ABSTRACT
Francisella tularensis causes lethal pneumonia following infection of the lungs by targeting macrophages for intracellular replication; however, macrophages stimulated with interferon gamma (IFN-γ) can resist infection in vitro. We therefore hypothesized that the protective effect of IFN-γ against F. tularensis in vivo requires macrophages receptive to stimulation. We found that the lethality of pulmonary F. tularensis LVS infection was exacerbated under conditions of alveolar macrophage depletion and in mice with a macrophage-specific defect in IFN-γ signaling (termed mice with macrophages insensitive to IFN-γ [MIIG mice]). We previously found that treatment with exogenous interleukin 12 (IL-12) protects against F. tularensis infection; this protection was lost in MIIG mice. MIIG mice also exhibited reduced neutrophil recruitment to the lungs following infection. Systemic neutrophil depletion was found to render wild-type mice highly sensitive to respiratory F. tularensis infection, and depletion beginning at 3 days postinfection led to more pronounced sensitivity than depletion beginning prior to infection. Furthermore, IL-12-mediated protection required NADPH oxidase activity. These results indicate that lung macrophages serve a critical protective role in respiratory F. tularensis LVS infection. Macrophages require IFN-γ signaling to mediate protection, which ultimately results in recruitment of neutrophils to further aid in survival from infection.
KEYWORDS: interferons, lung defense, lung infection, macrophages, neutrophils, tularemia
INTRODUCTION
The tier 1 biothreat Francisella tularensis is a Gram-negative bacterium that is capable of replicating within phagocytes (1–3). In respiratory infection, alveolar macrophages have been reported to be the primary host cell for replication (4–6). It has been suggested that alveolar phagocytes are therefore detrimental to the host during respiratory infection, and a 2005 study reported that depletion of alveolar phagocytes following high challenge doses of LVS resulted in a modestly delayed time to death (7). The course of disease is characterized by a delayed immune response, followed by systemic dissemination and sepsis (8–10). Consequently, the prevailing opinion is that F. tularensis evades destruction by innate immunity and subverts myeloid cells, particularly macrophages, for its own benefit.
Despite the proficiency of F. tularensis in subverting and exploiting host immunity, it is possible to stimulate innate immunity to successfully counter F. tularensis infection. Macrophages significantly contribute to in vitro bacterial killing if they are stimulated with interferon gamma (IFN-γ) (2, 11–13). Correspondingly, IFN-γ is known to be required for protection in vivo (14). Treatment with exogenous interleukin 12 (IL-12) has been shown to protect mice, and mice lacking either the IL-12p35 or IL-12p40 subunit are highly susceptible to LVS infection; protection mediated by exogenous IL-12 treatment has been shown to be dependent upon IFN-γ signaling (14). Furthermore, it was recently reported that lethal and sublethal infections recruit myeloid populations with different phenotypic compositions and different levels of maturity (15). This report concluded that mature phagocytic cell populations are essential for protection in sublethal respiratory F. tularensis infection.
Although it is increasingly apparent that macrophages and IFN-γ can protect against respiratory F. tularensis infection under some circumstances, the exact mechanism of this protection is unclear. IFN-γ-stimulated alveolar macrophages are capable of killing F. tularensis in a manner that is not dependent on nitric oxide species (11). IFN-γ-stimulated alveolar macrophages also secrete tumor necrosis factor alpha (TNF-α); while it is not necessary for these cells to control F. tularensis LVS in vitro, it is required for protection in vivo (16). This suggests the involvement of other cell populations, possibly neutrophils, which are recruited to the lung within 3 days of F. tularensis infection (4, 13).
Although neutrophils are essential for survival following intravenous or intradermal F. tularensis infection, their specific role during lung infection is less clear, and it has in fact been reported that neutrophil depletion does not alter the bacterial burden in mice infected via aerosol challenge (17, 18). In addition, it has been suggested that neutrophils are actually detrimental to the host following pulmonary Francisella infection due to induction of overwhelming inflammation (19, 20). However, following protective IL-12 treatment and F. tularensis LVS infection, neutrophils are recruited to the lungs 1 day earlier in treated than in untreated mice, and a beneficial role for NADPH oxidase in respiratory F. tularensis infection has been observed (13, 21).
We sought to clarify the protective mechanism of macrophages and IFN-γ in vivo during respiratory F. tularensis infection. Here, we report that during sublethal respiratory F. tularensis LVS infection, IFN-γ exerts a protective effect through stimulation of alveolar macrophages and through recruitment of other phagocytes, particularly neutrophils.
RESULTS
Alveolar macrophage depletion is detrimental to survival in pulmonary F. tularensis LVS infection.
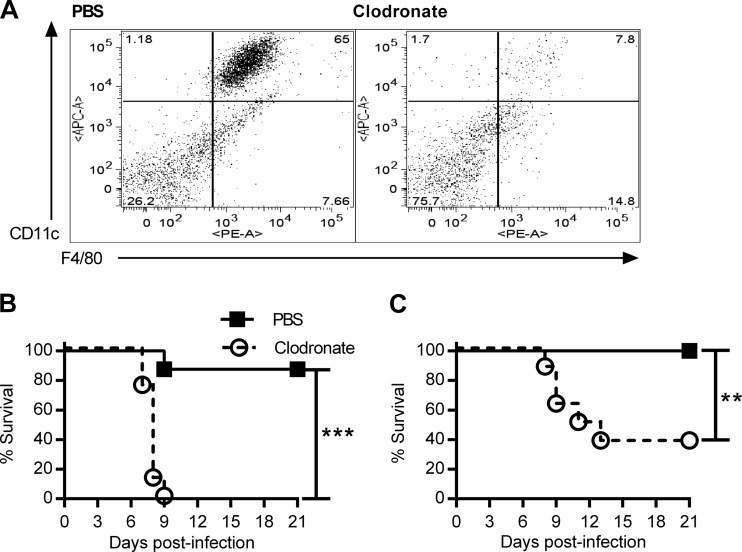
A previous report indicated a delayed time to death in clodronate-treated mice infected with a lethal dose of F. tularensis LVS (7). However, recent evidence suggests that sublethal infection with F. tularensis LVS elicits a greater proportion of mature phagocytes, which exhibit a protective effect (15). Reasoning that mature resident phagocytes may play a protective role against low doses of F. tularensis LVS, we treated mice intranasally (i.n.) with clodronate liposomes or phosphate-buffered saline (PBS) liposomes to deplete alveolar macrophages. Depletion was confirmed by flow cytometric analysis of bronchoalveolar lavage fluid (BALF) (Fig. 1A). Two days after the final treatment, we infected BALB/c mice i.n. with two sub-50% lethal doses (sub-LD50s) of F. tularensis LVS (500 CFU [Fig. 1B] or 100 CFU [Fig. 1C]) and monitored survival for 21 days. These infectious doses were minimally lethal for PBS liposome-treated mice but highly lethal for clodronate liposome-treated mice.
FIG 1.
Depletion of alveolar macrophages increases susceptibility to LVS. (A) BALB/c mice were treated i.n. with clodronate liposomes or PBS liposomes on days 2 and 1 prior to infection, and depletion of alveolar macrophages in BALF was confirmed by flow cytometry. (B and C) At 2 days after treatment, mice (8 mice/group) were infected i.n. with 500 CFU (B) or 100 CFU (C) of F. tularensis LVS. Survival was monitored for 21 days. P values were determined by the log-rank test. **, P < 0.01; ***, P < 0.001.
Protection from F. tularensis LVS requires macrophage-specific IFN-γ sensitivity.
Earlier work demonstrated that IFN-γ, a known stimulator of macrophage cytolytic activity, is essential for survival from respiratory F. tularensis infection (14, 22). To determine if macrophages are the primary effectors of IFN-γ-mediated protection, we exploited mice that express a truncated IFN-γ receptor specifically in the CD68+ cell subset, with the result being that IFN-γ signaling is inhibited in macrophages; these mice are termed mice with macrophages insensitive to IFN-γ (MIIG mice) (23). It was found that MIIG C57BL/6 mice had dramatically increased mortality rates compared to wild-type (WT) C57BL/6 mice after infection with 1 LD50 or 0.5 LD50 of F. tularensis LVS (Fig. 2A and B). Bacterial burdens in the lungs, liver, and spleen were also assessed 4 days after i.n. infection, at which time point MIIG mice contained higher numbers of bacterial CFU in the lungs and noticeably (though not statistically significantly) increased burdens in the liver and spleen (Fig. 2C to E). These results indicate that protection against pulmonary F. tularensis LVS infection specifically requires an IFN-γ-responsive CD68+ population.
FIG 2.
MIIG mice are highly sensitive to LVS respiratory tularemia. MIIG mice contain macrophages which do not respond to IFN-γ stimulation. (A and B) MIIG and WT C57BL/6 mice (8 mice/group) were infected i.n. with 1 LD50 (A) or 0.5 LD50 (B) of F. tularensis LVS. Survival was monitored for 21 days. P values were determined by the log-rank test. *, P < 0.05; ***, P < 0.001. (C to E) MIIG and WT C57BL/6 mice (3 mice/group) were infected i.n. with 1 LD50 of F. tularensis LVS. Lungs (C), livers (D), and spleens (E) were harvested at 4 days postinfection, and bacterial burdens were assessed. *, P < 0.05 by two-tailed t test. (F) MIIG and WT C57BL/6 mice (6 to 8 mice/group) were treated i.n. with IL-12 or PBS 1 day prior to i.n. infection with 1 LD100 of F. tularensis LVS. Survival was monitored for 21 days. P values were determined by the log-rank test. ***, P < 0.001; NS, not significant. (G) C57BL/6 and WT mice (3 or 4 mice/group) were treated with IL-12 or PBS prior to infection as described above. At 1 day postinfection, lungs were harvested for analysis of IFN-γ-positive (IFN-γ+) NK cells by flow cytometry. No significant differences were detected between WT and MIIG mice.
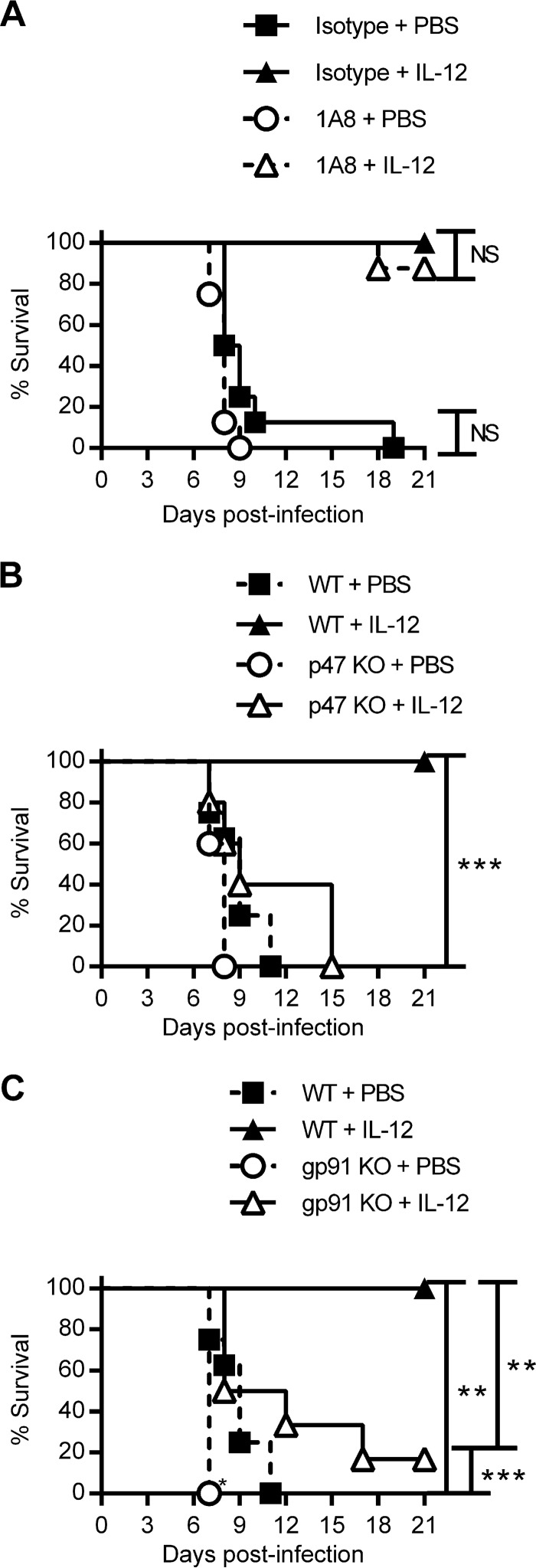
Mice can be protected against F. tularensis LVS by exogenous IL-12 treatment, and this treatment is known to be dependent upon IFN-γ (14). We therefore investigated whether the protective effects of IL-12 require downstream participation by IFN-γ-stimulated macrophages. To investigate whether IL-12-mediated protection was dependent specifically on IFN-γ stimulation of macrophages, WT and MIIG C57BL/6 mice were treated i.n. with IL-12 or PBS 1 day prior to lethal LVS infection, and survival was monitored. All WT mice treated with PBS succumbed to infection, while all IL-12-treated WT mice survived (Fig. 2F), consistent with our previous results (14). However, IL-12 failed to protect MIIG mice, with 100% lethality being seen within 8 days. NK cells are activated by IL-12 (24) and also during pulmonary LVS infection (25, 26). Thus, we determined whether NK cell activation might be defective in MIIG mice. It was found that NK cells in WT and MIIG mice expressed equivalent amounts of IFN-γ following LVS infection and IL-12 treatment (Fig. 2G). Thus, the loss of IL-12 protective efficacy in MIIG mice was not a result of impeded NK cell activity and was observed only in the presence of IFN-γ-mediated stimulation of macrophages.
Macrophage sensitivity to IFN-γ mediates neutrophil recruitment to the lungs in F. tularensis LVS infection.
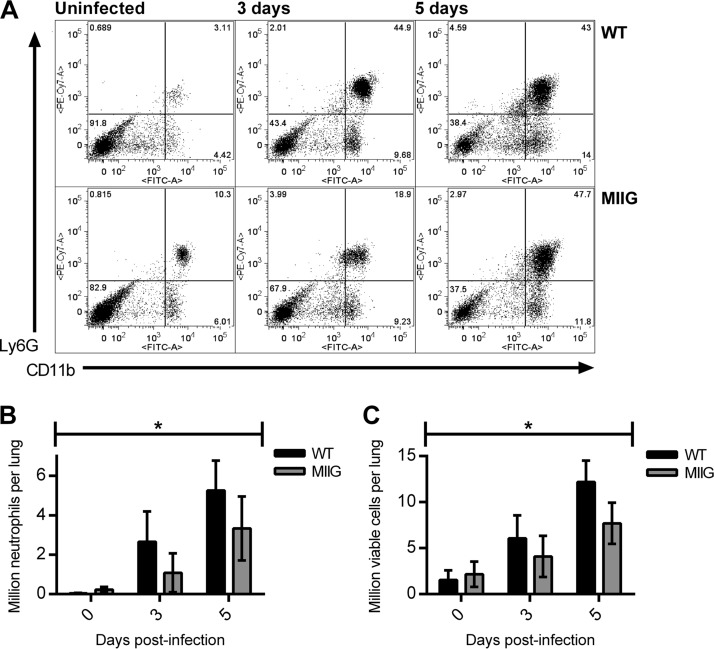
One consequence of IL-12 treatment is recruitment of neutrophils to the lungs up to 24 h earlier than what is normally observed in pulmonary F. tularensis LVS infection (13). Although neutrophils have been postulated to be a source of lung tissue damage in F. tularensis pulmonary infection (19), they are known to be protective against F. tularensis systemic infection (18). We hypothesized that IL-12 treatment would result in IFN-γ-mediated stimulation of macrophages, which in turn would promote neutrophil recruitment to the lungs. We tested this by infecting WT and MIIG C57BL/6 mice i.n. with 1 LD50 of F. tularensis LVS. Lungs were harvested at 3 and 5 days postinfection, and the numbers of Ly6G-positive (Ly6G+) CD11b+ cells were enumerated by flow cytometry (Fig. 3A). The results showed a statistically significantly lower number of neutrophils in the lungs of MIIG mice throughout the infection (Fig. 3B). This was supported by a statistically significant difference in the total number of viable cells (Fig. 3C). This suggests that IFN-γ-mediated activation of macrophages results in early neutrophil recruitment into the lungs.
FIG 3.
Neutrophil recruitment requires stimulation of CD68+ cells by IFN-γ. (A to C) MIIG and WT C57BL/6 mice were infected i.n. with 1 LD50 of F. tularensis LVS (5 mice/group, except 3 mice/group for uninfected MIIG mice). At the indicated time points, lung Ly6G+ CD11b+ cells were enumerated by flow cytometry. (A) Representative flow cytometry plots of MIIG and WT mouse lungs after gating on live cells; (B) total lung neutrophils; (C) total viable cells. *, P < 0.05 for genotype by two-way ANOVA.
Neutrophils are required for survival in F. tularensis LVS infection.
Early neutrophil recruitment is observed in a model of IL-12-mediated protection against F. tularensis LVS, suggesting a possible beneficial role early in infection (13). Based on the work of others (17), we hypothesized that depletion of neutrophils would hinder control of infection, leading to increased mortality. To assess whether neutrophils influence survival in respiratory F. tularensis LVS infection, BALB/c mice were depleted of neutrophils by treatment with anti-Ly6G monoclonal antibody (MAb) 1A8 either prior to infection with 1 LD50 of LVS or beginning at 3 days after infection, at a time when neutrophil infiltration is usually most pronounced. Neutrophil depletion significantly increased susceptibility in both cases (Fig. 4A). Neutrophil depletion beginning at 3 days after infection had the greatest effect, with all mice succumbing to infection by day 8. Depletion of neutrophils before infection also suggested reduced survival, although the effects on time to death were less noticeable and one neutrophil-depleted mouse survived the infection. Depletion using the less specific RB6 anti-Gr1 MAb, which also depletes inflammatory monocytes (27, 28), showed similar results, although in this case, anti-Gr1 depletion resulted in 100% lethality following infection regardless of whether cell depletion was performed prior to or 3 days after bacterial challenge (Fig. 4B). For both antibodies, late treatment caused a significant reduction in survival compared to that with early treatment, with P being <0.01 in both cases. The decreased effects of performing depletion prior to bacterial challenge were not due to neutrophil repopulation during infection; flow cytometry analysis showed that the depletion procedure resulted in a reduction in the number of detectable lung neutrophils for at least 6 days in F. tularensis LVS-infected mice (Fig. 4C and D). These results indicate that neutrophils exert a protective and not a detrimental role in respiratory infection.
FIG 4.
Depletion of neutrophils increases susceptibility to LVS respiratory tularemia. (A and B) BALB/c mice (8 mice/group) were treated i.p. with the neutrophil-depleting 1A8 MAb (A), with the RB6-8C5 MAb (B), or with isotype-matched IgG. Mice were treated either 1 day before and after infection (preinfection) or 3 and 5 days after infection (day 3). The infectious dose was 1 LD50. P values for the significance of the differences between the indicated groups were determined by the log-rank test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C and D) BALB/c mice (3 mice/group) were infected i.n. with 1 LD50 of F. tularensis LVS and given i.p. injections of the 1A8 MAb or an isotype-matched IgG. At the indicated time points, lung Gr1-positive CD11b+ cells were enumerated by flow cytometry. (C) Representative flow cytometry plots of 1A8- and isotype control-treated lungs; (D) quantification of neutrophils as a percentage of live lung cells. ****, P < 0.001 by two-way ANOVA with a multiple-comparison test comparing means at each time point.
IL-12-mediated protection from F. tularensis LVS requires NADPH oxidase.
Treatment of mice with IL-12 prior to infection is known to protect mice against intranasal challenge with F. tularensis LVS and to reduce the lung bacterial burden (13, 14). IL-12 treatment also results in the earlier recruitment of neutrophils to the lungs following infection (13). We thus hypothesized that the protective effect of IL-12 is due to the early recruitment of neutrophils to the lungs. To test this hypothesis, we examined whether IL-12 enhanced the survival of neutrophil-depleted mice. BALB/c mice received intraperitoneal (i.p.) injections of 1A8 or an isotype-matched control with or without i.n. IL-12 prior to infection with a lethal dose of F. tularensis LVS. Survival was monitored for 21 days. Although neutrophil-depleted mice not treated with IL-12 succumbed to infection, as described above, administration of IL-12 protected both neutrophil-depleted and nondepleted mice (Fig. 5A). These results indicate that despite the observed protective role of neutrophils, macrophage stimulation in the absence of neutrophils is sufficient to protect against LVS challenge.
FIG 5.
The protective effect of IL-12 treatment requires NADPH oxidase but not neutrophils. (A) BALB/c mice (8 mice/group) were treated i.n. with IL-12 or PBS and given i.p. injections of the 1A8 MAb or an isotype-matched IgG. Mice were then infected i.n. with 1 LD100 of F. tularensis LVS. Survival was monitored for 21 days. (B and C) Survival between WT C57BL/6 mice (8 mice/group) and p47phox−/− mice (5 mice/group) (B) or gp91phox−/− mice (6 mice/group) (C) was compared after i.n. treatment with IL-12 or PBS 1 day prior to i.n. infection with 1 LD100 CFU of F. tularensis LVS. Survival was monitored for 21 days. P values for the significance of the differences between the indicated groups were determined by the log-rank test. **, P < 0.01; ***, P < 0.001; NS, not significant. KO, knockout.
The proteins p47phox and gp91phox are components of the NADPH oxidase complex, which is essential for the phagocyte respiratory burst. Mutations in these genes in humans result in chronic granulomatous disease, primarily a defect in neutrophil function (29–32). We infected WT, p47phox−/−, and gp91phox−/− C57BL/6 mice with a lethal dose of F. tularensis LVS with or without IL-12 i.n. prior to infection. IL-12 was again highly protective for WT mice but, surprisingly, failed to protect p47phox−/− or gp91phox−/− mice, indicating that the respiratory burst, likely within macrophages, is essential for control of F. tularensis infection and plays an important role in IL-12-mediated protection (Fig. 5B and C).
We conclude that alveolar macrophages are essential for protection against sublethal infectious doses of F. tularensis LVS and that this protection is mediated at least in part through the recruitment and activation of neutrophils and other phagocytes.
DISCUSSION
Innate immunity can protect against pulmonary F. tularensis LVS infection in a manner involving Th1-associated cytokines. Although this protection has been demonstrated both in vitro (2, 11–13) and in vivo (14, 26), the mechanism of this protection in vivo is incompletely understood. Here, we investigated the cellular mechanism contributing to IFN-γ-mediated protection against pulmonary F. tularensis LVS infection in mice. Our results demonstrate that alveolar macrophages, long considered detrimental in this infection, are required for protection against sublethal infectious doses. Protection also requires neutrophil recruitment to the infected lungs, and this recruitment is dependent upon IFN-γ stimulation of macrophages.
To test the assumption that intracellular macrophage replication is a key factor in F. tularensis virulence, we depleted alveolar macrophages in order to restrict bacterial replication within these cells following pulmonary infection. Liposomal clodronate is phagocytized by macrophages, and the clodronate is then converted to an ATP analogue and causes cell apoptosis (33, 34). Contrary to the concept that macrophage depletion would enhance survival by removing the bacterial replication niche, clodronate depletion actually resulted in decreased survival following LVS infection. We were aware of the possibility that macrophage debris resulting from this process could cause inflammation and potentially explain the increased susceptibility to infection. However, we observed the same heightened sensitivity to LVS infection in MIIG mice, which have a defect in IFN-γ signaling specific to macrophage lineage cells and which do not require cell depletion.
It has been reported that depletion of lung cells with liposomal clodronate prior to pulmonary challenge with F. tularensis LVS increases the mean time to death (7), a conclusion that is opposite to our findings. However, the authors of that study used a far greater bacterial inoculum in their study (5 × 104 CFU per mouse), which was lethal with or without clodronate treatment, and death was delayed by only 1 to 2 days. It is known that an excessive inflammatory response is a key feature of death from F. tularensis infection (8, 9, 19, 20). Although at more physiologically relevant infectious doses macrophages appear to be essential for survival, at higher doses in which control of infection becomes impossible with or without macrophages, the absence of macrophages (and macrophage-derived inflammatory cytokines) may merely delay lethal sepsis. Finally, we have obtained independent confirmatory results regarding the essential protective role of macrophages by use of MIIG mice.
Although neutrophils are known to exert a protective effect against cutaneous and systemic F. tularensis infection (17), the role of neutrophils in pneumonic F. tularensis infection has also remained somewhat controversial. The survival experiments in our study show unequivocally that neutrophils play a protective role in pneumonic F. tularensis LVS infection. This result contrasts with earlier work reporting that depletion of neutrophils using the monoclonal antibody RB6-8C5 prior to aerosol infection with LVS did not affect the bacterial burden in the lungs (18). However, the same work indicated that depletion of neutrophils did significantly increase the bacterial burden in the liver and also resulted in a trend toward an increased burden in the spleen. Other work by the same group indicated that NADPH oxidase, which is involved in the killing of bacteria by neutrophils, extends survival following SchuS4 challenge since gp91phox−/− mice, which are deficient in NADPH oxidase, exhibited a reduced mean time to death after i.n. SchuS4 challenge (21).
IL-12 is expressed in the lungs of F. tularensis-infected mice within 48 to 72 h after infection (35). Treatment with exogenous IL-12 prior to i.n. F. tularensis LVS infection induces early neutrophil recruitment to the lungs, which is associated with improved control of the bacterial burden relative to that in untreated mice (13). Our present work showed that neutrophil influx was correlated with IFN-γ activation of macrophages. After F. tularensis infection, MIIG mice showed statistically significant differences in the amounts of total lung neutrophils relative to WT mice. Recruitment of neutrophils was found to be essential for survival following infection; mice systemically depleted of neutrophils prior to infection or beginning at 3 days after infection showed increased mortality relative to mock-depleted mice. Nevertheless, neutrophil-depleted mice that were treated i.n. with IL-12 to induce IFN-γ secretion in the lungs exhibited protection similar to that seen in nondepleted mice treated with IL-12, which suggests that early stimulation of macrophages with IFN-γ is sufficient for protection in the absence of neutrophils. Transgenic mice with defects in NADPH oxidase activity succumbed to infection regardless of IL-12 treatment. Although in humans neutrophils are believed to be the primary source of NADPH-derived reactive oxygen, it is likely that reactive oxygen species in mice are produced by other phagocytes which are not affected by 1A8 treatment, specifically, macrophages or monocytes (36).
Based on this work, we propose that infection results in increased IL-12 secretion, which induces NK cells, CD8+ T cells, or both, to secrete IFN-γ. While unstimulated macrophages can be targeted by bacteria for replication, macrophages stimulated with IFN-γ are resistant to infection and are capable of killing intracellular bacteria, as shown by in vitro experiments (2, 11, 12, 37). In vivo, IFN-γ stimulation of macrophages also induces recruitment of neutrophils to the lungs, providing a further level of protection, although neutrophil recruitment is not essential given a sufficient level of macrophage stimulation. The precise mechanism by which IFN-γ-stimulated macrophages mediate protection remains to be elucidated, as do the relative contributions of neutrophils within and outside the lungs. However, an important role for reactive oxygen species in survival is likely.
Taken together, our results challenge the current understanding of alveolar macrophages as a passive or wholly detrimental bacterial reservoir during respiratory F. tularensis LVS infection and instead demonstrate that macrophages can play an essential protective role.
MATERIALS AND METHODS
Ethics statement.
All animal procedures followed those in the Guide for the Care and Use of Laboratory Animals (8th edition) of the National Research Council (38) and were approved by the Institutional Animal Care and Use Committee of Albany Medical College (protocol 12-03011).
Bacteria.
The original stock of LVS was obtained from Karen Elkins, FDA, Bethesda, MD. F. tularensis was grown in Mueller-Hinton broth (Becton Dickinson) supplemented with IsoVitaleX (Becton Dickinson) or brain heart infusion broth (Becton Dickinson) adjusted to pH 6.8, as described by Hazlett et al. (39). The bacteria were grown to log phase at 37°C and frozen at −80°C.
Mice.
BALB/c and C57BL/6 mice were purchased from Taconic, The Jackson Laboratory, and Charles River Laboratories under a contract with the National Cancer Institute. p47phox−/− and gp91phox−/− C57BL/6 mice were purchased from The Jackson Laboratory. MIIG C57BL/6 mice were generated at Cincinnati Children's Hospital Medical Center (23) and were bred and maintained at the Albany Medical College. In experiments using MIIG mice, wild-type (WT) C57BL/6 littermates were used as controls. Mice were anesthetized prior to infection, alveolar macrophage depletion, and/or IL-12 treatment by i.p. injection of a 2% ketamine–0.5% xylazine solution in PBS (both reagents were from Vedco).
Infections.
Infectious doses were prepared from frozen stocks and diluted to 40 to 50 μl/mouse in PBS. The infectious doses were confirmed by plating on chocolate agar. Six to eight mice were used for the survival experiments and three to five mice per time point were used for the bacterial burden and flow cytometry experiments, except where noted otherwise in the figure legends. BALB/c mice are somewhat more resistant to LVS infection than C57BL/6 mice, and thus, higher challenge doses were required to reach an LD50 (26).
Alveolar macrophage depletion.
Clodronate liposomes or control PBS liposomes (FormuMax) were administered i.n. in a volume of 50 μl 1 day prior to infection. Mice were anesthetized prior to treatment. Depletion was confirmed by flow cytometry 1 day after treatment. Of note, no weight loss was associated with liposomal clodronate treatment in the absence of infection, indicating that these animals maintained good health.
Bacterial burden analysis.
Mice were sacrificed by i.p. injection of pentobarbital (Fort Dodge Laboratories), followed by cervical dislocation. For assessment of the bacterial burdens in lung, liver, and spleen, the organs were harvested and homogenized in 1 ml of PBS using a Minibeadbeater (Biospec Products). Homogenates were centrifuged, and the supernatants were plated on chocolate agar for enumeration.
IL-12 treatment.
At 1 day prior to infection, mice were treated i.n. with 0.5 μg of IL-12 in 25 μl PBS with 1% normal mouse serum as a protein carrier, as described previously (40). Treatment with PBS in 1% normal mouse serum was used as a control.
Analysis of IFN-γ expression in NK cells.
Mice were treated with IL-12 and infected with F. tularensis LVS as described above. At 1 day postinfection, lungs were harvested, digested with collagenase D (Sigma) for 30 min at 37°C, and passed through 40-μm-pore-size nylon filters (BD Falcon). Cells were restimulated in vitro with F. tularensis at a multiplicity of infection of 100 and IL-12 at a concentration of 5 ng/ml for 1 h at 37°C. Cytokine secretion was inhibited by incubation with the BD GolgiPlug protein transport inhibitor for 1 h at 37°C. Cells were stained with eFluor780-conjugated fixable viability dye (FVD; eBioscience) and phycoerythrin (PE)-conjugated anti-NK1.1 MAb and then fixed and permeabilized with BD Cytofix and stained with allophycocyanin (APC)-conjugated anti-IFN-γ MAb. The fluorescent intensity was determined using a FACSCanto flow cytometer (Becton Dickinson), and the data were analyzed using FlowJo software (TreeStar).
Neutrophil depletion.
Neutrophils were depleted using 100 μg of the RB6-8C5 Gr1-specific antibody (Maine Biotechnology and BioXCell) or 500 μg of the 1A8 Ly6G-specific antibody (BioXCell), as described previously (41, 42). One set of mice was depleted at 1 day prior to infection and then at 1 day postinfection, followed by injections of isotype-matched control Ig at 3 days and 5 days postinfection. Another set of mice was depleted beginning at 3 days after infection; these animals were injected with isotype-matched control Ig at 1 day prior to infection and 1 day postinfection, followed by depletion of antibody at 3 days and 5 days postinfection.
To assess of the effects of IL-12 on neutropenic mice, the 1A8 MAb was injected at 1 day prior to infection, 1 day postinfection, and 3 days postinfection. Rat IgG was used as a control in all cases.
Neutrophil expression was assessed by harvesting the lungs 1 day after the last dose of depleting MAb. Lungs were digested in collagenase D (Sigma) for 30 min at 37°C and separated into a suspension of live cells by passage through 40-μm-pore-size nylon filters (BD Falcon). Cell suspensions were stained with APC-conjugated anti-Gr1 MAb and peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated anti-CD11b MAb. The fluorescent intensity was determined using a FACSCanto flow cytometer (Becton Dickinson), and the data were analyzed using FlowJo software (TreeStar). Cells which were positive for both Ly6G and CD11b were considered to be neutrophils.
Neutrophil recruitment assay.
Mice were infected i.n. and sacrificed at several time points after infection, as described in Results. The lungs were harvested, digested in collagenase D (Sigma) for 30 min at 37°C, and separated into a suspension of live cells by passage through 40-μm-pore-size nylon filters (BD Falcon). Cells were stained with eFluor780-conjugated FVD (eBioscience), fluorescein isothiocyanate (FITC)-conjugated anti-CD11b MAb, and PE-Cy7-conjugated anti-Ly6G MAb. The fluorescent intensity was determined using a FACSCanto flow cytometer (Becton Dickinson), and the data were analyzed using FlowJo software (TreeStar). A gate identifying FVD-negative cells as live was drawn, and cells within this gate which were positive for both Ly6G and CD11b were considered to be neutrophils.
Statistical analyses.
Survival was analyzed by Mantel-Cox log-rank tests, using the Bonferroni correction for multiple comparisons. Bacterial burdens were analyzed by a 2-tailed t test. Comparisons of IFN-γ-positive NK cells and of neutrophil levels between different groups of mice were analyzed by two-way analysis of variance (ANOVA), with Bonferroni's correction for multiple comparisons being used where appropriate. All statistics were determined using GraphPad Prism software. Statistical significance was considered to be a P value of <0.05.
ACKNOWLEDGMENTS
We are indebted to Anthony Hickey, Girish Kirimanjeswara, Sean Roberts, and Sharon Salmon for technical assistance.
This work was supported by NIH grant PO1 A1056320.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Shepard CC. 1959. Nonacid-fast bacteria and HeLa cells: their uptake and subsequent intracellular growth. J Bacteriol 77:701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortier AH, Polsinelli T, Green SJ, Nacy CA. 1992. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun 60:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolger CE, Forestal CA, Italo JK, Benach JL, Furie MB. 2005. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. J Leukoc Biol 77:893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- 4.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. 2008. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun 76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts LM, Tuladhar S, Steele SP, Riebe KJ, Chen CJ, Cumming RI, Seay S, Frothingham R, Sempowski GD, Kawula TH, Frelinger JA. 2014. Identification of early interactions between Francisella and the host. Infect Immun 82:2504–2510. doi: 10.1128/IAI.01654-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai XH, Golovliov I, Sjostedt A. 2001. Francisella tularensis induces cytopathogenicity and apoptosis in murine macrophages via a mechanism that requires intracellular bacterial multiplication. Infect Immun 69:4691–4694. doi: 10.1128/IAI.69.7.4691-4694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosio CM, Dow SW. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol 175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 8.Mares CA, Ojeda SS, Morris EG, Li Q, Teale JM. 2008. Initial delay in the immune response to Francisella tularensis is followed by hypercytokinemia characteristic of severe sepsis and correlating with upregulation and release of damage-associated molecular patterns. Infect Immun 76:3001–3010. doi: 10.1128/IAI.00215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma J, Li Q, Mishra BB, Pena C, Teale JM. 2009. Lethal pulmonary infection with Francisella novicida is associated with severe sepsis. J Leukoc Biol 86:491–504. doi: 10.1189/jlb.1208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma J, Mares CA, Li Q, Morris EG, Teale JM. 2011. Features of sepsis caused by pulmonary infection with Francisella tularensis type A strain. Microb Pathog 51:39–47. doi: 10.1016/j.micpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polsinelli T, Meltzer MS, Fortier AH. 1994. Nitric oxide-independent killing of Francisella tularensis by IFN-gamma-stimulated murine alveolar macrophages. J Immunol 153:1238–1245. [PubMed] [Google Scholar]
- 12.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. 2007. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol 179:532–539. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 13.Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. 2008. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol Rev 225:244–255. doi: 10.1111/j.1600-065X.2008.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckett NS, Olmos S, Durrant DM, Metzger DW. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Periasamy S, Avram D, McCabe A, MacNamara KC, Sellati TJ, Harton JA. 2016. An immature myeloid/myeloid-suppressor cell response associated with necrotizing inflammation mediates lethal pulmonary tularemia. PLoS Pathog 12:e1005517. doi: 10.1371/journal.ppat.1005517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sjostedt A, North RJ, Conlan JW. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142(Pt 6):1369–1374. [DOI] [PubMed] [Google Scholar]
- 17.Sjostedt A, Conlan JW, North RJ. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun 62:2779–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conlan JW, KuoLee R, Shen H, Webb A. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb Pathog 32:127–134. doi: 10.1006/mpat.2001.0489. [DOI] [PubMed] [Google Scholar]
- 19.Malik M, Bakshi CS, McCabe K, Catlett SV, Shah A, Singh R, Jackson PL, Gaggar A, Metzger DW, Melendez JA, Blalock JE, Sellati TJ. 2007. Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J Immunol 178:1013–1020. doi: 10.4049/jimmunol.178.2.1013. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz JT, Barker JH, Kaufman J, Fayram DC, McCracken JM, Allen LA. 2012. Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan. J Immunol 188:3351–3363. doi: 10.4049/jimmunol.1102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KuoLee R, Harris G, Conlan JW, Chen W. 2011. Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect 13:447–456. doi: 10.1016/j.micinf.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 59:2922–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, Karp CL, Aliberti J, Flick MJ, Jordan MB. 2010. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol 184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 25.Elkins KL, Colombini SM, Krieg AM, De Pascalis R. 2009. NK cells activated in vivo by bacterial DNA control the intracellular growth of Francisella tularensis LVS. Microbes Infect 11:49–56. doi: 10.1016/j.micinf.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Lopez MC, Duckett NS, Baron SD, Metzger DW. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol 232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Dunay IR, Fuchs A, Sibley LD. 2010. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun 78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. 2011. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol 41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomax KJ, Leto TL, Nunoi H, Gallin JI, Malech HL. 1989. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science 245:409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- 30.Dinauer MC, Curnutte JT, Rosen H, Orkin SH. 1989. A missense mutation in the neutrophil cytochrome b heavy chain in cytochrome-positive X-linked chronic granulomatous disease. J Clin Invest 84:2012–2016. doi: 10.1172/JCI114393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolscher BG, de Boer M, de Klein A, Weening RS, Roos D. 1991. Point mutations in the beta-subunit of cytochrome b558 leading to X-linked chronic granulomatous disease. Blood 77:2482–2487. [PubMed] [Google Scholar]
- 32.Casimir CM, Bu-Ghanim HN, Rodaway AR, Bentley DL, Rowe P, Segal AW. 1991. Autosomal recessive chronic granulomatous disease caused by deletion at a dinucleotide repeat. Proc Natl Acad Sci U S A 88:2753–2757. doi: 10.1073/pnas.88.7.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, Azhayev A, Vaananen HK, Hassinen IE. 2002. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol 61:1255–1262. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 34.van Rooijen N, Hendrikx E. 2010. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol 605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 35.Elkins KL, Cooper A, Colombini SM, Cowley SC, Kieffer TL. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect Immun 70:1936–1948. doi: 10.1128/IAI.70.4.1936-1948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Vliet A. 2008. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44:938–955. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger DW, Bakshi CS, Kirimanjeswara G. 2007. Mucosal immunopathogenesis of Francisella tularensis. Ann N Y Acad Sci 1105:266–283. doi: 10.1196/annals.1409.007. [DOI] [PubMed] [Google Scholar]
- 38.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 39.Hazlett KR, Caldon SD, McArthur DG, Cirillo KA, Kirimanjeswara GS, Magguilli ML, Malik M, Shah A, Broderick S, Golovliov I, Metzger DW, Rajan K, Sellati TJ, Loegering DJ. 2008. Adaptation of Francisella tularensis to the mammalian environment is governed by cues which can be mimicked in vitro. Infect Immun 76:4479–4488. doi: 10.1128/IAI.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun K, Salmon SL, Lotz SA, Metzger DW. 2007. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun 75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun K, Metzger DW. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14:558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 42.Tate MD, Deng YM, Jones JE, Anderson GP, Brooks AG, Reading PC. 2009. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 183:7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]