ABSTRACT
Mycoplasma gallisepticum, known primarily as a respiratory pathogen of domestic poultry, has emerged since 1994 as a significant pathogen of the house finch (Haemorhous mexicanus) causing severe conjunctivitis and mortality. House finch-associated M. gallisepticum (HFMG) spread rapidly and increased in virulence for the finch host in the eastern United States. In the current study, we assessed virulence in domestic poultry with two temporally distant, and yet geographically consistent, HFMG isolates which differ in virulence for house finches—Virginia 1994 (VA1994), the index isolate of the epidemic, and Virginia 2013 (VA2013), a recent isolate of increased house finch virulence. Here we report a significant difference between VA1994 and VA2013 in their levels of virulence for chickens; notably, this difference correlated inversely to the difference in their levels of virulence for house finches. VA1994, while moderately virulent in house finches, displayed significant virulence in the chicken respiratory tract. VA2013, while highly virulent in the house finch, was significantly attenuated in chickens relative to VA1994, displaying less-severe pathological lesions in, and reduced bacterial recovery from, the respiratory tract. Overall, these data indicate that a recent isolate of HFMG is greatly attenuated in the chicken host relative to the index isolate, notably demonstrating a virulence phenotype in chickens inversely related to that in the finch host.
KEYWORDS: Mycoplasma gallisepticum, chicken, house finch, pathology, virulence
INTRODUCTION
Mycoplasma gallisepticum is a bacterial pathogen associated with acute and chronic respiratory disease in domestic poultry. Previously, M. gallisepticum was thought to be relatively host specific and pathogenic only for gallinaceous birds (1). The emergence of M. gallisepticum infection in a wild North American songbird host—the house finch (Haemorhous mexicanus)—was first reported in 1994 in Virginia and Maryland and was associated with severe and chronic lymphoplasmacytic conjunctivitis, sinusitis, and rhinitis, contrasting with the respiratory form of M. gallisepticum disease primarily observed in poultry (2–4). The epidemic quickly spread throughout the eastern, or introduced, house finch range across the mid-Atlantic and the eastern states, and it eventually spread to the native house finch range in the western United States (2, 5–8). House finch-associated M. gallisepticum (HFMG) has been associated with dramatic declines in house finch populations, likely as a result of affecting the host's ability to forage or to avoid predation (3, 9–11). This well-documented spread of HFMG has enabled dynamic modeling of various aspects of the epidemic as an example of the spread of an emergent pathogen (12, 13).
House finches represent the first recognized M. gallisepticum reservoir sustained in the wild (14). Samples have been collected from wild birds spanning both the geographic and temporal ranges of the epidemic, and while other wild bird species can be affected by M. gallisepticum, house finches are considered the primary host of HFMG (14, 15). Indeed, bacterial multilocus genetic analysis has revealed a closely related, monophyletic HFMG clade relative to known poultry isolates, indicating that a single M. gallisepticum introduction became established and successfully spread in the North American house finch epidemic (16).
Notably, HFMG isolates from disparate geotemporal spaces in the epidemic exhibit different virulence phenotypes. These differences have indicated a decrease in HFMG virulence during transmission from eastern to western range hosts and parallel increases in HFMG virulence during local spread within eastern and western range host populations (17–19). Genomic changes occurring in the HFMG strain during the epidemic have been examined, again matching geotemporal patterns of isolation with phylogeny (20, 21), and with the most dramatic genomic changes involving regions encoding phase-variable, surface-expressed lipoprotein (21). Thus, HFMG virulence is evolving as it spreads and becomes endemic in the novel house finch host.
Given the spread of HFMG in the wild, the potential transmission of M. gallisepticum from wild bird reservoirs to domestic poultry populations is of concern. Such potential has been indicated experimentally and through genetic typing of wild bird and poultry isolates (16, 22). Experimentally, HFMG was able to transmit by direct contact from infected house finches captured early in the epizootic to contact chickens, but no disease or pathology was observed in chickens and transmission was inferred from bacterial isolation and seroconversion (22). Also, experimental inoculation of an “early” HFMG isolate (K4058, Georgia 1996) into chickens induced respiratory pathology which was attenuated relative to that produced by virulent poultry strain R (23). Through genotyping, certain isolates from domestic turkeys appeared to be derived from HFMG within a decade of emergence (16). How HFMG isolates of evolved virulence phenotypes may infect or cause disease in domestic poultry is currently unknown.
To better understand threats associated with potential reintroduction of evolved HFMG into poultry species, we conducted experiments to compare the levels of virulence of house finch-adapted M. gallisepticum strains in chickens. Two HFMG isolates were compared in domestic poultry hosts through experimental challenge and assessment of pathology and bacterial recovery. One of these isolates, Virginia 1994 (VA1994), represents the known index isolate of the HFMG epidemic and is of moderate virulence in house finches. The second, Virginia 2013 (VA2013), is a more recent isolate of high virulence in house finches. Here we report that VA2013 is less virulent in chickens than VA1994.
RESULTS
Gross anatomic lesions in chickens.
As previously described (23), early epidemic HFMG induced air sac anatomic gross lesions in chickens, as caseous exudates were present in and around the abdominal air sacs in chickens infected with VA1994 (data not shown). Air sac exudates observed in chickens infected with virulent poultry strain Rlow were largely associated with thoracic, rather than abdominal, air sacs. Gross lesions were absent in all air sacs of chickens inoculated with either late-epidemic HFMG isolate VA2013 or with growth medium alone. Additionally, no ocular lesions were observed in any inoculated chickens.
Histologic lesion scores in chickens.
Chicken tracheal histologic lesion scores were significantly different (P < 0.05) between the two HFMG isolates (Fig. 1A). Chickens inoculated with the VA1994 isolate demonstrated tracheal lesion scores that were higher than those of medium control chickens and statistically the same as those of chickens inoculated with virulent strain Rlow. Chickens inoculated with VA2013, however, demonstrated lesion scores that were significantly lower than those from VA1994-inoculated chickens and were, in fact, not significantly different from those from the medium control chickens. Chickens inoculated with VA1994 demonstrated mild mucosal thickening resulting from multiple lymphofollicular infiltrates, while those inoculated with VA2013 demonstrated minimal focal lymphofollicular infiltrates. Chickens inoculated with Rlow displayed moderate mucosal thickening resulting from diffuse lymphocytic and histiocytic infiltrates of the lamina propria. Medium controls challenged with medium alone showed no significant histologic lesions. Similarly, lung histologic lesion scores were significantly higher in chickens inoculated with VA1994 than in those inoculated with VA2013 or the control medium (Fig. 1B).
FIG 1.
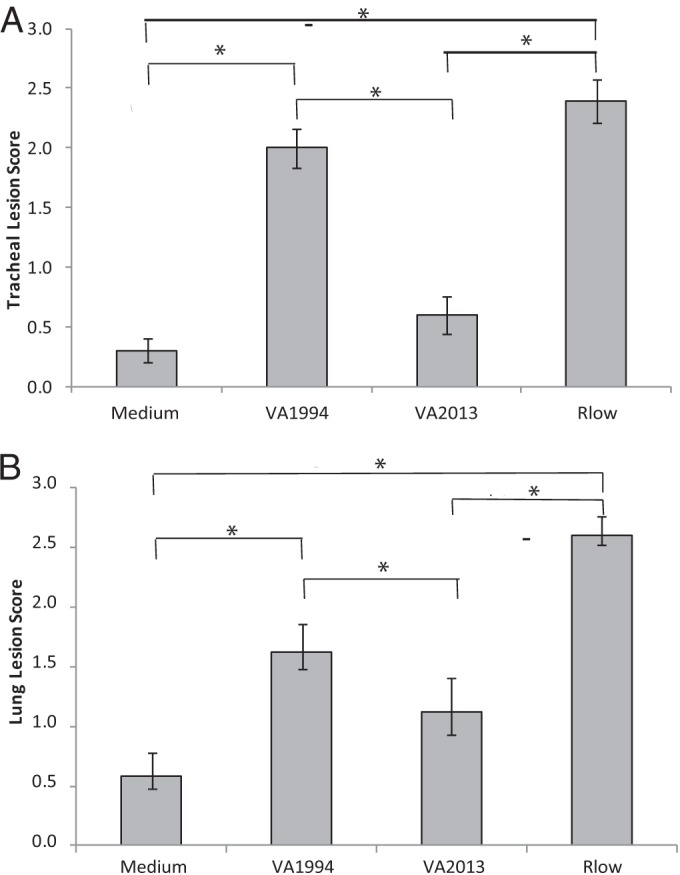
Lesion scores from (A) tracheas or (B) lungs of M. gallisepticum-infected or medium control chickens at 14 dpi. n = 20 total animals per group (n = 10 per group per experiment). Error bars indicate standard errors of the means (SEM), and asterisks (*) and brackets indicate groups significantly different (P < 0.05) in pairwise multiple comparisons.
Taking the data together, the histologic lesion scores of chicken respiratory tissues indicated a reduction in the virulence of VA2013 compared to VA1994. Again, consistent with previous results, neither strain caused the severity of lesions induced by virulent poultry strain Rlow, suggesting continued reduction of HFMG virulence for chickens after the initial adaptation to house finches.
Tracheal thickness in chickens.
As an objective second measure of tracheal histopathology, tracheal thickness was assessed. Chickens inoculated with VA1994 demonstrated mild mucosal thickening, with measurements significantly (P < 0.05) higher than in medium controls but significantly lower than in those inoculated with virulent Rlow (Fig. 2). Chickens inoculated with VA2013 showed minimal mucosal thickening—significantly less than the thickening seen with chickens inoculated with Rlow but intermediate with respect to and statistically indistinguishable from the thickening seen with those inoculated with either VA1994 or medium control (Fig. 2). Chickens inoculated with Rlow displayed moderate mucosal thickening.
FIG 2.
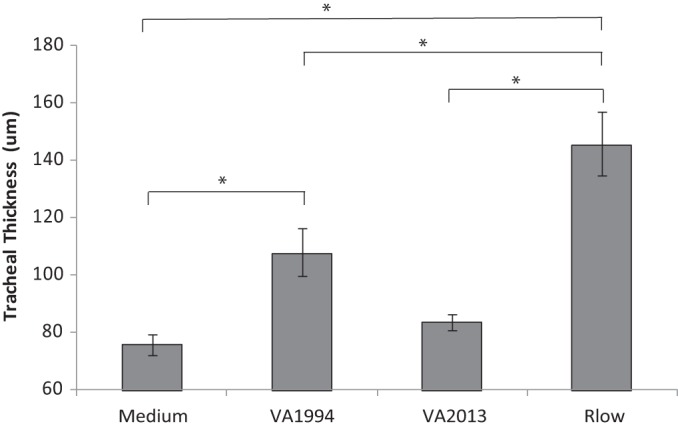
Tracheal thickness of M. gallisepticum-infected or medium control chickens at 14 dpi. n = 20 total animals per group (n = 10 per group per experiment). Error bars show SEM, and asterisks (*) and brackets indicate groups significantly different (P < 0.05) in pairwise multiple comparisons.
Bacterial recovery from chickens.
There was a difference in the levels of recovery of M. gallisepticum from respiratory tissues of infected chickens at 14 days postinoculation (dpi) (Fig. 3). Mycoplasmas were isolated from tracheas of 70% of the VA1994-inoculated chickens but, notably, not from those of VA2013-inoculated chickens. Similarly, mycoplasmas were recovered from lungs of 65% of VA1994-inoculated chickens but not from those of VA2013-inoculated chickens (Fig. 3). Mycoplasmas were recovered from both types of respiratory tissues of 80% to 100% of virulent Rlow-inoculated chickens but not from those of control medium-inoculated chickens.
FIG 3.
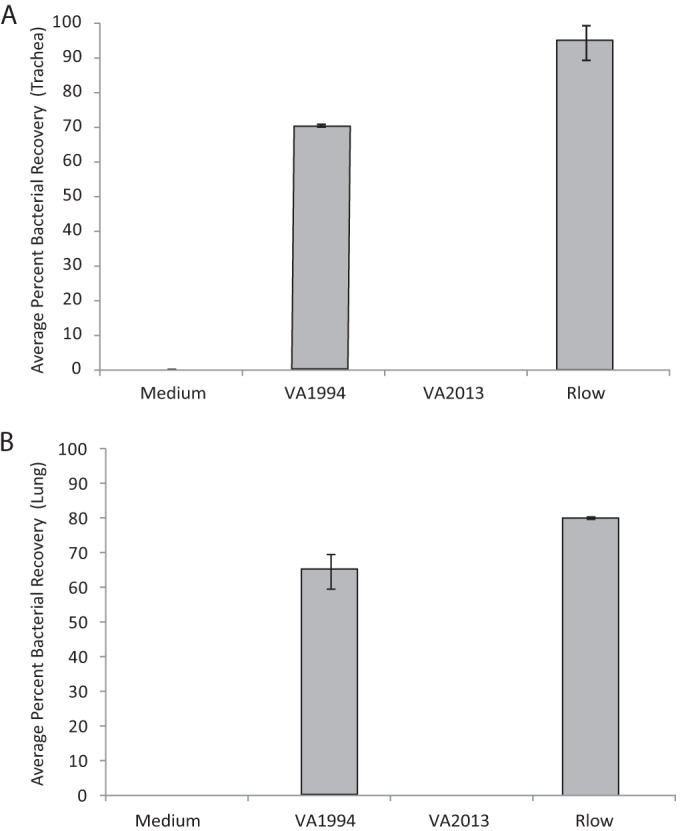
Average percentages of chickens in which M. gallisepticum was recovered from the (A) trachea or (B) lung. n = 20 total animals per group (n = 10 per group per experiment). Error bars indicate SEM in comparisons between two experiments.
Virulence of VA2013 for house finches.
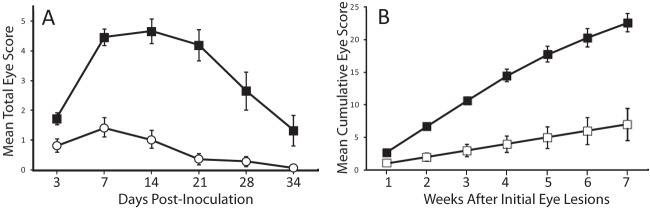
To verify the increased virulence of VA2013 for house finches relative to VA1994, data from multiple independent experiments were compiled (Fig. 4). In a single experiment using directly inoculated finches, VA2013 was demonstrated to be more virulent than NC1995 (for isolate treatment, F1,28.17 = 52.0, P < 0.0001), an isolate shown previously to be more virulent than VA1994 (18) (Fig. 4A). In experiments where sentinel finches were placed in contact with directly inoculated index finches, VA2013 induced higher cumulative eye lesion scores than did VA1994 (Fig. 4B). Starting the second week after the initial lesions were observed in sentinel finches, cumulative lesion scores were significantly higher in finches exposed to VA2013 than in those exposed to VA1994 (week 2, U = 11, P = 0.02; week 3, U = 4, P = 0.002; week 4, U = 6.5, P = 0.06; week 5, U = 5.5, P = 0.004; week 6, U = 6, P = 0.005; week 7, U = 6, P = 0.005). These combined data confirm increased virulence of VA2013 for the house finch relative to VA1994.
FIG 4.
Virulence of HFMG strains for house finches. (A) Mean total (left plus right) eye lesion scores in house finches directly inoculated with VA2013 (filled squares) and VA1995 (open circles), an isolate more virulent than VA1994 (18) (n = 15 total animals per group). (B) Mean cumulative eye lesion scores in sentinel house finches in contact with index birds directly inoculated with VA2013 (filled squares, 2014 experiment) and with VA1994 (open squares, 2010 experiment), starting at first appearance of lesions (n = 10 animals per group). Error bars indicate SEM.
DISCUSSION
Data presented here show that two temporally distant HFMG strains are significantly different in virulence for chickens, notably in a manner reciprocal to their virulence for house finches. We found that HFMG strain VA2013, indicated here to be more virulent than VA1994 in the house finch eye, was significantly less virulent than VA1994 in the chicken respiratory tract as measured by tracheal and lung histopathology. Most striking was the complete lack of M. gallisepticum recovery from chickens challenged with VA2013, since chickens inoculated with VA1994 retained recoverable mycoplasma at 14 dpi. These data indicate that VA2013, while more virulent in and better able to colonize the eyes of the house finch host, was less able than VA1994 to colonize and cause pathology in the lower respiratory tract of chickens. Collectively, these data indicate that the levels of M. gallisepticum virulence for house finch and poultry hosts are not comparable, and they suggest that an inverse relationship is possible. The differential virulence results described here for HFMG isolates from early and late in the house finch epidemic for different hosts have implications for M. gallisepticum virulence evolution—a topic for which HFMG has become a well-established model.
M. gallisepticum virulence for house finches has previously been shown to increase over time in a given geographic region (18). Data presented here confirm previous findings in that a recent HFMG isolate, VA2013, continues to be highly virulent in house finches. Notably, data presented here now indicate that the virulence for chickens of a recent isolate (VA2013) is attenuated relative to that of an early isolate (VA1994, the index isolate of the ancestral HFMG genotype [21]), suggesting that a finch-adapted HFMG isolate with increased virulence for finches has developed a commensurately reduced capacity to induce disease in chickens. While the bacterial determinants that are important for the differences in HFMG virulence for chickens and house finches remain to be identified, specific genetic features—insertion/deletions and variations in repetitive elements and genes, notably those for surface lipoproteins—have previously been characterized as variable and potentially under diversifying pressure in HFMG clade M. gallisepticum from across the house finch epidemic (21). Similar features could conceivably affect the virulence differences between VA1994 and VA2013 observed here.
While data presented here are limited to two isolates, they represent isolates adapted on either end of the epizootic across which virulence evolution is well established. Previous studies have shown that HFMG virulence evolution is not constant but is instead dynamic and dependent on ecological context (e.g., expanding versus established geographic ranges) (18, 24). It is clear that M. gallisepticum was virulent for finches by the time that it was first noted and collected as VA1994 (coalescence analysis has indicated that HFMG first truly emerged in house finches 4 to 7 years prior [20, 21]); however, here it is indicated that the period of time in which HFMG was circulating in finches prior to 1994 was insufficient to dramatically attenuate HFMG virulence for chickens. The 19 years since 1994 appear to have resulted in altered virulence phenotypes for the VA2013 isolate; however, the rates at which virulence for different hosts may have changed are not known. While increases in HFMG virulence for finches have been shown to be more rapid in a given geographic location (18), how changes in virulence for chickens may be related requires further study. Assessment of larger numbers of HFMG isolates for virulence for both house finch and poultry hosts, and concurrent assessment of HFMG genomics for identification of specific elements associated with evolution in each host, would be of interest for future studies.
Of particular interest regarding HFMG virulence are the changes in tissue tropism observed along with altered virulence for different hosts. HFMG virulence in the finch eye is positively correlated with pathogen load and disease transmission, fitting a virulence tradeoff model where virulence evolves to trade host fitness for pathogen transmission (17, 18, 25). The attenuation of HFMG for the chicken respiratory system may possibly redirect such a tradeoff in chickens toward increased host fitness and lower levels of pathogen transmission. Conceivably, shifts in HFMG tissue tropism could impact different mechanisms of transmission in different hosts. HFMG transmission is known to be impacted by bird feeder use (26), and ocular disease and bacterial shedding could possibly impact direct or fomite transmission at feeders, thus making feeder use a potential driving factor for changes in HFMG virulence in the eye (3). Conversely, HFMG does not cause conjunctivitis in chickens (23), and data presented here suggest that HFMG may evolve in finches away from virulence and the ability to colonize in the chicken lower respiratory tract. Thus, while other studies have indicated that HFMG appears to adapt to the ocular niche in finches, our data from the VA2013 isolate suggest that adaptation to the finch eye may potentially be at the expense of the ability to colonize and cause disease in the poultry respiratory tract. Tissue tropism and transmission effects potentially play significant roles in this change.
Finally, natural occurrences of HFMG reintroduction into individual domestic poultry have been reported (16), suggesting that such occurrences may pose a potential threat to domestic poultry populations as a whole. Our data indicate that domestic poultry remain susceptible to infection with house finch-adapted strains from free-ranging birds; however, they also suggest that as M. gallisepticum adapts to wild bird species it becomes less virulent for domestic poultry. With a decrease in HFMG virulence for poultry, the disease and epidemiological risk associated with HFMG reintroduction from wild species and spread in poultry populations may conceivably be reduced.
MATERIALS AND METHODS
Bacterial culture.
For chicken experiments, M. gallisepticum stocks were prepared by inoculating HFMG strain VA1994 (North Carolina State University [NCSU] ADRL no. 7994-1) or VA2013 (NCSU ADRL no. 2013-089-15), or poultry strain Rlow, into fresh Hayflick's medium and incubating at 37°C until mid-log-phase growth was reached. Titers of cultures were determined, and 1-ml stock aliquots were frozen at −80°C for future use. Before challenge, one aliquot of each M. gallisepticum stock (Rlow, VA1994, and VA2013) was grown at 37°C with shaking at 130 rpm in fresh Hayflick's complete medium for 5 h prior to inoculation. M. gallisepticum concentrations were estimated by determination of the optical density at 620 nm (OD620) and were adjusted to a challenge concentration of 5 × 108 CFU/ml in Hayflick's complete medium as described previously (27). Viable titers of challenge inocula were confirmed by 10-fold serial dilution and culture for endpoint assessment of color-changing units (CCU).
For house finch experiments, M. gallisepticum stocks were prepared and cryopreserved for the strains described above plus for VA1995 (NCSU ADRL no. 13295-2) at North Carolina State University (NCSU) as described previously (18, 19, 28, 29) and shipped frozen to Virginia Tech and Cornell.
Animal infection.
For chicken experiments, 4-week-old female specific-pathogen-free White Leghorn chickens were purchased (Spafas, North Franklin, CT). Upon receipt, chickens were divided into groups of 10 per HEPA-filtered isolator, tagged, and allowed to acclimate for 1 week prior to experimentation. Nonmedicated feed and water were provided ad libitum throughout the experimental period. All animal procedures were conducted in accordance with state and federal policies to ensure the humane use and care of research animals as approved by the University of Connecticut Institutional Animal Care and Use Committee (IACUC) (approval no. A15-056).
Groups of 10 chickens were inoculated intratracheally with 200 μl (1 × 108 CFU) of M. gallisepticum in Hayflick's complete medium per bird on days 0 and 2 (27). Medium control chickens received 200 μl Hayflick's complete medium on days 0 and 2. The experiment was repeated once in full to validate initial results.
For house finch experiments, wild house finches were captured. Capture was conducted with mist nets or cage traps in Virginia under permits from the Virginia Department of Game and Inland Fisheries (VDGIF) (permit 044569) and the United States Fish and Wildlife Service (USFWS) (MB158404-1) and in New York under permit license 39 (New York State Fish and Wildlife, Albany, NY) and permit 22669 (United States Geological Survey, Department of the Interior [Laurel, MD]). All sampling procedures were approved by Cornell University's Institutional Animal Care and Use Committee (permit 2006-094). Finches were held in groups during quarantine, during which they were monitored for clinical signs of M. gallisepticum and at the end of which they were tested for prior M. gallisepticum exposure via enzyme-linked immunosorbent assay (ELISA) (30, 31) and quantitative PCR (qPCR) (32) as done previously. Animals with signs of disease or seropositivity were excluded from the experiment. As done previously, house finches were housed singly indoors (Virginia Tech) or in groups of 12 in large semioutdoor aviaries (Cornell) and were provided food and water ad libitum as approved by university IACUCs. House finches were inoculated by diluting cryopreserved inocula to a concentration of 1 to 2 × 107 CCU/ml immediately prior to use and inoculating bilaterally in the palpebral conjunctiva with 0.04 to 0.05 ml of an HFMG strain. At Virginia Tech, all birds were directly inoculated and observed daily for clinical lesion scores; at Cornell, two index animals were directly inoculated, and 10 in-contact sentinel animals were observed daily for clinical lesion scores.
Lesion scores and tracheal thickness.
Chickens were humanely euthanized and immediately subjected to necropsy on day 14 after the second round of infection. Gross anatomic lesions were noted, and tracheas and lungs were collected for histologic processing, examination, and scoring in a blind fashion based on criteria adapted from Nunoya et al. (33) and described by Gates et al. (27). Lung scoring was done on the basis of the presence of increasing amounts of lymphofollicular (B-cell follicles) and disseminated lymphocytic accumulations around the primary bronchi and interlobular septa with some epithelial hyperplasia. Additionally, tracheal thickness measurements were determined for all chickens as previously described (27).
Finches were examined regularly postinoculation or postcontact to assign scores corresponding to the severity of inflammatory eye lesions as described by Sydenstricker et al. (28). Lesions were scored on a scale of 0 to 3 as follows: 0, no detectable swelling or eversion; 1, minor swelling around the eye ring; 2, moderate swelling and eversion of the conjunctival tissue; 3, the eye nearly hidden by swelling and crusted exudate. Lesion scores for each eye were summed within individuals for a given sampling day.
Bacterial recovery.
At chicken necropsy, a ring from the distal portion of the trachea and a caudal portion of lung were collected directly into Hayflick's complete medium and incubated for 5 h at 37°C. After the incubation period, cultures were passed through 0.45-μM-pore-size filters to remove nonmycoplasmal contaminates, adjusted to pH 7.8, and reincubated at 37°C. Samples were considered positive for M. gallisepticum recovery if the color shifted to yellow within 30 days.
Statistical analysis.
Histological data were subjected to nonparametric Kruskal-Wallis one-way analysis of variance (ANOVA) in the ranks test, in which all pairwise multiple-comparison procedures were performed using the Student-Newman-Keuls method for groups of equal sizes or Dunn's method for groups of unequal sizes. Statistical tests were conducted using SigmaPlot 11.0 (Systat Software, San Jose, CA). Eye lesion scores in individually housed birds inoculated directly with distinct M. gallisepticum isolates were compared using a mixed model in JMP 13.0 (SAS Institute, Cary, NC) that considered treatment and days postinoculation and their pairwise interaction. Individual bird identifiers (ID) were included as a random effect to account for repeated, nonindependent observations from the same individuals (n = 15 per isolate) over time. Cumulative eye lesion scores in sentinel house finches exposed to different M. gallisepticum isolates were compared using a Wilcoxon rank sum test in STATISTIX 10 (Analytical Software, Tallahassee, FL).
ACKNOWLEDGMENTS
We thank W. M. Hochachka at Cornell University, A. Dobson at Princeton University, and M. May at the University of New England for support and useful discussion.
REFERENCES
- 1.Raviv Z, Ley DH. 2013. Mycoplasma gallisepticum infection, p 1423 In Glisson JR, McDougald LR, Swayne DE, Nolan LK, Suarez DL, Nair V (ed), Diseases of poultry, 13th ed Wiley, Ames, IA. [Google Scholar]
- 2.Fischer JR, Stallknecht DE, Luttrell P, Dhondt AA, Converse KA. 1997. Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population. Emerg Infect Dis 3:69–72. doi: 10.3201/eid0301.970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley DH, Berkhoff JE, McLaren JM. 1996. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis 40:480–483. doi: 10.2307/1592250. [DOI] [PubMed] [Google Scholar]
- 4.Ley DH, Berkhoff JE, Levisohn S. 1997. Molecular epidemiologic investigations of Mycoplasma gallisepticum conjunctivitis in songbirds by random amplified polymorphic DNA analyses. Emerg Infect Dis 3:375–380. doi: 10.3201/eid0303.970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhondt AA, Tessaglia DL, Slothower RL. 1998. Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. J Wildl Dis 34:265–280. doi: 10.7589/0090-3558-34.2.265. [DOI] [PubMed] [Google Scholar]
- 6.Dhondt AA, Badyaev AV, Dobson AP, Hawley DM, Driscoll MJL, Hochachka WM, Ley DH. 2006. Dynamics of mycoplasmal conjunctivitis in the native and introduced range of the host. EcoHealth 3:95. doi: 10.1007/s10393-006-0019-7. [DOI] [Google Scholar]
- 7.Duckworth RA, Badyaev AV, Farmer KL, Hill GE, Roberts SR. 2003. First case of Mycoplasma gallisepticum infection in the western range of the house finch (Carpodacus mexicanus). Auk 120:528–530. doi: 10.2307/4090206. [DOI] [Google Scholar]
- 8.Ley DH, Sheaffer DS, Dhondt AA. 2006. Further western spread of Mycoplasma gallisepticum infection of house finches. J Wildl Dis 42:429–431. doi: 10.7589/0090-3558-42.2.429. [DOI] [PubMed] [Google Scholar]
- 9.Adelman JS, Mayer C, Hawley DM. 2017. Infection reduces anti-predator behaviors in house finches. J Avian Biol 48:001–010. doi: 10.1111/jav.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochachka WM, Dhondt AA. 2000. Density-dependent decline of host abundance resulting from a new infectious disease. Proc Natl Acad Sci U S A 97:5303–5306. doi: 10.1073/pnas.080551197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luttrell MP, Fischer JR, Stallknecht DE, Kleven SH. 1996. Field investigation of Mycoplasma gallisepticum infections in house finches (Carpodacus mexicanus) from Maryland and Georgia. Avian Dis 40:335–341. doi: 10.2307/1592229. [DOI] [PubMed] [Google Scholar]
- 12.Altizer S, Hochachka WM, Dhondt AA. 2004. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J Animal Ecol 73:309–322. doi: 10.1111/j.0021-8790.2004.00807.x. [DOI] [Google Scholar]
- 13.Faustino CR, Jennelle CS, Connolly V, Davis AK, Swarthout EC, Dhondt AA, Cooch EG. 2004. Mycoplasma galllisepticum infection dynamics in a house finch population: empirical analysis of seasonal variation in survival, encounter and transmission rate. J Anim Ecol 73:651–669. doi: 10.1111/j.0021-8790.2004.00840.x. [DOI] [Google Scholar]
- 14.Hartup BK, Kollias GV, Ley DH. 2000. Mycoplasmal conjunctivitis in songbirds from New York. J Wildl Dis 36:257–264. doi: 10.7589/0090-3558-36.2.257. [DOI] [PubMed] [Google Scholar]
- 15.Ley DH, Hawley DM, Geary SJ, Dhondt AA. 2016. House finch (Haemorhous mexicanus) conjunctivitis, and Mycoplasma spp. isolated from North American wild birds, 1994–2015. J Wildl Dis 52:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochachka WM, Dhondt AA, Dobson A, Hawley DM, Ley DH, Lovette IJ. 2013. Multiple host transfers, but only one successful lineage in a continent-spanning emergent pathogen. Proc Biol Sci 280:20131068. doi: 10.1098/rspb.2013.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley DM, Dhondt KV, Dobson AP, Grodio JL, Hochachka WM, Ley DH, Osnas EE, Schat KA, Dhondt AA. 2010. Common garden experiment reveals pathogen isolate but no host genetic diversity effect on the dynamics of an emerging wildlife disease. J Evol Biol 23:1680–1688. doi: 10.1111/j.1420-9101.2010.02035.x. [DOI] [PubMed] [Google Scholar]
- 18.Hawley DM, Osnas EE, Dobson AP, Hochachka WM, Ley DH, Dhondt AA. 2013. Parallel patterns of increased virulence in a recently emerged wildlife pathogen. PLoS Biol 11:e1001570. doi: 10.1371/journal.pbio.1001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grodio JL, Hawley DM, Osnas EE, Ley DH, Dhondt KV, Dhondt AA, Schat KA. 2012. Pathogenicity and immunogenicity of three Mycoplasma gallisepticum isolates in house finches (Carpodacus mexicanus). Vet Microbiol 155:53–61. doi: 10.1016/j.vetmic.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Delaney NF, Balenger S, Bonneaud C, Marx CJ, Hill GE, Ferguson-Noel N, Tsai P, Rodrigo A, Edwards SV. 2012. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet 8:e1002511. doi: 10.1371/journal.pgen.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tulman ER, Liao X, Szczepanek SM, Ley DH, Kutish GF, Geary SJ. 2012. Extensive variation in surface lipoprotein gene content and genomic changes associated with virulence during evolution of a novel North American house finch epizootic strain of Mycoplasma gallisepticum. Microbiology 158:2073–2088. doi: 10.1099/mic.0.058560-0. [DOI] [PubMed] [Google Scholar]
- 22.Stallknecht DE, Luttrell MP, Fischer JR, Kleven SH. 1998. Potential for transmission of the finch strain of Mycoplasma gallisepticum between house finches and chickens. Avian Dis 42:352–358. doi: 10.2307/1592485. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor RJ, Turner KS, Sander JE, Kleven SH, Brown TP, Gomez L Jr, Cline JL. 1999. Pathogenic effects on domestic poultry of a Mycoplasma gallisepticum strain isolated from a wild house finch. Avian Dis 43:640–648. doi: 10.2307/1592732. [DOI] [PubMed] [Google Scholar]
- 24.Osnas EE, Hurtado PJ, Dobson AP. 2015. Evolution of pathogen virulence across space during an epidemic. Am Nat 185:332–342. doi: 10.1086/679734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams PD, Dobson AP, Dhondt KV, Hawley DM, Dhondt AA. 2014. Evidence of trade-offs shaping virulence evolution in an emerging wildlife pathogen. J Evol Biol 27:1271–1278. doi: 10.1111/jeb.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adelman JS, Moyers SC, Farine DR, Hawley DM. 2015. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc Biol Sci 282:20151429. doi: 10.1098/rspb.2015.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gates AE, Frasca S, Nyaoke A, Gorton TS, Silbart LK, Geary SJ. 2008. Comparative assessment of a metabolically attenuated Mycoplasma gallisepticum mutant as a live vaccine for the prevention of avian respiratory mycoplasmosis. Vaccine 26:2010–2019. doi: 10.1016/j.vaccine.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Sydenstricker KV, Dhondt AA, Ley DH, Kollias GV. 2005. Re-exposure of captive house finches that recovered from Mycoplasma gallisepticum infection. J Wildl Dis 41:326–333. doi: 10.7589/0090-3558-41.2.326. [DOI] [PubMed] [Google Scholar]
- 29.Sydenstricker KV, Dhondt AA, Hawley DM, Jennelle CS, Kollias HW, Kollias GV. 2006. Characterization of experimental Mycoplasma gallisepticum infection in captive house finch flocks. Avian Dis 50:39–44. doi: 10.1637/7403-062805R.1. [DOI] [PubMed] [Google Scholar]
- 30.Hawley DM, Grodio J, Frasca S, Kirkpatrick L, Ley DH. 2011. Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian Pathol 40:321–327. doi: 10.1080/03079457.2011.571660. [DOI] [PubMed] [Google Scholar]
- 31.Grodio JL, Buckles EL, Schat KA. 2009. Production of house finch (Carpodacus mexicanus) IgA specific anti-sera and its application in immunohistochemistry and in ELISA for detection of Mycoplasma gallisepticum-specific IgA. Vet Immunol Immunopathol 132:288–294. doi: 10.1016/j.vetimm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Grodio JL, Dhondt KV, O'Connell PH, Schat KA. 2008. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathol 37:385–391. doi: 10.1080/03079450802216629. [DOI] [PubMed] [Google Scholar]
- 33.Nunoya T, Tajima M, Yagihashi T, Sannai S. 1987. Evaluation of respiratory lesions in chickens induced by Mycoplasma gallisepticum. Nippon Juigaku Zasshi 49:621–629. doi: 10.1292/jvms1939.49.621. [DOI] [PubMed] [Google Scholar]