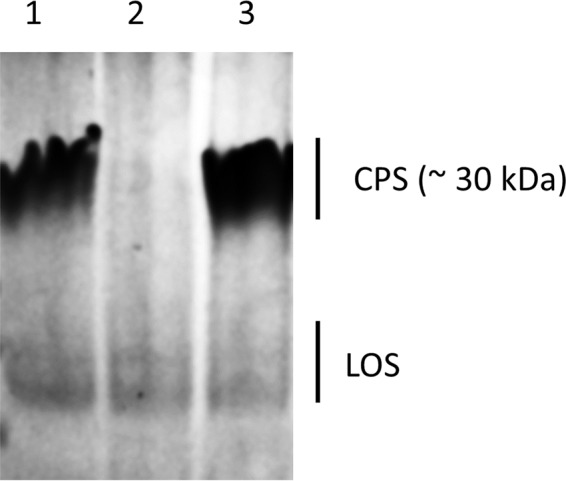
Analysis of capsule expression of C. jejuni IA3902 with alcian blue staining. Lanes: 1, wild-type strain IA3902; 2, capsule mutant strain (ΔkpsS); 3, complemented mutant strain (ΔkpsS-C). Positions of capsule (CPS) and lipooligosaccharide (LOS) are shown.
FIG 3.
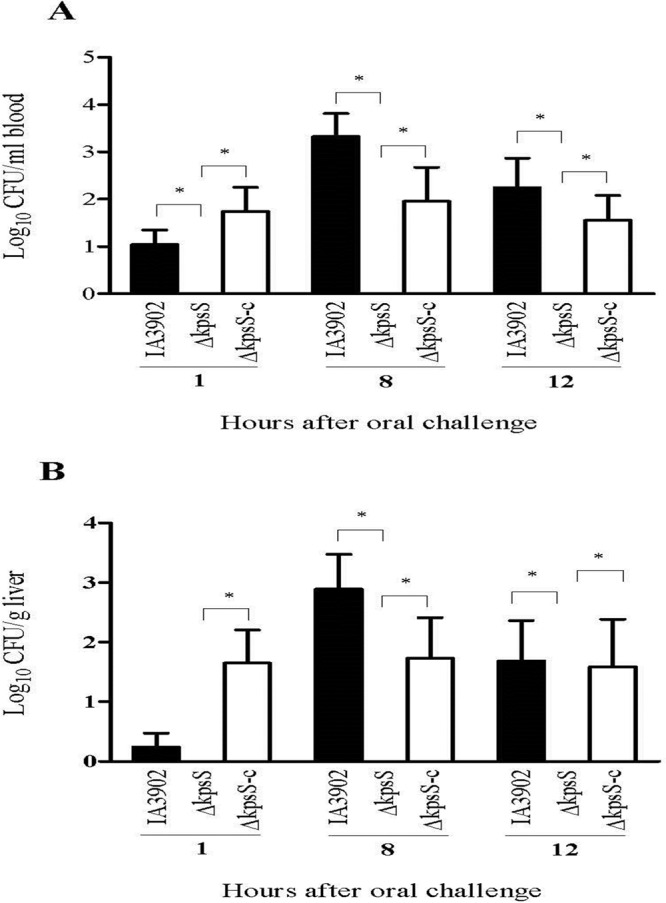
Effect of CPS on systemic infection of mice by C. jejuni. CD-1 mice (n = 8/group) were challenged via gastric gavage with 108 CFU of either wild-type strain IA3902 or the ΔkpsS or ΔkpsS-C mutant, and CFU were determined at different time points. Each bar represents the log10 CFU per milliliter of blood (A) or gram of liver (B) (means ± SEM) for 5 mice. *, P < 0.05 (statistically significant). Data were collected in two different trials, which were performed under similar conditions.
Capsule is required for induction of abortion in pregnant guinea pigs.
Next, we examined the role of capsule in abortion induction using an established pregnant guinea pig model (10, 34). Animals were confirmed to be free of Campylobacter by culturing of rectal swabs before inoculation. All of the pregnant guinea pigs (n = 10) orally inoculated with C. jejuni IA3902 aborted, with the first abortion occurring on day 4 p.i. (Fig. 4A). The majority of abortions (n = 7) occurred within the next 3 days (5 to 7 days p.i.), while the remaining two took place on days 9 and 11 p.i., respectively. In contrast, none of the pregnant guinea pigs (n = 8; 2 animals that had no identifiable pregnancy at ultrasound preinoculation were found to be nonpregnant at necropsy and were excluded from the calculations) orally challenged with the ΔkpsS isogenic capsule mutant aborted for the entire period of the study (Fig. 4A). The complemented strain (ΔkpsS-C) induced abortion in 8 of the 9 inoculated pregnant guinea pigs starting as early as day 4 p.i. (Fig. 4A). Pregnant animals in the control group inoculated with NCTC 11168 did not have any abortion during the entire experiment (results not shown). The abortion rates for the groups inoculated with IA3902 (100%) and the ΔkpsS-C strain (88.8%) were not significantly different (P > 0.05) but were significantly higher (P < 0.05) than that for the group inoculated with the ΔkpsS strain (0%).
FIG 4.
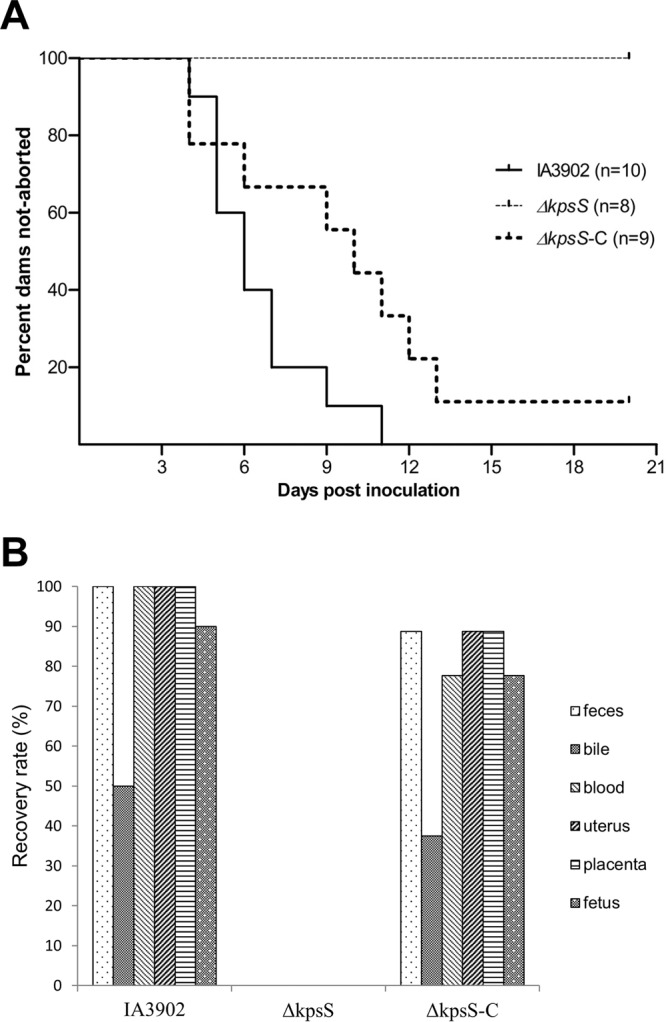
(A) Abortion rates following oral inoculation with the C. jejuni IA3902 wild-type, capsule mutant (ΔkpsS), and complemented capsule mutant (ΔkpsS-C) strains in pregnant guinea pigs. (B) Percentages of culture-positive pregnant guinea pigs (n = 10, 8, and 9 in the wild-type, mutant, and complemented groups, respectively) by each sample type processed at necropsy following abortion or at termination of the experiment 21 days after oral inoculation with the C. jejuni IA3902 wild-type, capsule mutant (ΔkpsS), or complemented capsule mutant (ΔkpsS-C) strain.
Semiquantitative culture results indicated the recovery of high numbers of Campylobacter cells (over 1,000 CFU on culture plates) from the majority of uteruses and placentas of the aborted guinea pigs inoculated with IA3902 or the ΔkpsS-C strain. Moderate levels (500 to 1,000 CFU on culture plates) of Campylobacter bacteria were also recovered from maternal blood, whereas the numbers of bacteria cultured from maternal intestinal contents and bile as well as fetal lung/liver were low (less than 500 CFU). In contrast, none of the samples (including the intestinal contents) from the pregnant guinea pigs inoculated with the ΔkpsS strain yielded any Campylobacter growth, consistent with the lack of clinical abortion in this group. It should be pointed out that the capsule mutant exhibited growth (as determined by colony size, CFU counts, and optical density at 600 nm [OD600] measurements for 48 h) and motility (as determined on motility agar) in culture media comparable to those of the wild-type strain (data not shown). Thus, the lack of infection in animals was not due to a growth or motility defect. The culture results (i.e., percentage of positive samples from pregnant animals among groups) are summarized in Fig. 4B. Histological examination revealed that the uteruses and placentas from the aborted guinea pigs inoculated with IA3902 or the ΔkpsS-C strain produced prominent microscopic inflammatory lesions, whereas those from animals inoculated with the ΔkpsS strain had no obvious microscopic lesions. Microscopic uterine lesions included suppurative endometritis, metritis, edema, and hemorrhage of various severities. Microscopic placental lesions included a combination of hemorrhage, necrosuppurative inflammation, and necrosis. These changes were evident on both the maternal and fetal sides of the placenta (results not shown). Nonpregnant guinea pigs and nonaborted pregnant animals were all Campylobacter negative upon culture and did not manifest any pathological findings on histopathology.
Capsule contributes to resistance to serum bactericidal activity.
Survival in blood is a key pathogenic step for the systemic spread of Campylobacter during abortion induction. To examine if capsule was involved in resistance to the bactericidal activity of serum, C. jejuni IA3902, its isogenic capsule mutant (ΔkpsS), and the complemented strain (ΔkpsS-C) were treated with fresh (containing complement activity) guinea pig serum free of Campylobacter-specific antibody. Following 2 h of incubation with serum, the capsule mutant showed a reduction in CFU counts of approximately 2.5 log10 units compared to the numbers at 0 h (Fig. 5A). This difference in CFU was statistically significant (P < 0.05). In contrast, the wild-type strain led to only a marginal reduction in bacterial counts upon treatment with guinea pig serum (P > 0.05). Additionally, the ΔkpsS-C strain fully restored the serum resistance level to that of the wild-type isolate (Fig. 5A). Serum sensitivity analysis was also performed by using fresh whole sheep blood, which revealed that the ΔkpsS mutant strain was no longer recoverable after 4 h of incubation in sheep blood, while the wild-type and ΔkpsS-C strains showed only modest reductions in CFU counts, and this reduction was not significant (P > 0.05) compared to values at time zero (Fig. 5B). These results indicated that capsule production is required for the optimal survival of C. jejuni within guinea pig serum and sheep blood.
FIG 5.
Survival of the C. jejuni IA3902 wild-type, capsule mutant (ΔkpsS), and complemented capsule mutant (ΔkpsS-C) strains in 20% fresh guinea pig serum (A) or in whole sheep blood (B). Data from a single experiment are shown (triplicates); similar results were obtained from an independent experiment. Significant differences (*, P < 0.05) are indicated.
Characteristics of the CPS locus of C. jejuni clone SA. (i) Genetic features.
The complete genome sequence of IA3902 (a clone SA isolate), including the entire CPS locus, was reported previously (19). Similar to other C. jejuni CPS loci studied so far, the capsular locus of IA3902 is organized into three regions in which the variable biosynthetic region is flanked by the conserved regions responsible for capsule transport and assembly (20, 22, 35, 36). The biosynthetic CPS locus of IA3902 (located between kpsC and kpsF, excluding these two genes) is approximately 26.4 kb long and comprises 22 genes of various predicted functions involved in capsule synthesis and modification (Table 1). Most of the predicted proteins encoded by the genes in the biosynthetic CPS locus of IA3902 exhibit high homology (over 85% amino acid identity) to at least one other counterpart in CPS loci from different C. jejuni strains (Table 1). As with many other biosynthetic CPS loci in C. jejuni, the four conserved genes (CJSA_1346 to CJSA_1349) involved in the biosynthesis of phosphoramidate (MeOPN) are present in IA3902, indicating the likelihood of this modification in IA3902. Although the CPS locus of IA3902 harbors a gene (gmhB) involved in the heptose pathway, it lacks the other conserved genes (hddC, gmhA, and hddA) necessary for heptose biosynthesis, indicating that this strain may not be able to synthesize this structure, which is found in many other CPS loci of C. jejuni strains (20, 22, 35). Another feature that is frequently found among the capsules of C. jejuni strains is the presence of glycerol phosphate residues, which are also suspected to be present in the capsule of IA3902 since the CPS locus of this strain harbors the two genes (tagD and tagF) involved in the biosynthesis of this structure (20, 35).
TABLE 1.
List of genes in the entire CPS locus of C. jejuni IA3902 (HS:1,8)d
| Genea | Product size (aa)e | Similarity to strain (serotype)b |
Annotation | |||||
|---|---|---|---|---|---|---|---|---|
| C. jejuni G1 (HS:1) | C. jejuni CJJ5070 (HS:4 complex) | C. jejuni subsp. doylei 269.97 (HS:17) | C. jejuni X (untypeable) | C. jejuni ATCC 43436 (HS:8) | C. jejuni NCTC 11168 (HS:2) | |||
| kpsS | 394 | Y | Y | Y | Y | Y | Y | Capsule polysaccharide export protein KpsS |
| kpsC | 689 | Y | Y | Y | Y | Y | Y | Capsule polysaccharide export protein KpsC |
| cysC | 170 | Y | Y | Y | Y | D | Y | Adenylyl sulfate kinase |
| CJSA_1347 | 253 | Y | Y | Y | Y | N | Y | Putative sugar-phosphate nucleotidyltransferase |
| CJSA_1348 | 200 | Y | Y | Y | Y | N | Y | Putative amidotransferase |
| CJSA_1349 | 779 | Y | Y | Y | Y | N | Y | Putative transferase |
| CJSA_1350 | 253 | Y | Y | Y | Y | N | Y | Putative methyltransferase |
| CJSA_1351 | 257 | Y | Y | Y | Y | N | Y | Putative methyltransferase |
| CJSA_1352 | 612 | D | D | N | D | N | D | Putative sugar transferase |
| CJSA_1353 | 240 | N | Y | Yc | Y | N | N | Capsular polysaccharide biosynthesis protein |
| CJSA_1354 | 507 | N | Y | Yc | Y | N | N | Capsular polysaccharide biosynthesis protein |
| gmhB | 132 | N | Y | Yc | Y | N | N | Heptose biphosphate phosphatase |
| CJSA_1356 | 639 | N | N | N | Y | D | N | Putative capsular polysaccharide biosynthesis protein |
| CJSA_1357 | 619 | N | N | N | N | N | N | Putative sugar nucleotidyltransferase |
| CJSA_1358 | 109 | N | Y | Yc | N | N | N | Conserved hypothetical protein |
| CJSA_1359 | 111 | N | Y | Yc | N | N | N | Conserved hypothetical protein |
| CJSA_1360 | 241 | N | Y | Yc | N | N | N | Putative nucleotidyltransferase |
| CJSA_1361 | 212 | N | Y | Yc | N | N | N | HADf superfamily hydrolase |
| CJSA_1362 | 212 | N | Y | Yc | N | N | N | Conserved hypothetical protein |
| CJSA_1363 | 637 | Y | N | N | N | N | D | Putative sugar transferase |
| CJSA_1364 | 851 | Y | N | N | N | N | N | Putative sugar transferase |
| tagF | 1,095 | Y | N | N | D | D | N | Putative CDP glycerol glycerophosphotransferase TagF |
| CJSA_1366 | 402 | Y | N | N | Y | Y | N | Conserved hypothetical protein |
| tagD | 129 | Y | N | N | Y | Y | N | Putative glycerol-3-phosphate cytidylyltransferase TagD |
| kpsF | 315 | Y | Y | Y | Y | Y | Y | Arabinose 5-phosphate isomerase |
| kpsD | 552 | Y | Y | Y | Y | Y | Y | Capsular polysaccharide ABC transporter KpsD |
| kpsE | 372 | Y | Y | Y | Y | Y | Y | Capsular polysaccharide ABC transporter KpsE |
| kpsT | 220 | Y | Y | Y | Y | Y | Y | Capsular polysaccharide ABC transporter KpsT |
| kpsM | 260 | Y | Y | Y | Y | Y | Y | Capsular polysaccharide ABC transporter KpsM |
Based on the gene name/locus tag of the sequenced genome of C. jejuni IA3902 (GenBank accession no. CP001876.1).
Y, yes (present with over 85% identity at the amino acid level); D, divergent (present with less than 85% identity); N, no (not present).
These genes are located in tandem as a separate cluster outside the CPS locus in this strain.
The entire CPS cluster of IA3902 is virtually identical to that of C. jejuni PT14 (NCBI GenBank accession no. NC_018709.2) and highly homologous to that of C. jejuni 01/51 (NCBI GenBank accession no. ERS742291 and ERS742289). Thus, these two strains are not included in the table.
aa, amino acids.
HAD, haloacid dehalogenase.
Previously, IA3902 was determined to belong to Penner serotype HS:1,8 (reacting with HS:1 and, to a lesser extent, HS:8 antisera) (11). This is, to our knowledge, the only C. jejuni strain with this serotype combination, suggesting the uniqueness of its CPS structure. As shown in Table 1, the biosynthetic CPS locus of IA3902 appears to be a mosaic of those of C. jejuni G1 (which belongs to HS:1; isolated from a patient with Guillain-Barre syndrome) and, to a lesser extent, other strains, including C. jejuni 5070 (associated with the HS:4 Penner complex; belongs to the “hyperinvasive” sequence type 677 [ST-677] clonal complex), C. jejuni subsp. doylei 269.97 (a serostrain of HS:17; isolated from human blood), and strain X (untypeable; cultured from a human patient with severe bloody diarrhea).
(ii) Genetic stability of the CPS locus.
The findings presented above indicated the uniqueness of the CPS locus in C. jejuni IA3902. To determine whether other clone SA isolates possessed the same CPS genes, we carried out a sequence-based comparison of the CPS locus of IA3902 and those of other C. jejuni strains from multiple sources, including clone SA isolates from sheep (Table 2). Of note, the CPS sequences of these strains were previously determined to a near-complete stage with paired-end reads (2 by 100 bp) on an Illumina HiSeq 2000 machine (37). The results showed that all the sequenced clone SA isolates (n = 63) had nucleotide sequences in their CPS loci that were virtually identical to that of IA3902 regardless of their isolation sources (sheep abortion, bovine abortion, goat abortion, sheep feces/bile, chicken feces, and human gastroenteritis) or years (1991 to 2013) (Table 2). This finding indicates that the CPS locus in clone SA is genetically stable.
TABLE 2.
Sequence-based comparison of CPS loci among C. jejuni strains in reference to clone SA isolate IA3902a
| Group | No. of isolates | Yr of isolation | Source | ST(s) | CC(s) | Description |
|---|---|---|---|---|---|---|
| Reference | ||||||
| IA3902 | 1 | 2006 | Sheep abortion | 8 | 21 | Penner serotype HS:1,8; whole genome sequenced |
| Clone SA | ||||||
| Early-SA | 11 | 1991–1993 | Sheep abortion | 8 | 21 | CPS locus identical to that of IA3902 |
| Mid-SA | 3 | 1999–2000 | Sheep abortion | 8 | 21 | CPS locus identical to that of IA3902 |
| Recent-SA | 27 | 2003–2013 | Sheep abortion | 8 | 21 | CPS locus identical to that of IA3902 |
| Bovine-SA | 5 | 2003–2009 | Bovine abortion | 8 | 21 | CPS locus identical to that of IA3902 |
| Goat-SA | 4 | 2005–2010 | Goat abortion | 8 | 21 | CPS locus identical to that of IA3902 |
| Sheep-SA | 4 | 2008 | Sheep feces/bile | 8 | 21 | CPS locus identical to that of IA3902 |
| Bird-SA | 2 | 2004 | Chicken feces | 8 | 21 | CPS locus identical to that of IA3902 |
| Human-SA | 7 | 2003–2010 | Human enteritis | 8 | 21 | CPS locus identical to that of IA3902 |
| Non-clone SA | ||||||
| Early-U.S. | 1 | 1993 | Sheep abortion | 21 | 21 | Rare; predicted progenitor of clone SA; CPS locus identical to that of IA3902 |
| Early-U.S. | 1 | 1993 | Sheep abortion | 441 | UA | Rare, CPS locus divergent from that of IA3902 |
| Early/mid-U.S. | 6 | 1993–2004 | Sheep abortion | 50 | 21 | Rare; 4 are identical to IA3902; 2 are divergent from IA3902 |
| Recent-U.S. | 10 | 2003–2010 | Sheep abortion | 38, 42, 43, 45, 607, 806, 982, 5189 | 48, 42, 21, 45, 607, 61 | CPS locus divergent from that of IA3902 |
| U.K. | 9 | 2003–2008 | Sheep abortion from U.K. | 21, 42, 45, 50, 206, 227, 270 | 21, 42, 45, 206, 403 | CPS locus divergent from that of IA3902, except ST-50 |
| Other | ||||||
| Cont-1 | 1 | 1985 | Human enteritis | 43 | 21 | NCTC 11168; divergent; nonabortifacient in guinea pigs |
| Cont-2 | 1 | 2006 | Sheep feces | 61 | 61 | OF48; divergent; nonabortifacient in guinea pigs |
ST, sequence type; CC, clonal complex; UA, unassigned.
Production of capsule.
To examine the production of capsule in clone SA isolates from sheep abortions, proteinase-treated whole-cell extracts were separated on a Tricine SDS-PAGE gel and stained with alcian blue as described previously (23). As shown in Fig. 6, all six clone SA strains produced an intense capsule band, the size and intensity of which are different from those of other strains of different genotypes, suggesting the presence of a distinct capsular structure in C. jejuni clone SA.
FIG 6.
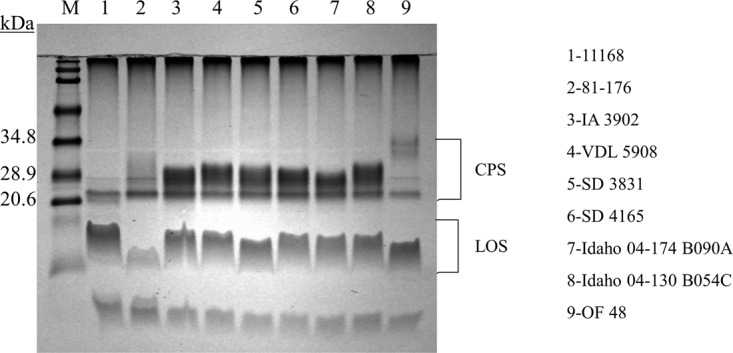
Capsule of C. jejuni as detected by Tricine SDS-PAGE with alcian blue staining. Clone SA isolates of sheep abortions from Iowa (lanes 3 and 4), South Dakota (lanes 5 and 6), and Idaho (lanes 7 and 8) are depicted. As controls for non-clone SA isolates, NCTC 11168 (lane 1), 81-176 (lane 2), and OF48 (lane 9) (from feces of healthy sheep) are included. M, prestained protein size markers (Bio-Rad). Positions of CPS and lipooligosaccharide (LOS) are shown on the right.
DISCUSSION
This study provides the first direct experimental evidence for a role of capsule in the pathogenesis of bacteremia, systemic infection, and abortion caused by a hypervirulent C. jejuni clone. Although C. jejuni is mainly considered a gastrointestinal pathogen in humans, it can occasionally cause severe conditions, including neurological complications (e.g., Guillain-Barre and Miller Fisher syndromes), bacteremia, and systemic infections such as abortion and newborn meningitis (38, 39). In ruminants, C. jejuni is becoming an important etiological factor for abortion. However, bacterial factors involved in the pathogenesis of extraintestinal infections due to C. jejuni are understudied. Even though the capsule of C. jejuni was presumed to play a crucial role in systemic disease due to its involvement in resistance to serum killing and phagocytosis as determined by in vitro studies (22, 32, 40), direct in vivo evidence for its contribution to the induction of bacteremia and systemic infection had not been demonstrated prior to this study. Here, we provide compelling evidence demonstrating that capsule is a key virulence determinant in a highly pathogenic C. jejuni clone (clone SA) using two different animal models, i.e., mouse bacteremia and guinea pig abortion. In both models, the capsule mutant showed a severe phenotypic defect, while complementation of the mutant restored its ability to induce bacteremia and abortion (Fig. 3 and 4).
There are several possible mechanisms underlying how capsule may contribute to systemic infection and abortion. Results from this study suggest that capsule plays a role in the survival and persistence of C. jejuni in the bloodstream and subsequent fetoplacental invasion, since the noncapsular mutant displayed a clear defect in serum resistance, bacteremia, and abortion induction (Fig. 3 to 5). Additionally, data from this study suggest a role of capsule in the colonization and/or translocation of intestinal mucosa and entering the bloodstream, as the noncapsular C. jejuni mutant was not recovered from any site, including cecal contents, bile, heart blood, uterus, and placenta, in pregnant guinea pigs (Fig. 4B). This notion is supported by findings from previous studies in which the capsule of C. jejuni was shown to play an important role in adhesion to/invasion of intestinal epithelial cells in vitro, colonization of chickens and mice, and induction of diarrhea in ferrets (29, 31, 40–42). The lack of capsule in C. jejuni was also associated with increased cytokine production by murine dendritic cells in vitro (43) and by lamina propria CD4 T cells from mouse intestine (31), which play an important role in the innate immune response to enteric bacterial pathogens (44, 45), suggesting the involvement of capsule in protection against the induction of immune responses in the gut. Altogether, these results demonstrate the significance of capsule in the evasion of host innate immunity present in the gut mucosa and systemic circulation by a hypervirulent C. jejuni clone.
It is most likely that the presence of a functional capsule, regardless of its structure, is required for systemic infection and abortion induction. This notion is supported by findings from a very recent study (37), which identified the key role of specific mutations in porA, encoding the major outer membrane protein (MOMP), in abortion induction by C. jejuni clone SA. Specifically, transferring specific mutations in porA of C. jejuni IA3902 (HS:1,8) to NCTC 11168 (HS:2), a nonabortifacient strain, converted it into a fully virulent strain (37), despite the fact that IA3902 and NCTC 11168 have different CPS sequences (Table 1). These findings indicate that the function of CPS is required but is not sufficient for abortion induction. The genetic and structural variations of CPS may contribute to other phenotypes such as antigenic variation and immune evasion, which remain to be determined in future studies.
Both sequence-based comparisons and phenotypic analysis (Table 2 and Fig. 6) indicated the presence of a unique CPS structure possessed by clone SA isolates. Considering the overall diversity of capsule loci detected among C. jejuni strains (20, 27), it is quite remarkable that all clone SA isolates had almost identical CPS sequences regardless of the time (spanning over 20 years), host (sheep, cattle, goat, chicken, and human), or site (aborted materials, feces, and bile) of isolation. In contrast to our findings, a recent study analyzing the CPS loci of hyperinvasive C. jejuni strains discovered a high degree of variability in capsule locus architectures even among strains that were very similar on the genome level (46). More specifically, among the six hyperinvasive strains studied, four of them were identical at the level of the core genome phylogeny (all belonging to ST-21) but had substantial genetic diversity in the capsule loci (46). The localized variations in the capsule loci of otherwise clonal strains are likely due to horizontal gene transfer and homologous recombination, which were reported in previous studies (20, 35). The high stability of the capsule locus in clone SA isolates over the years suggests that it is necessary for the adaptation of clone SA in the ruminant reservoir.
In agreement with the high degree of genetic similarity, phenotypic analysis with alcian blue staining also suggested the presence of stable and similar CPS structures among the different isolates of clone SA (Fig. 6). Different levels of intensity of CPS shown with alcian blue (a cationic dye) staining were previously observed (23), and it was suggested that high intensity was associated with the presence of glycerol phosphate residues (negatively charged) in the CPSs of certain C. jejuni isolates (20). The intensive staining of the CPSs of clone SA isolates is consistent with the fact that clone SA contains the tagD and tagF genes responsible for the biosynthesis of glycerol phosphate (Table 1). It should be pointed out that alcian blue also stains capsules that lack glycerol phosphate but contain MeOPN (also negatively charged), as was the case with strains 11168 and 81-176 (Fig. 6).
Ovine abortion poses a major economic burden on sheep producers worldwide, and C. jejuni clone SA has been consistently the predominant cause of this important disease in the United States for over a decade (4, 5). Whole-cell-based killed vaccines (along with antibiotics) have been used for the control of Campylobacter-associated abortions in sheep; however, their efficacy appears to be poor and varies widely in the United States (47, 48). Previous studies indicated that CPS is a protective antigen, as capsule is a proven virulence determinant of C. jejuni, and a capsule-based conjugate vaccine was highly immunogenic and protective against disease in both mice and monkeys (33, 49). Considering the findings of this study showing that the CPS is a crucial virulence factor in systemic infection (Fig. 3 and 4) and is highly conserved and stable over time (Table 2), capsule may be used as a potential target for the development of an efficacious vaccine to prevent sheep abortion in the United States.
In summary, our results demonstrate that capsule plays an indispensable role in the pathogenesis of bacteremia and fetoplacental infection, the hallmarks of hypervirulent C. jejuni clone SA that has emerged during the last decade as the predominant cause of sheep abortion in the United States. We showed that the isogenic acapsular IA3902 mutant was totally attenuated in the induction of bacteremia in the mouse model and of abortion in the pregnant guinea pig model, which correlated with its impaired survival in serum and whole blood. We further identified that the CPS locus of IA3902 has a mosaic organization and contains several genes that are common to other Campylobacter strains with perceived high virulence. The CPS locus of clone SA was found to be strikingly stable, as all the strains of this clone had virtually identical capsule sequences regardless of the time or source of isolation, suggesting a role for capsule in bacterial persistence and fitness. Altogether, these findings establish the capsule as a feasible candidate for the development of effective antibacterial means against systemic infections associated with C. jejuni, a zoonotic pathogen causing disease in both humans and animals worldwide.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni IA3902 is a clinical isolate of clone SA originally derived from an ovine abortion case in Iowa in 2006, as described previously (4). The complete genome of IA3902 has been sequenced (19). C. jejuni NCTC 11168 and 81-176 are both human isolates (25, 50), nonabortifacient, and genome sequenced and were used as controls. All C. jejuni strains were grown on Mueller-Hinton (MH) agar under microaerobic (5% oxygen, 10% carbon dioxide, and 85% nitrogen) conditions at 42°C for up to 48 h. When needed, the culture medium was supplemented with Campylobacter-selective agents and supplements (catalog no. SR084E and SR117E; Oxoid). Kanamycin (50 μg/ml) or chloramphenicol (20 μg/ml) was added to MH medium as needed.
Construction of a CPS mutant.
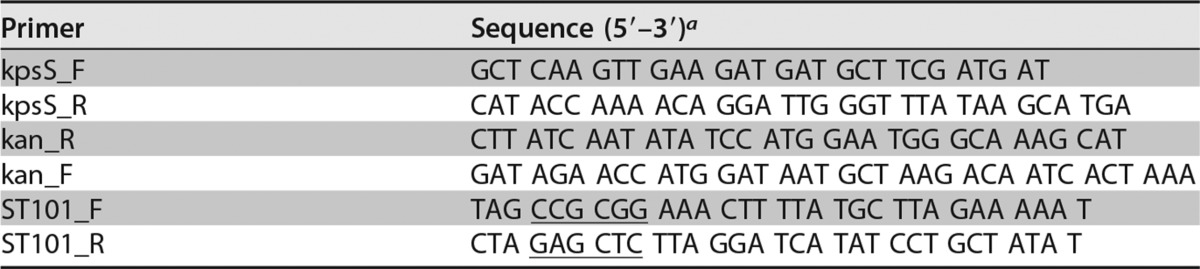
Insertion-deletion mutagenesis in the kpsS gene (encoding an ABC transporter involved in capsule transport) of IA3902 was performed in order to define the role of CPS in studies performed here according to our previously reported methods (51). Briefly, a 1,693-bp region harboring kpsS (CJSA_1344) (1,182 bp) of IA3902 was PCR amplified with primers kpsS_F and kpsS_R (Table 3) and cloned into the commercial vector pGEM-T (Promega, Madison, WI) to yield pGEM-T::kpsS. The resulting construct was digested with SwaI and ligated with the aphA-3 gene that was amplified from pMW10 (52) with primers Kan_F and Kan_R (Table 3). The suicide vector was delivered to C. jejuni IA3902 via natural transformation, and mutants were selected on MH agar plates containing kanamycin (50 μg/ml). ΔkpsS mutants were confirmed by PCR analysis and alcian blue staining (Fig. 2), as described below.
TABLE 3.
PCR primers used in this study
The underlined sequences indicate the restriction sites for SacI (GAGCTC) and SacII (CCGCGG).
Complementation of the kpsS mutant in trans.
The construction of the complementing plasmid for the kpsS mutant was based on methods reported in our previous study (53). The entire coding region of the kpsS gene was amplified from strain IA3902 by PCR using primers ST101_F and ST101_R (Table 3). The PCR product was digested with SacI and SacII and cloned into the plasmid construct pRY112-pABC (54, 55) to generate pRY112-kpsS, in which the kpsS gene was fused to the constitutively expressed promoter of cmeABC. The constructed plasmid was confirmed by PCR. For complementation, the shuttle plasmid pRY112-kpsS was introduced into the ΔkpsS mutant by conjugation. The complemented strain was selected on MH agar containing chloramphenicol (20 μg/ml) and named the ΔkpsS-C strain. PCR analysis and alcian blue staining were performed to confirm the complementation of capsule expression (Fig. 2). Both the ΔkpsS and ΔkpsS-C mutant strains grew on nonselective and Campylobacter-selective agar plates comparably to the wild type, and each strain exhibited wild-type levels of motility (results not shown).
Alcian blue staining for CPS.
Preparation of CPS samples for alcian blue staining was performed as previously described (23). Wild-type and mutant C. jejuni cells were harvested following 24 h of growth on MH agar, weighed, and solubilized in 300 μl of lysis buffer (2% sodium dodecyl sulfate, 4% 2-mercaptoethanol, 10% glycerol, 1 M Tris-HCl [pH 6.8], and 10 mg bromophenol blue) for 10 min at 100°C. After centrifugation at 13,000 × g for 5 min, 20-μl aliquots were taken, mixed with proteinase K (Sigma, St. Louis, MO) to a final concentration of 1 μg/μl, incubated at 50°C for 1 h, and fractionated by SDS-PAGE. CPS was visualized by alcian blue staining (0.1% alcian blue dye, 40% ethanol, and 5% acetic acid).
Mouse experiments.
Eight- to ten-week-old wild-type CD-1 (outbred) female mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained by Laboratory Animal Resources at Iowa State University. The mice were housed for a minimum of 3 days before being used for experiments. Following oral inoculation, mice were housed in groups of four or five in sterile polycarbonate microisolator cages with autoclaved bedding and provided with water and feed ad libitum (except that feed was withdrawn overnight prior to bacterial challenge). C. jejuni bacteria used for inoculations were recovered from freezer stocks, plated onto selective medium, and incubated for 48 h microaerobically. Bacterial cultures obtained from these plates were subpassaged under the same conditions for 18 to 20 h. Fresh cultures were harvested and suspended in MH broth, diluted to the desired concentration based on the optical density, and subsequently confirmed by viable plate counts. Each mouse received 100 μl bacterial culture (approximately 1 × 108 CFU) via gastric gavage using a curved, ball-tipped, 18-gauge, 2-inch needle under light sedation with 2% isoflurane, as previously described (56).
At 1, 8, 12, 24, and 48 h p.i., mice were deeply anesthetized via intraperitoneal (i.p.) injection of a ketamine-xylazine mixture, followed by exsanguination. Samples harvested for Campylobacter culture included cardiac blood and liver. Blood was collected by the use of a sterile tuberculin syringe with a 22-gauge needle and placed into blood collection tubes (0.5-ml Greiner Vacuette MiniCollect K3 EDTA; Fisher Scientific). Within 2 h of sample collection, 250 μl of undiluted blood and appropriate serial dilutions were plated onto Campylobacter-selective medium and incubated for 48 h. Since liver has a potential role in clearing C. jejuni from the bloodstream, liver tissues were also cultured. Liver tissues were placed into a separate sterile plastic bag (Whirl-Pak; Nasco, Fort Atkinson, WI), weighed, homogenized in sterile MH broth, serially diluted, plated onto selective medium, and incubated for 48 h microaerobically. C. jejuni recovery is expressed as log10 CFU per milliliter of blood or gram of liver. Fecal swabs were taken before challenge, and a noninoculated control group (n = 5) was sacrificed at the final time point (48 h) to confirm that all mice were Campylobacter free and that no cross-contamination occurred. Additionally, recovered isolates were subjected to PCR/antibiotic susceptibility confirmation to ensure that the source of infection was oral inoculation and not environmental contamination or cross-contamination.
Pregnant guinea pig experiments.
Palpably pregnant (at approximately 30 to 35 days of gestation) Hartley guinea pigs were obtained from a commercial source (ELM Hill Labs, Chelmsford, MA) for use in this study according to overall protocols described previously (10). Animals were housed individually in standard plastic cages with wood chip bedding and provided with a commercial guinea pig diet and water ad libitum, except that the feed was withdrawn overnight prior to bacterial challenge. Rectal swabs were obtained and cultured to confirm that animals were free of Campylobacter prior to experimental inoculation. Within 48 h of housing, the animals were subjected to ultrasound to confirm pregnancy. The animals were then divided into 3 groups (each consisting of 10 subjects) and inoculated orally by employing the same type of feeding needle as the one used on mice with a fresh culture (grown for about 20 h on MH agar) of C. jejuni strain IA3902 (wild type), the ΔkpsS mutant (isogenic capsule mutant), or the ΔkpsS-C strain (complemented capsule mutant strain). Each guinea pig within a group received 1 ml MH broth containing approximately 2 × 108 CFU of the respective strain. As a negative-control group, 5 animals were orally inoculated with C. jejuni NCTC 11168, which was shown to be nonabortifacient in our previous study (10).
Following inoculation, the guinea pigs were monitored twice daily for signs of abortion (vaginal bleeding and/or expelled fetuses/placentas). Once an animal was found to have signs of abortion, it was euthanized via i.p. injection of a commercial sodium pentobarbital solution (Fatal-Plus 390 mg/ml; Vortech Pharmaceuticals, Dearborn, MI). At necropsy, samples were collected for semiquantitative bacterial culture (maternal blood from the heart, bile, intestinal contents, uterus, placenta, and fetal lung/liver) and histopathology (uterus and placenta). Semiquantitative culture involved plating out approximately 250 μl of fluid samples or streaking a cotton swab that had been macerated into approximately 5 g of tissue samples over the entire surface of the agar plate. Growth was then recorded in a “semiquantitative” fashion such that “high” designates >1,000 CFU/plate, “moderate” indicates 500 to 1,000 CFU/plate, and “low” specifies <500 CFU/plate. The culture conditions for Campylobacter (the selective media used and incubation) were the same as those described above for the mouse experiments. On day 21 postinoculation, all nonaborted animals were euthanized and necropsied for culture and histopathology as described above. The isolates recovered from the tissues were confirmed to be the inoculated strain for each group by using specific PCR and culture on antibiotic-containing media. All of the procedures and protocols involving animal studies (both mice and guinea pigs) were approved by the institutional animal care and use committee (IACUC) at Iowa State University prior to the start of experiments.
Serum bactericidal assay.
To determine the role of capsule in resistance to the bactericidal activity of guinea pig serum, sera were collected from five Campylobacter-free pregnant guinea pigs, confirmed to be free of Campylobacter-specific antibodies by immunoblotting, pooled, filter sterilized with a 0.22-μm filter, and kept frozen in small aliquots at −80°C until use. Bacterial inocula were prepared from fresh cultures of C. jejuni IA3902, the ΔkpsS mutant, and the ΔkpsS-C strain grown overnight, and the OD600 was adjusted to 0.01 to give approximately 1 × 107 CFU/ml of each strain. The bactericidal assay was performed in sterile 96-well culture plates at 37°C with microaerobic incubation according to previously described methods (32, 57). Each reaction well (3 wells per strain) was supplemented with 200 μl of the pooled serum (at a final concentration of 20% serum) and the bacterial inoculum (final cell concentration of approximately 5 × 105 CFU/ml). Bacterial counts for each strain were determined via plate culture before (0 h) and after 2 h of incubation under the conditions described above. Log10-converted CFU at 0 h and 2 h of incubation were used to determine the amount of killing by serum for each strain. The experiment was repeated twice. Similar experiments were also performed with fresh sheep blood, in which the bacterial counts were determined after incubation in whole blood for 4 h.
Genetic features and distribution of CPS in clone SA isolates.
Characteristics of the CPS locus, including genetic organization, gene content, and predicted structural modifications, were determined by using the complete genome sequence of strain IA3902 of C. jejuni clone SA (GenBank accession no. CP001876.1). Comparative genomic analysis of the CPS locus was carried out by utilizing the near-complete genome sequences of a large number of clone SA isolates determined previously (37). The distribution of the CPS loci among clone SA genomes and relevant Campylobacter strains were ascertained by using the available sequence data. Alcian blue staining (23) was used to determine the overall production/structure of the CPS of clone SA isolates and for comparison to other strains with known phenotypes.
Statistical analysis.
Systemic infection results for mice are expressed as mean log10 CFU per milliliter of blood or gram of liver, with error bars denoting the standard errors of the means (SEM). The significance of differences in the levels of systemic infection between groups inoculated with different wild-type or mutant strains was determined by using the Wilcoxon rank sum test. Statistical analysis of serum bactericidal assay results was performed with the Student t test. Log rank and Wilcoxon rank sum tests were used to compare abortion rates among groups of pregnant guinea pigs inoculated with different strains. P values of less than 0.05 were considered statistically significant for all analyses.
ACKNOWLEDGMENT
This work was supported by AFRI Animal Health competitive grant no. 2013-67015-20368 from the USDA National Institute of Food and Agriculture.
REFERENCES
- 1.Kaakoush NO, Castano-Rodriguez N, Mitchell HM, Man SM. 2015. Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skirrow MB. 1994. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol 111:113–149. doi: 10.1016/S0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 3.USDA. 2001, Part II: reference of sheep health in the United States. USDA, APHIS, VS, CEAH, National Animal Health Monitoring System, Forth Collins, CO. [Google Scholar]
- 4.Sahin O, Plummer PJ, Jordan DM, Sulaj K, Pereira S, Robbe-Austerman S, Wang L, Yaeger MJ, Hoffman LJ, Zhang Q. 2008. Emergence of a tetracycline-resistant Campylobacter jejuni clone associated with outbreaks of ovine abortion in the United States. J Clin Microbiol 46:1663–1671. doi: 10.1128/JCM.00031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, Sippy R, Sahin O, Plummer P, Vidal A, Newell D, Zhang Q. 2014. Genetic diversity and antimicrobial susceptibility of Campylobacter jejuni isolates associated with sheep abortion in the United States and Great Britain. J Clin Microbiol 52:1853–1861. doi: 10.1128/JCM.00355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley K, Jones K. 2003. Cattle and sheep farms as reservoirs of Campylobacter. J Appl Microbiol 94(Suppl):104S–113S. doi: 10.1046/j.1365-2672.94.s1.12.x. [DOI] [PubMed] [Google Scholar]
- 7.Milnes AS, Stewart I, Clifton-Hadley FA, Davies RH, Newell DG, Sayers AR, Cheasty T, Cassar C, Ridley A, Cook AJ, Evans SJ, Teale CJ, Smith RP, McNally A, Toszeghy M, Futter R, Kay A, Paiba GA. 2008. Intestinal carriage of verocytotoxigenic Escherichia coli O157, Salmonella, thermophilic Campylobacter and Yersinia enterocolitica, in cattle, sheep and pigs at slaughter in Great Britain during 2003. Epidemiol Infect 136:739–751. doi: 10.1017/S0950268807009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acik MN, Cetinkaya B. 2006. Heterogeneity of Campylobacter jejuni and Campylobacter coli strains from healthy sheep. Vet Microbiol 115:370–375. doi: 10.1016/j.vetmic.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Mannering SA, West DM, Fenwick SG, Marchant RM, O'Connell K. 2006. Pulsed-field gel electrophoresis of Campylobacter jejuni sheep abortion isolates. Vet Microbiol 115:237–242. doi: 10.1016/j.vetmic.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Burrough ER, Sahin O, Plummer PJ, Zhang QJ, Yaeger MJ. 2009. Pathogenicity of an emergent, ovine abortifacient Campylobacter jejuni clone orally inoculated into pregnant guinea pigs. Am J Vet Res 70:1269–1276. doi: 10.2460/ajvr.70.10.1269. [DOI] [PubMed] [Google Scholar]
- 11.Sahin O, Fitzgerald C, Stroika S, Zhao S, Sippy RJ, Kwan P, Plummer PJ, Han J, Yaeger MJ, Zhang Q. 2012. Molecular evidence for zoonotic transmission of an emergent highly pathogenic Campylobacter jejuni clone in the United States. J Clin Microbiol 50:680–687. doi: 10.1128/JCM.06167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton DJ. 2015. Campylobacter virulence and survival factors. Food Microbiol 48:99–108. doi: 10.1016/j.fm.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Young KT, Davis LM, DiRita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5:665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 14.Gilbreath JJ, Cody WL, Merrell DS, Hendrixson DR. 2011. Change is good: variations in common biological mechanisms in the epsilonproteobacterial genera Campylobacter and Helicobacter. Microbiol Mol Biol Rev 75:84–132. doi: 10.1128/MMBR.00035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konkel ME, Monteville MR, Rivera-Amill V, Joens LA. 2001. The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr Issues Intest Microbiol 2:55–71. [PubMed] [Google Scholar]
- 16.Poly F, Guerry P. 2008. Pathogenesis of Campylobacter. Curr Opin Gastroenterol 24:27–31. doi: 10.1097/MOG.0b013e3282f1dcb1. [DOI] [PubMed] [Google Scholar]
- 17.Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 18.Grogono-Thomas R, Dworkin J, Blaser MJ, Newell DG. 2000. Roles of the surface layer proteins of Campylobacter fetus subsp. fetus in ovine abortion. Infect Immun 68:1687–1691. doi: 10.1128/IAI.68.3.1687-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Sahin O, Shen Z, Liu P, Miller WG, Zhang Q. 2013. Multi-omics approaches to deciphering a hypervirulent strain of Campylobacter jejuni. Genome Biol Evol 5:2217–2230. doi: 10.1093/gbe/evt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlyshev AV, Quail MA, Parkhill J, Wren BW. 2013. Unusual features in organisation of capsular polysaccharide-related genes of C. jejuni strain X. Gene 522:37–45. doi: 10.1016/j.gene.2013.03.087. [DOI] [PubMed] [Google Scholar]
- 21.Guerry P, Szymanski CM. 2008. Campylobacter sugars sticking out. Trends Microbiol 16:428–435. doi: 10.1016/j.tim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. 2012. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol 2:7. doi: 10.3389/fcimb.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlyshev AV, Wren BW. 2001. Detection and initial characterization of novel capsular polysaccharide among diverse Campylobacter jejuni strains using alcian blue dye. J Clin Microbiol 39:279–284. doi: 10.1128/JCM.39.1.279-284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlyshev AV, Linton D, Gregson NA, Lastovica AJ, Wren BW. 2000. Genetic and biochemical evidence of a Campylobacter jejuni capsular polysaccharide that accounts for Penner serotype specificity. Mol Microbiol 35:529–541. doi: 10.1046/j.1365-2958.2000.01717.x. [DOI] [PubMed] [Google Scholar]
- 25.Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 26.Poly F, Serichantalergs O, Kuroiwa J, Pootong P, Mason C, Guerry P, Parker CT. 2015. Updated Campylobacter jejuni capsule PCR multiplex typing system and its application to clinical isolates from South and Southeast Asia. PLoS One 10:e0144349. doi: 10.1371/journal.pone.0144349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pike BL, Guerry P, Poly F. 2013. Global distribution of Campylobacter jejuni Penner serotypes: a systematic review. PLoS One 8:e67375. doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones MA, Marston KL, Woodall CA, Maskell DJ, Linton D, Karlyshev AV, Dorrell N, Wren BW, Barrow PA. 2004. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect Immun 72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol 40:769–777. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 30.van Alphen LB, Wenzel CQ, Richards MR, Fodor C, Ashmus RA, Stahl M, Karlyshev AV, Wren BW, Stintzi A, Miller WG, Lowary TL, Szymanski CM. 2014. Biological roles of the O-methyl phosphoramidate capsule modification in Campylobacter jejuni. PLoS One 9:e87051. doi: 10.1371/journal.pone.0087051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, Ferderber JS, Porter CK, Trent MS, Guerry P. 2013. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 81:665–672. doi: 10.1128/IAI.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keo T, Collins J, Kunwar P, Blaser MJ, Iovine NM. 2011. Campylobacter capsule and lipooligosaccharide confer resistance to serum and cationic antimicrobials. Virulence 2:30–40. doi: 10.4161/viru.2.1.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. 2009. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun 77:1128–1136. doi: 10.1128/IAI.01056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrough ER, Sahin O, Plummer PJ, DiVerde K, Zhang Q, Yaeger MJ. 2010. Comparison of two commercial ovine Campylobacter vaccines and an experimental bacterin in a guinea pig model. Am J Vet Res 72:799–805. doi: 10.2460/ajvr.72.6.799. [DOI] [PubMed] [Google Scholar]
- 35.Karlyshev AV, Champion OL, Churcher C, Brisson JR, Jarrell HC, Gilbert M, Brochu D, St Michael F, Li J, Wakarchuk WW, Goodhead I, Sanders M, Stevens K, White B, Parkhill J, Wren BW, Szymanski CM. 2005. Analysis of Campylobacter jejuni capsular loci reveals multiple mechanisms for the generation of structural diversity and the ability to form complex heptoses. Mol Microbiol 55:90–103. doi: 10.1111/j.1365-2958.2004.04374.x. [DOI] [PubMed] [Google Scholar]
- 36.Poly F, Serichatalergs O, Schulman M, Ju J, Cates CN, Kanipes M, Mason C, Guerry P. 2011. Discrimination of major capsular types of Campylobacter jejuni by multiplex PCR. J Clin Microbiol 49:1750–1757. doi: 10.1128/JCM.02348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Periaswamy B, Sahin O, Yaeger M, Plummer P, Zhai W, Shen Z, Dai L, Chen SL, Zhang Q. 2016. Point mutations in the major outer membrane protein drive hypervirulence of a rapidly expanding clone of Campylobacter jejuni. Proc Natl Acad Sci U S A 113:10690–10695. doi: 10.1073/pnas.1605869113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JL. 2002. Campylobacter jejuni infection during pregnancy: long-term consequences of associated bacteremia, Guillain-Barre syndrome, and reactive arthritis. J Food Prot 65:696–708. doi: 10.4315/0362-028X-65.4.696. [DOI] [PubMed] [Google Scholar]
- 39.Blaser MJ, Perez GP, Smith PF, Patton C, Tenover FC, Lastovica AJ, Wang WI. 1986. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J Infect Dis 153:552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- 40.Wong A, Lange D, Houle S, Arbatsky NP, Valvano MA, Knirel YA, Dozois CM, Creuzenet C. 2015. Role of capsular modified heptose in the virulence of Campylobacter jejuni. Mol Microbiol 96:1136–1158. doi: 10.1111/mmi.12995. [DOI] [PubMed] [Google Scholar]
- 41.Bachtiar BM, Coloe PJ, Fry BN. 2007. Knockout mutagenesis of the kpsE gene of Campylobacter jejuni 81116 and its involvement in bacterium-host interactions. FEMS Immunol Med Microbiol 49:149–154. doi: 10.1111/j.1574-695X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 42.Grant AJ, Coward C, Jones MA, Woodall CA, Barrow PA, Maskell DJ. 2005. Signature-tagged transposon mutagenesis studies demonstrate the dynamic nature of cecal colonization of 2-week-old chickens by Campylobacter jejuni. Appl Environ Microbiol 71:8031–8041. doi: 10.1128/AEM.71.12.8031-8041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose A, Kay E, Wren BW, Dallman MJ. 2012. The Campylobacter jejuni NCTC11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol 201:137–144. doi: 10.1007/s00430-011-0214-1. [DOI] [PubMed] [Google Scholar]
- 44.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 45.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, Girardin SE. 2011. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med 17:837–844. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 46.Baig A, McNally A, Dunn S, Paszkiewicz KH, Corander J, Manning G. 2015. Genetic import and phenotype specific alleles associated with hyper-invasion in Campylobacter jejuni. BMC Genomics 16:852. doi: 10.1186/s12864-015-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delong WJ, Jaworski MD, Ward AC. 1996. Antigenic and restriction enzyme analysis of Campylobacter spp. associated with abortion in sheep. Am J Vet Res 57:163–167. [PubMed] [Google Scholar]
- 48.Williams CE, Renshaw HW, Meinershagen WA, Everson DO, Chamberlain RK, Hall RF, Waldhalm DG. 1976. Ovine campylobacterosis: preliminary studies of the efficacy of the in vitro serum bactericidal test as an assay for the potency of Campylobacter (Vibrio) fetus subsp. intestinalis bacterins. Am J Vet Res 37:409–415. [PubMed] [Google Scholar]
- 49.Maue AC, Poly F, Guerry P. 2014. A capsule conjugate vaccine approach to prevent diarrheal disease caused by Campylobacter jejuni. Hum Vaccin Immunother 10:1499–1504. doi: 10.4161/hv.27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofreuter D, Tsai J, Watson RO, Novik V, Altman B, Benitez M, Clark C, Perbost C, Jarvie T, Du L, Galan JE. 2006. Unique features of a highly pathogenic Campylobacter jejuni strain. Infect Immun 74:4694–4707. doi: 10.1128/IAI.00210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeon B, Muraoka W, Scupham A, Zhang Q. 2009. Roles of lipooligosaccharide and capsular polysaccharide in antimicrobial resistance and natural transformation of Campylobacter jejuni. J Antimicrob Chemother 63:462–468. doi: 10.1093/jac/dkn529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittwer M, Keller J, Wassenaar TM, Stephan R, Howald D, Regula G, Bissig-Choisat B. 2005. Genetic diversity and antibiotic resistance patterns in a Campylobacter population isolated from poultry farms in Switzerland. Appl Environ Microbiol 71:2840–2847. doi: 10.1128/AEM.71.6.2840-2847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han J, Sahin O, Barton YW, Zhang Q. 2008. Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog 4:e1000083. doi: 10.1371/journal.ppat.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oakland M, Jeon B, Sahin O, Shen ZQ, Zhang QJ. 2011. Functional characterization of a lipoprotein-encoding operon in Campylobacter jejuni. PLoS One 6:e20084. doi: 10.1371/journal.pone.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao R, Alm RA, Trust TJ, Guerry P. 1993. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene 130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- 56.Blaser MJ, Duncan DJ, Warren GH, Wang WL. 1983. Experimental Campylobacter jejuni infection of adult mice. Infect Immun 39:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahin O, Zhang Q, Meitzler JC, Harr BS, Morishita TY, Mohan R. 2001. Prevalence, antigenic specificity, and bactericidal activity of poultry anti-Campylobacter maternal antibodies. Appl Environ Microbiol 67:3951–3957. doi: 10.1128/AEM.67.9.3951-3957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]