ABSTRACT
Acanthamoeba castellanii is a ubiquitous free-living amoeba with a worldwide distribution that can occasionally infect humans, causing particularly severe infections in immunocompromised individuals. Dissecting the immunology of Acanthamoeba infections has been considered problematic due to the very low incidence of disease, despite the high exposure rates. While macrophages are acknowledged as playing a significant role in Acanthamoeba infections, little is known about how this facultative parasite influences macrophage activity. Therefore, in this study we investigated the effects of Acanthamoeba on the activation of resting macrophages. Consequently, murine bone marrow-derived macrophages were cocultured with trophozoites of either the laboratory Neff strain or a clinical isolate of A. castellanii. In vitro real-time imaging demonstrated that trophozoites of both strains often established evanescent contact with macrophages. Both Acanthamoeba strains induced a proinflammatory macrophage phenotype characterized by the significant production of interleukin-12 (IL-12) and IL-6. However, macrophages cocultured with the clinical isolate of Acanthamoeba produced significantly less IL-12 and IL-6 than the Neff strain. The utilization of macrophages derived from MyD88-, TRIF-, Toll-like receptor 2 (TLR2)-, TLR4-, and TLR2/4-deficient mice indicated that Acanthamoeba-induced proinflammatory cytokine production was through MyD88-dependent, TRIF-independent, TLR4-induced events. This study shows for the first time the involvement of TLRs expressed on macrophages in the recognition of and response to Acanthamoeba trophozoites.
KEYWORDS: Acanthamoeba, macrophages
INTRODUCTION
Acanthamoeba castellanii is a ubiquitous free-living amoeba that has been isolated from both outdoor and indoor environments. It exists as actively feeding, dividing trophozoites and the dormant environmentally resistant cyst. Despite its ability to proliferate and survive as a free-living organism, Acanthamoeba is also a facultative parasite of humans, most frequently causing a painful, potentially blinding infection of the eye, called Acanthamoeba keratitis (AK), in immunocompetent individuals. Acanthamoeba is also an opportunistic parasite, and in immunocompromised individuals it is responsible for an often fatal infection of the brain, named granulomatous amoebic encephalitis (GAE) (1). The ability of the vast majority of immunocompetent humans to resist infection coupled with the susceptibility of the immunocompromised demonstrates the importance of the immune system in resistance to infection. However, and surprisingly, there are few available data regarding the immune response to Acanthamoeba, although approximately 50 to 100% of people are known to be seropositive (2).
Acanthamoeba preferentially infects immune-privileged sites, such as the brain and the eye, which are characterized by a limited regenerative capability (3). It has been demonstrated that both the innate and the adaptive immune responses are involved during Acanthamoeba infection (2, 4). Among those elements of the innate immune response that have been implicated in protective immunity are the phagocytic cells, primarily neutrophils and macrophages, both of which are capable of killing Acanthamoeba (5). However, macrophages have been demonstrated to persist at the site of infection (6, 7), and therefore, they not only may be involved in initiating and maintaining an effective immune response, but also may have a role in tissue repair (8). To date, the majority of studies have examined the interaction between corneal epithelial cells (9–11) and Acanthamoeba, with comparatively few examining the interaction of these organisms with macrophages. Macrophages either can be long-lived cells patrolling the host's tissues (resident macrophages) or can originate from recruited blood-derived monocytes at the site of infection (elicited macrophages) (12). Resident macrophages, strategically distributed within all tissues, are responsible for the first recognition of pathogens through pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). The interaction of these PRRs with pathogen-associated molecular patterns (PAMPs) is important in initiating effector mechanisms for the eradication of pathogens and also directing the developing adaptive immune response and initiating tissue repair (13). To date, the role of TLRs expressed on macrophages in recognizing and responding to Acanthamoeba has not been addressed. To address this in this study, bone marrow-derived (BMD) macrophages were coincubated with either a classical laboratory strain of A. castellanii named Neff or a clinical isolate of A. castellanii isolated from a case of bilateral keratitis. Both strains utilized were of the T4 genotype, which has been associated with the majority of human infections. The kinetics of proinflammatory cytokines released by macrophages upon exposure to trophozoites of A. castellanii were quantified, and the TLRs and key signaling molecules responsible for these immunological events were identified.
RESULTS
In vitro real-time imaging of A. castellanii infection shows cell-cell interactions between murine BMD macrophages and trophozoites of either the Neff strain or the clinical isolate.
Macrophages and Neff strain trophozoites were found to engage in mutual interactions a few minutes after infection. Trophozoites showed characteristic pseudopodia and amoeboid locomotion. Macrophages were healthy and actively moving, and their lamellipodia and filopodia were visible. However, macrophages were generally less motile than trophozoites (see Movie S1a in the supplemental material). Neff trophozoites were observed to establish contact with macrophages and to roll on the macrophage surface. Although macrophages briefly attached to trophozoites with either lamellipodia or filopodia, they were unable to maintain this contact and to phagocytose them (Movie S1b). Indeed, no phagocytic invagination was observed at any time during observation for up to 1 h (Movies S1a and b). In contrast, the real-time interaction between macrophages and trophozoites of the clinical isolate showed some differences from that during macrophage infection with the Neff strain. In this instance, trophozoites of the clinical isolate did not show rolling behavior over the macrophage surface (Movies S2a and b); nevertheless, macrophages were able to interact with trophozoites through lamellipodia and filopodia (Movies S2a and b), and this sometimes resulted in trophozoite disruption (Movie S2a), but we also observed trophozoite division during the observation period (Movie S2b).
A. castellanii Neff induces IL-12 and IL-6 production in murine BMD macrophages in a time- and trophozoite density-dependent manner.
In order to evaluate if the production of proinflammatory cytokines depends on trophozoite density or on the duration of infection, murine macrophages were coincubated with Neff trophozoites at three different ratios of trophozoite/macrophage (1:1, 1:2, 1:10), and the production of interleukin-12 (IL-12) and IL-6 was assessed at 4, 6, 8, 10, and 24 h after infection.
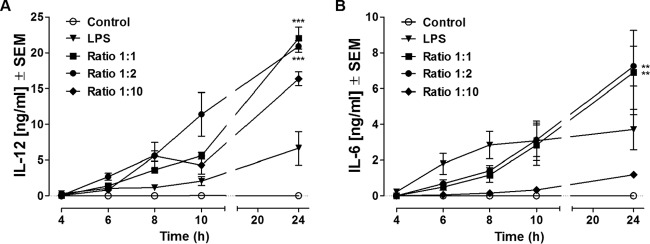
IL-12 production increased progressively throughout the course of the study in all trophozoite-infected experimental groups, reaching the highest concentrations at 24 h postinfection, when the concentrations were compared to those in unstimulated control cultures. At 24 h, trophozoite/macrophage coculture ratios of 1:1 and 1:2 had induced similar levels of IL-12 and levels significantly higher than those for the other experimental groups (22.08 ng/ml [P < 0.0005] and 20.92 ng/ml [P < 0.0005], respectively). Lipopolysaccharide (LPS)-stimulated macrophages produced IL-12 in a time-dependent manner, with the concentrations peaking at 24 h poststimulation. No IL-12 was detectable in control macrophage cultures at any time (Fig. 1A).
FIG 1.
Release of IL-12 (A) and IL-6 (B) at 4, 6, 8, 10, and 24 h after coincubation with A. castellanii Neff trophozoites. Murine macrophages (1 × 106) obtained from BALB/c mice were challenged with either 1 × 106 (Ratio 1:1), 5 × 105 (Ratio 1:2), or 1 × 105 (Ratio 1:10) trophozoites of the A. castellanii Neff strain. LPS was used as a positive control at a concentration of 200 ng/ml, whereas uninfected macrophages (Control) were considered the negative control. Experiments were repeated three times. Results represent the mean ± standard error (n = 6). Two-way ANOVA could not be applied, since the interaction between the stimuli and time was statistically significant, and the results of statistical analysis for the time and stimulus effects were therefore difficult to interpret. For this reason, one-way ANOVA was applied for each time point. Tukey's multiple-comparison test was performed to evaluate differences within the condition means at each time point. In the graphs, significant differences within the different conditions are indicated as follows: **, P < 0.005; ***, P < 0.0005. Note that higher trophozoite/macrophage infection ratios induce higher levels of cytokine production and IL-6 and IL-12 production peaks at 24 h.
IL-6 production by murine macrophages cocultured with trophozoites increased throughout the 24-h time course of study, and the levels recorded from trophozoite/macrophage coculture ratios of 1:1 and 1:2 were significantly greater than those recorded from control macrophage cultures from 8 h. Trophozoite/macrophage coculture ratios of 1:1 and 1:2 were equally effective at inducing IL-6 production and significantly more effective than a trophozoite/macrophage coculture ratio of 1:10 (Fig. 1B). Interestingly, the dynamics and profile of macrophage IL-6 production induced by LPS were quite dissimilar from those induced by trophozoite coculture. LPS induced a significantly more rapid IL-6 production than trophozoite/macrophage coculture ratios of 1:1 and 1:2, which peaked at 8 h poststimulation. No IL-6 was detectable in noninfected macrophage cultures at any time.
A clinical isolate of A. castellanii belonging to genotype T4 stimulates lower levels of cytokine production than Neff, the classical laboratory T4 genotype strain.
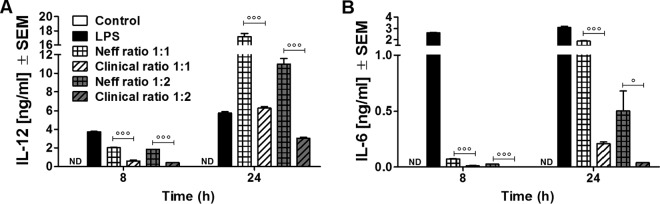
Differences in the levels of cytokines induced by macrophages cocultured with either the Neff or the clinical strain were investigated. On the basis of the effectiveness of cytokine induction in the studies described above, murine BMD macrophages were infected at trophozoite/macrophage ratios of 1:1 and 1:2. Samples were collected at 8 h and 24 h after coincubation, which were early and late time points, respectively. The level of IL-12 production by macrophages was greater following incubation with Neff strain trophozoites than trophozoites of the clinical isolate at both 8 h and 24 h (P < 0.0005) (Fig. 2A). These results were consistent at both trophozoite/macrophage infection levels. IL-6 production by macrophages was significantly induced by Neff strain trophozoites compared with the level of production by noninfected macrophage cultures at both 8 and 24 h (P < 0.0005) postinfection. In addition, macrophages incubated with Neff strain trophozoites produced significantly more IL-6 than macrophages incubated with clinical strain trophozoites at both 8 h and 24 h (P < 0.0005) postinfection. Clinical strain trophozoites failed to induce IL-6 production to levels significantly above those produced by unstimulated control macrophages at 8 h postinfection (Fig. 2B). In addition, Neff strain trophozoites induced IL-6 at a later time point than the LPS control.
FIG 2.
Release of IL-12 (A) and IL-6 (B) at 8 and 24 h after coincubation with A. castellanii trophozoites. Murine macrophages (1 × 106) obtained from BALB/c mice were challenged with 1 × 106 or 5 × 105 trophozoites of either the Neff strain (Neff ratio 1:1 and Neff ratio 1:2, respectively) or the clinical isolate (Clinical ratio 1:1 and Clinical ratio 1:2, respectively). LPS at a concentration of 200 ng/ml was used as a positive control, whereas uninfected macrophages (Control) were considered the negative control. The experiment was repeated twice. Results represent the mean ± standard error (n = 3). One-way ANOVA was applied for each time point, and Tukey's multiple-comparison test was performed to evaluate differences within the condition means at each time point. In the graphs, significant differences between Neff and clinical strains are indicated as follows: °, P < 0.05; °°°, P < 0.0005. Values below the detectable levels are indicated in the graphs as ND (not detected). Note that the Acanthamoeba Neff strain induces higher levels of macrophage proinflammatory cytokines than the Acanthamoeba clinical isolate. This event was observed at both the early time points (8 h after coincubation) and the later time points (24 h after coincubation).
Acanthamoeba-induced IL-12 and IL-6 production is MyD88 dependent and partially TRIF dependent.
Studies were performed using gene-deficient mice to identify the key signaling pathways involved in the induction of cytokines by Acanthamoeba. BMD macrophages were obtained from C57BL/6 wild-type (WT) mice or C57BL/6 mice deficient in either the MyD88 or TRIF gene and coincubated with Neff strain or clinical isolate trophozoites at ratios of 1:1 and 1:2. As positive controls, macrophages were stimulated with LPS, the natural TLR4 ligand, or with the synthetic double-stranded RNA poly(I·C), a selective TLR3 agonist. Production of IL-12 and IL-6 was then measured at 24 h after the coculture.
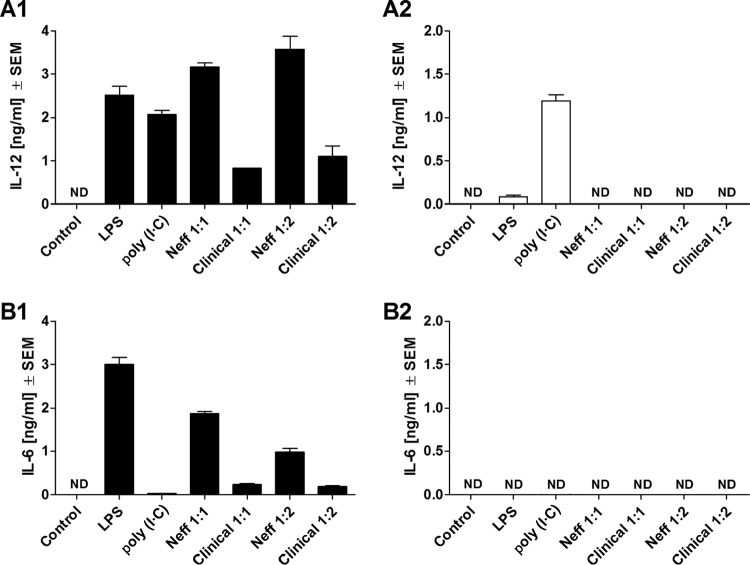
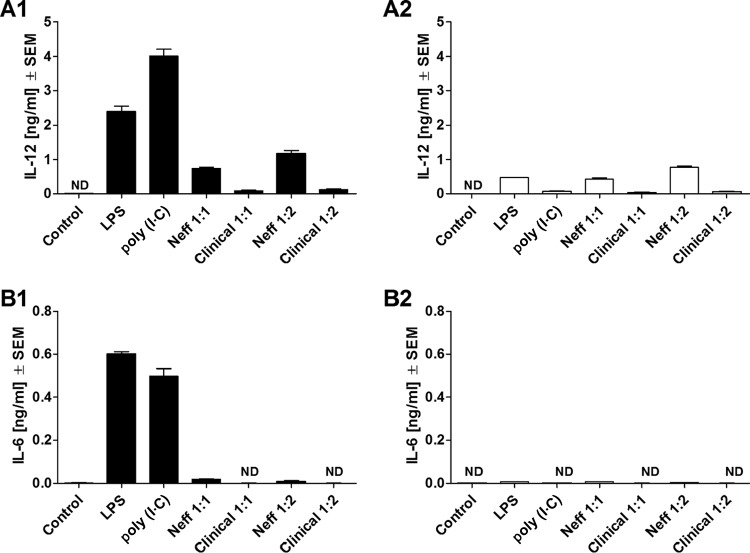
Significant production of IL-12 and IL-6 by WT macrophages was induced by trophozoites of either the Neff strain or the clinical isolate at both coculture ratios studied (Fig. 3A1 and B1). However, MyD88−/− macrophages coincubated with trophozoites of either the Neff strain or the clinical isolate failed to produce detectable levels of either IL-12 or IL-6 (Fig. 3A2 and B2). By comparison, IL-12 production was significantly inhibited, though not ablated, in TRIF−/− macrophages coincubated with trophozoites of either the Neff strain or the clinical isolate compared to that in WT macrophages (Fig. 4A1 and A2). In addition, IL-6 production by the Neff strain significantly decreased in TRIF−/− macrophages (P = 0.0014) (Fig. 4B1 and B2).
FIG 3.
Release of IL-12 (A) and IL-6 (B) by comparison of C57BL/6 WT (A1 and B1) and C57BL/6 MyD88−/− (A2 and B2) mouse macrophages at 24 h after coincubation with Acanthamoeba trophozoites. Murine macrophages (1 × 106) obtained from either C57BL/6 WT or C57BL/6 MyD88−/− mice were challenged with 1 × 106 or 5 × 105 trophozoites of either the A. castellanii Neff strain (Neff 1:1 and Neff 1:2, respectively) or the clinical isolate (Clinical 1:1 and 1:2, respectively). LPS and poly(I·C) at 200 ng/ml and 10 μg/ml, respectively, were used as positive controls to stimulate macrophages. Uninfected macrophages (Control) were considered the negative control. The experiment was performed twice. Results represent the mean ± standard error (n = 3). Student's t test was applied to evaluate differences between C57BL/6 WT and the C57BL/6 MyD88−/− macrophage cytokine production. Values below the detectable levels are indicated in the graphs as ND (not detected). IL-12 and IL-6 production under trophozoite/MyD88−/− macrophage coincubation conditions was completely ablated in comparison to that under trophozoite/WT macrophage coincubation conditions, suggesting that the production of these proinflammatory cytokines by macrophages in response to Acanthamoeba is MyD88 dependent.
FIG 4.
Release of IL-12 (A) and IL-6 (B) by comparison of C57BL/6 WT (A1 and B1) and C57BL/6 TRIF−/− (A2 and B2) mouse macrophages at 24 h after coincubation with Acanthamoeba trophozoites. Murine macrophages (1 × 106) obtained from either C57BL/6 WT or C57BL/6 TRIF−/− mice were challenged with 1 × 106 or 5 × 105 trophozoites of either the A. castellanii Neff strain (Neff 1:1 and Neff 1:2, respectively) or the clinical isolate (Clinical 1:1 and Clinical 1:2, respectively). LPS and poly(I·C) at 200 ng/ml and 10 μg/ml, respectively, were used as positive controls to stimulate macrophages. Uninfected macrophages (Control) were considered the negative control. The experiment was performed twice. Results represent the mean ± standard error (n = 3). Student's t test was applied to evaluate differences between C57BL/6 WT and the C57BL/6 TRIF−/− macrophages. Values below the detectable levels are indicated in the graphs as ND (not detected). IL-12 and IL-6 production under trophozoite/TRIF−/− macrophage coincubation conditions was significantly diminished in comparison with that under trophozoite/WT macrophage coincubation conditions, suggesting that the production of these proinflammatory cytokines by macrophages in response to Acanthamoeba might be, in part, TRIF dependent, although this does not appear to be the main signaling pathway involved during Acanthamoeba stimulation.
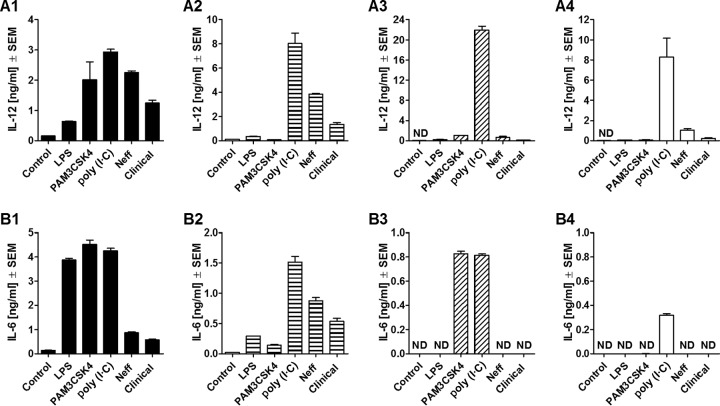
Acanthamoeba-induced macrophage IL-12 and IL-6 production is TLR4 dependent but TLR2 independent.
TLR2 and TLR4 were considered potential targets for trophozoite-induced macrophage cytokine induction since they are localized on the cell surface and associated with the MyD88-dependent signaling pathway. Therefore, macrophages derived from C57BL/6 mice deficient in TLR2, TLR4, or TLR2/4 were utilized to determine their specific roles in this process. Neff strain and clinical strain trophozoites were used in coincubations with isolated macrophages at a ratio of 1:1. As positive controls, macrophages were stimulated with either LPS, PAM3CKS4, or the RNA poly(I·C). The levels of production of IL-12 and IL-6 were then measured at 24 h after infection.
C57BL/6 WT macrophages produced IL-12 and IL-6 when induced by trophozoites of either the Neff or the clinical strain (Fig. 5A1 and B1). Production of these cytokines was not diminished in TLR2−/− macrophages (Fig. 5A2 and B2), whereas IL-12 production by TLR4−/− macrophages was significantly lower than that by WT macrophages (for the Neff strain, P = 0.0009; for the clinical strain, P = 0.0002) (Fig. 5A3), and the production of IL-6 was completed ablated (Fig. 5B3). The simultaneous absence of both TLR2 and TLR4 on macrophages was characterized by significantly lower levels of IL-12 production when macrophages were coincubated with either Neff strain (P = 0.0009) or clinical isolate (P = 0.0002) trophozoites (Fig. 5A4) and no IL-6 production compared to the results obtained with WT macrophages (Fig. 5B4).
FIG 5.
Release of IL-12 (A) and IL-6 (B) by comparison of C57BL/6 WT (A1 and B1) and C57BL/6 TLR2−/− (A2 and B2), TLR4−/− (A3 and B3), and TLR2/4−/− (A4 and B4) mouse macrophages at 24 h after coincubation with Acanthamoeba trophozoites. Murine macrophages (1 × 106) obtained from C57BL/6 WT and knockout mice were challenged with 1 × 106 trophozoites of either the A. castellanii Neff strain (Neff) or the clinical isolate (Clinical). LPS, PAM3CSK4, and poly(I·C) at 200 ng/ml, 320 ng/ml, and 10 μg/ml, respectively, were used as positive controls to stimulate macrophages. Uninfected macrophages (Control) were considered the negative control. The experiment was performed twice. Results represent the mean ± standard error (n = 3). Student's t test was applied to evaluate differences between C57BL/6 WT mice and the C57BL/6 TLR2−/−, TLR4−/−, and TLR2/4−/− mice. Values below the detectable levels are indicated in the graphs as ND (not detected). IL-12 and IL-6 production under trophozoite/TLR2−/− macrophage coincubation conditions was not significantly diminished in comparison with that under trophozoite/WT macrophage coincubation conditions, suggesting that the production of these proinflammatory cytokines by macrophages in response to Acanthamoeba is not TLR2 dependent. On the other hand, in the absence of TLR4, trophozoite-induced IL-12 production by macrophages was significantly decreased, whereas IL-6 production was completely ablated. The same pattern was observed when neither TLR2 nor TLR4 was expressed. Therefore, TLR4 appeared to be the main TLR involved in the recognition of and response to A. castellanii.
DISCUSSION
A. castellanii is a ubiquitous free-living microorganism and is so ubiquitous that individuals are constantly exposed to amoebae of this species in their everyday lives. Although the opportunities of becoming infected with this protist are high, few cases of facultative or opportunistic parasitism and disease have been reported. Patients with an immune deficiency are particularly susceptible to infection with these organisms, and they usually present the most severe and deadly amoebic disease, GAE. On the other hand, AK can also occur in immunocompetent individuals and occurs predominantly, but not exclusively, in contact lens wearers (14). While AK is generally attributed either to bad hygiene or to corneal trauma caused by the lens, studies have demonstrated that prolonged use of contact lenses may impair innate immunity at the ocular surface, providing an additional mechanism that potentially contributes to an increased incidence of AK in contact lens wearers (15, 16). Overall, therefore, it is the general consensus that the immune system is critical in determining whether infection occurs following an encounter with Acanthamoeba in humans.
Our studies have shown, for the first time, the profile and kinetics of Acanthamoeba-induced IL-12 and IL-6 production in murine BMD macrophages during coculture with trophozoites. These cytokines not only play important roles in the activation of the innate immune cell functions, but they are also involved in the activation and proliferation of the adaptive immune cells (17, 18). IL-12, in particular, plays a pivotal role in controlling microbial infections (19) as it stimulates gamma interferon production by natural killer (NK) cells and promotes type 1 immune responses and classical macrophage activation (20). IL-6 is known to play both early and late roles at the site of infection/inflammation (17). Thus, in the absence of IL-6, mice are highly susceptible to infection, as seen with a variety of organisms, including Listeria monocytogenes (21, 22), Candida albicans (23), and Toxoplasma gondii (24). However, IL-6 is also necessary to downregulate ongoing inflammatory responses by inhibiting the production of tumor necrosis factor alpha (TNF-α) and IL-1 by macrophages (25–28).
Acanthamoeba can grow axenically under laboratory conditions. However, this can lead to the loss of virulence factors, an encystment capability, and reduced susceptibility to drugs (29). Therefore, we compared the ability of the A. castellanii Neff strain, a classical laboratory strain, to that of an A. castellanii strain isolated from a case of bilateral keratitis to induce macrophage cytokine production. This clinical strain of Acanthamoeba was chosen as it is known to be capable of infecting immunocompetent hosts. Interestingly, while the clinical and Neff strains induced macrophage IL-12 and IL-6 production with similar kinetics, macrophages incubated with clinical isolate trophozoites produced significantly lower levels of cytokines than macrophages incubated with Neff strain trophozoites under similar conditions. While it could be argued that these results are predictable, as limiting the production of proinflammatory cytokines would enhance the virulence of a pathogen, it would require a more comprehensive study comparing a number of clinical and laboratory isolates of the T4 genotype to establish such a relationship. The lower levels of cytokine production by macrophages incubated with the clinical strain could have been due to the induction of comparatively more damage to macrophages by this strain than by the Neff strain, although this was not investigated in the present study. Certainly, real-time imaging experiments indicated differences in macrophage responses to the pathogenic and nonpathogenic Acanthamoeba strains. In particular, the results indicate that macrophages respond to trophozoites of the clinical isolate in a contact-dependent manner not observed with the laboratory strain, with some cytolysis being observed and phagocytosis being attempted.
When all of this information is considered, our data demonstrate that Acanthamoeba trophozoites induce the production of both IL-12 and IL-6 by murine macrophages. The higher levels of production were observed at 24 h after coincubation, in accordance with their role in modulating immune responses, at the highest coincubation ratios. In contrast, a recent study from our laboratories has demonstrated that Acanthamoeba trophozoites of the clinical strain fail to induce proinflammatory cytokines, such as TNF-α, IL-6, and IL-12, by human monocyte-derived macrophages. This discrepancy could be explained by differences between murine and human cells or the experimental techniques optimized for each cell type. This would include differences in the numbers of trophozoites used in the coincubation models, as well as the time of observations, each of which was optimized to allow coculture with minimal cell death but with a sufficient interaction to induce measurable levels of cytokines (30). In addition, we also investigated whether conditioned medium derived from Acanthamoeba cultures could mimic some of the effects observed on human cells (30, 31), but we found conditioned medium to be less effective with murine cells. These differences could be species dependent or due to differences in culture conditions, including medium components, such as the concentration of heat-inactivated fetal bovine serum.
The role of innate immune receptors, such as TLRs, during protist infections has been widely described. TLR2 and TLR4 are the main TLRs involved in the recognition of parasitic protists, such as Leishmania spp., Trypanosoma cruzi, T. gondii, Plasmodium falciparum, and Entamoeba histolytica. Studies have demonstrated that Acanthamoeba trophozoites can activate TLR4 expressed on corneal epithelial cells, inducing proinflammatory cytokines and chemokines at the ocular surface (10, 32). The activation of TLRs at the ocular surface by Acanthamoeba and the release of cytokines and chemokines can be the triggering events for the recruitment of innate immune cells, such as macrophages and neutrophils (33).
The results described herein demonstrate that recognition of Acanthamoeba trophozoites is predominately through TLR4, which induces MyD88-dependent signaling with a small contribution of TRIF-dependent signaling, culminating in cytokine production. This is in accordance with the unique ability of TLR4 to induce both MyD88-dependent and TRIF-dependent signaling pathways (34). Our study demonstrates that TLR4 is the main receptor involved in the recognition of and response to Acanthamoeba trophozoites in macrophages, and this is in agreement with the findings of studies utilizing corneal epithelial cells (10, 32).
The results obtained suggest that Acanthamoeba might present on its surface molecules that are recognized by TLR4, thus inducing an innate immunological response. Glycosylphosphatidylinositol anchors are highly expressed in several parasitic protists (35) and are highly immunogenic, inducing a response by cells of both the myeloid and lymphoid lineages (36). These structures are recognized by TLRs, mainly TLR2 and TLR4, and are therefore considered protist PAMPs (37). According to an early study, 31% of the mass of the plasma membrane of A. castellanii is composed of lipophosphoglycans (LPG) (38). These data have been confirmed and further characterized by a more recent study using gas chromatography-mass spectrometry techniques, where the chemical nature of acanthamoebic LPG was identified and recognized to be glycoinositolphosphosphingolipids (GIPSL) (39). Although it has yet to be confirmed, these structural moieties are strong candidates to be the Acanthamoeba-associated molecular patterns that stimulate the TLRs expressed on macrophages.
The studies described herein used murine bone marrow-derived macrophages, as these can consistently be generated in high numbers from both wild-type and gene-deficient mice. The results are intuitively relevant to systemic infections and most tissues where macrophages are known to be resident. Although macrophages are known to be present within the eye, they are predominantly found adjacent to the pigment epithelium of the iris, in the ciliary body, and in the retina. It was previously thought that antigen-presenting cells, such as macrophages and dendritic cells, are generally not present in the cornea, which is the site of AK. However, a series of recent studies has demonstrated the presence of resident macrophages, albeit in low numbers, within both murine and human corneas, and these studies are reviewed elsewhere (40). Furthermore, these macrophages have been found to be important in a murine model of Pseudomonas aeruginosa infection. Thus, F4/80+ cells present in the corneal stroma expressing TLR4, TLR5, and TLR2 were found to respond to Pseudomonas by releasing cytokines in a TLR4/TIRAP/MyD88 and TLR4/TRIF–NF-κB translocation-dependent manner (41). Thus, the studies described herein could also have relevance to AK.
In conclusion, we have demonstrated that Acanthamoeba interacts with and activates macrophages, inducing the production of IL-12 and IL-6 in a time- and density-dependent manner. Furthermore, the clinical isolate examined in this study induces lower levels of cytokine production by macrophages than the classical laboratory strain Neff. Both strains induce IL-12 and IL-6 production by macrophages through a predominantly TLR4- and MyD88-dependent mechanism. The study of the immunological mechanisms involved in these rare but insidious infectious diseases is essential to develop appropriate therapeutic strategies. Indeed, the modulation of receptor activity and of signaling pathways at the site of infection might help not only control infection but also mitigate overexuberant immune/inflammatory responses.
MATERIALS AND METHODS
Acanthamoeba castellanii cultures.
Trophozoites of the A. castellanii Neff strain, a classical laboratory strain isolated from soil over 60 years ago and kindly donated by the late Keith Vickerman (Glasgow, United Kingdom), and an A. castellanii genotype T4 strain isolated in 1992 from a patient affected by bilateral keratitis in Ancora, Italy (clinical isolate) (31, 42–44), were used in this study. Both strains were grown in a medium consisting of 0.9% (wt/vol) d-(+)-maltose monohydrate (95%; AlfaAesar, Heysham UK) and 2% (wt/vol) mycological peptone (Oxoid, Basingstoke, UK) supplemented with 125 μg/ml of penicillin-streptomycin (Sigma Chemical Co., Poole, UK), and the Neff strain was also treated with 125 μg/ml amphotericin B (Sigma Chemical Co., Poole, UK). Trophozoites were cultured in 75-cm2 tissue culture flasks (Corning, NY, USA) and incubated at room temperature. They were used when confluent and harvested by mechanical detachment.
Culture of BDM macrophages.
Bone marrow-derived (BMD) macrophages, obtained from the femurs of 7-week-old male BALB/c or C57BL/6 mice deficient in Toll-like receptor 4 (TLR4−/−), Toll like receptor 2 (TLR2−/−), Toll like receptor 2/4 (TLR2/4−/−), the myeloid differentiation primary response gene 88 (MyD88 −/−), or the TIR domain-containing adapter-inducing beta interferon (TRIF−/−) and their wild-type counterparts, were used to perform the experiments. Briefly, bone marrow stem cells were flushed from the femur with 5 ml of Dulbecco's modified Eagle medium (DMEM; Life Technologies, Paisley, UK) supplemented with 20% (vol/vol) heat-inactivated fetal calf serum (HI-FCS; Biosera, Sussex, UK), 30% (vol/vol) L-cell-conditioned medium, 2 mM l-glutamine (Sigma Chemical Co., Poole, UK), 125 U/ml penicillin, and 125 μg/ml streptomycin and grown for 10 days at 37°C in a 5% CO2 atmosphere. L-cell-conditioned medium was obtained by harvesting the metabolized medium from cultured cells of the murine fibroblastic cell line L-929. This conditioned medium provides a source of macrophage colony-stimulating factor (M-CSF), necessary for the growth and differentiation of bone marrow stem cells into mature macrophages (45).
Coincubation of BMD macrophages with A. castellanii trophozoites.
At day 10, macrophages were harvested with RPMI 1640 medium (Lonza BioWhittaker, Verviers, Belgium) and centrifuged at 300 × g for 5 min. Pellets were resuspended in RPMI 1640 medium supplemented with 10% (vol/vol) HI-FCS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete RPMI 1640 medium [cRPMI]) and centrifuged at 300 × g for 5 min. The supernatants were discarded, and the macrophages were then resuspended in cRPMI and counted using a Neubauer chamber (Superior Marienfeld, Germany). Macrophage suspensions were prepared at the required density, seeded in the appropriate vessel, and incubated overnight at 37°C in 5% CO2 to allow the macrophages to adhere.
On the next day, trophozoites were harvested by mechanical detachment. Trophozoite suspensions were centrifuged at 360 × g for 10 min and subsequently washed once in sterile phosphate-buffered saline without Ca2+, Mg2+, or phenol red (PBS; Lonza, Walkersville, MD, USA) (360 × g, 5 min) and once in cRPMI (360 × g, 5 min). Trophozoites were then suspended in cRPMI and counted using the Neubauer chamber. Trophozoite suspensions were used for the coincubation experiments at specified ratios. Macrophages were coincubated with the trophozoite suspension in cRPMI of either the Neff strain or the clinical isolate.
Real-time microscopy of a coculture of murine BMD macrophages and trophozoites of A. castellanii at 37°C.
Murine macrophages were harvested and suspended to 7 × 105 cells/ml in cRPMI. Thirty microliters of the cell suspension was added into 3 channels of a μ-slide (vI0.4; ibidi GmbH, Martinsried, Germany). After replacing the lid to cover the reservoirs, the μ-slide was incubated at 37°C in 5% CO2 for 1 h 30 min to allow the macrophages to adhere. Afterwards, 60 μl of cRPMI was added to each well, and then the μ-slides were incubated at 37°C in 5% CO2 overnight. After incubation, the cRPMI was removed from the wells, and 30 μl of either Neff or clinical strain trophozoites (a suspension of 7 × 105 trophozoites/ml in cRPMI) was added. In the control channel (macrophages alone), 30 μl of cRPMI was applied; subsequently, 60 μl of cRPMI was added to all wells. Prepared μ-slides were then observed, using an inverted epifluorescence microscope (Eclipse TE300; Nikon) provided with a 37°C chamber that allowed maintenance of the samples at the appropriate temperature while images were acquired at a ×10, ×20, or ×40 magnification. After manual focusing, images were acquired every 30 s for a period of 1 h using the software MetaMorph (Molecular Devices, Sunnyvale, CA, USA). Thereafter, images were processed using the program Volocity (PerkinElmer, MA, USA).
Stimulation of BMD macrophages with specific agonists.
Lipopolysaccharide (LPS) from Salmonella enterica serotype Abortus Equi (Sigma Chemical Co., Poole, UK), the synthetic tripalmitoylated lipopeptide (PAM3CSK4; InvivoGen, San Diego, CA, USA), and synthetic double-stranded RNA polyinosinic-poly(C) [poly(I·C)] were used to stimulate macrophages via TLR4, TLR2, and TLR3, respectively, as positive controls. The final concentrations used in the wells were 200 ng/ml for LPS, 320 ng/ml for PAM3CSK4, and 10 μg/ml for poly(I·C).
Quantitative analysis of murine cytokine production.
At specific time points after coincubation, plates were centrifuged at 480 × g for 1 min, supernatants were collected, and an enzyme-linked immunosorbent assay (ELISA) was performed, using paired purified rat anti-mouse immunoglobulin primary antibody and secondary biotin rat anti-mouse immunoglobulin antibody (BD Bioscience), to determine the concentrations of IL-12 p40/p70 and IL-6 released by murine macrophages. The optical density (OD) at 405 nm of each well was determined using a SpectraMax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA) was used to obtain the values of OD and to calculate the cytokine concentration in relation to the standard curve (BD Bioscience). Cytokine concentrations were expressed in nanograms per milliliter.
Statistical analyses.
Experiments were performed in triplicate and repeated twice. Data are shown as the mean ± standard error of the mean (SEM) from 3 replicates. Statistical analyses were performed using the GraphPad Prism (version 5) program. Data that were normally distributed were analyzed using the parametric statistical tests one-way analysis of variance (ANOVA) and Student's t test according to the nature of the experiments and of the hypothesis to be investigated. One-way ANOVA was used to analyze statistical significance under several conditions (more than two), and post hoc tests were applied to set all pairwise comparisons (Tukey's test) and to compare each condition mean with the control (Dunnett's test). Student's t test was applied to evaluate significant differences between two conditions. Differences with a P value of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Andrew MacDonald, Lauren Webb for providing access to bone marrow from MyD88- and TRIF-knockout mice, and Clare Bryant and Pani Tourlomousis for providing access to bone marrow from the various TLR-knockout mice.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.01054-16.
REFERENCES
- 1.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cursons RT, Brown TJ, Keys EA, Moriarty KM, Till D. 1980. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun 29:401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederkorn JY. 2006. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol 7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Cursons RT, Brown TJ, Keys EA, Moriarty KM, Till D. 1980. Immunity to pathogenic free-living amoebae: role of cell-mediated immunity. Infect Immun 29:408–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurt M, Apte S, Leher H, Howard K, Niederkorn J, Alizadeh H. 2001. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun 69:2988–2995. doi: 10.1128/IAI.69.5.2988-2995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knickelbein JE, Kovarik J, Dhaliwal DK, Chu CT. 2013. Acanthamoeba keratitis: a clinicopathologic case report and review of the literature. Hum Pathol 44:918–922. doi: 10.1016/j.humpath.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Larkin DFP, Easty DL. 1991. Experimental Acanthamoeba keratitis. II. Immunohistochemical evaluation. Br J Ophthalmol 75:421–424. doi: 10.1136/bjo.75.7.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tripathi T, Smith AD, Abdi M, Alizadeh H. 2012. Acanthamoeba-cytopathic protein induces apoptosis and proinflammatory cytokines in human corneal epithelial cells by cPLA 2α activation. Invest Ophthalmol Vis Sci 53:7973–7982. doi: 10.1167/iovs.12-10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alizadeh H, Tripathi T, Abdi M, Smith AD. 2014. Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea. PLoS One 9:e92375. doi: 10.1371/journal.pone.0092375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi T, Abdi M, Alizadeh H. 2014. Protease-activated receptor 2 (PAR2) is upregulated by Acanthamoeba plasminogen activator (aPA) and induces proinflammatory cytokine in human corneal epithelial cells. Invest Ophthalmol Vis Sci 55:3912–3921. doi: 10.1167/iovs.14-14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon S, Plüddemann A, Martinez Estrada F. 2014. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013. Tissue-resident macrophages. Nat Immunol 14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. 2013. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol 29:181–187. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Pan H, Wu X. 2012. Hypoxia attenuates inflammatory mediators production induced by Acanthamoeba via Toll-like receptor 4 signaling in human corneal epithelial cells. Biochem Biophys Res Commun 420:685–691. doi: 10.1016/j.bbrc.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 16.Thakur A, Willcox MDP. 2000. Contact lens wear alters the production of certain inflammatory mediators in tears. Exp Eye Res 70:255–259. doi: 10.1006/exer.1999.0767. [DOI] [PubMed] [Google Scholar]
- 17.Ma X. 2001. TNF-α and IL-12: a balancing act in macrophage functioning. Microbes Infect 3:121–129. doi: 10.1016/S1286-4579(00)01359-9. [DOI] [PubMed] [Google Scholar]
- 18.Jones SA. 2005. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol 175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 19.Biron CA, Gazzinelli RT. 1995. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr Opin Immunol 7:485–496. doi: 10.1016/0952-7915(95)80093-X. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 22.Dalrymple SA, Lucian LA, Slattery R, McNeil T, Aud DM, Fuchino S, Lee F, Murray R. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun 63:2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. 1996. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med 183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jebbari H, Roberts CW, Ferguson D, Bluethmann H, Alexander J. 1998. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol 20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 25.Aderka D, Le J, Vilcek J. 1989. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol 143:3517–3523. [PubMed] [Google Scholar]
- 26.Akira S, Hirano T, Taga T, Kishimoto T. 1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J 4:2860–2867. [PubMed] [Google Scholar]
- 27.Ulich TR, Yin S, Guo K, Yi ES, Remick D, Del Castillo J. 1991. Intratracheal injection of endotoxin and cytokines. Am J Pathol 138:1097–1101. [PMC free article] [PubMed] [Google Scholar]
- 28.Tilg H, Dinarello CA, Mier JW. 1997. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today 18:428–432. doi: 10.1016/S0167-5699(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 29.Koehsler M, Leitsch D, Duchêne M, Nagl M, Walochnik J. 2009. Acanthamoeba castellanii: growth on human cell layers reactivates attenuated properties after prolonged axenic culture. FEMS Microbiol Lett 299:121–127. doi: 10.1111/j.1574-6968.2009.01680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattana A, Sanna M, Cano A, Delogu G, Erre G, Roberts CW, Henriquez FL, Fiori PL, Cappuccinelli P. 2016. Acanthamoeba castellanii genotype T4 stimulates the production of interleukin-10 as well as proinflammatory cytokines in THP-1 cells, human peripheral blood mononuclear cells and human monocyte-derived macrophages. Infect Immun 84:2953–2962. doi: 10.1128/IAI.00345-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattana A, Cappai V, Alberti L, Serra C, Fiori PL, Cappuccinelli P. 2002. ADP and other metabolites released from Acanthamoeba castellanii lead to human monocytic cell death through apoptosis and stimulate the secretion of proinflammatory cytokines. Infect Immun 70:4424–4432. doi: 10.1128/IAI.70.8.4424-4432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren M, Gao L, Wu X. 2010. TLR4: the receptor bridging Acanthamoeba challenge and intracellular inflammatory responses in human corneal cell lines. Immunol Cell Biol 88:529–536. doi: 10.1038/icb.2010.6. [DOI] [PubMed] [Google Scholar]
- 33.Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S. 2011. Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond) 120:441–450. doi: 10.1042/CS20100425. [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ropert C, Gazzinelli RT. 2000. Signaling of immune system cells by glycosylphosphatidylinositol (GPI) anchor and related structures derived from parasitic protozoa. Curr Opin Immunol 3:395–403. [DOI] [PubMed] [Google Scholar]
- 36.Uematsu S, Akira S. 2008. Toll-like receptors (TLRs) and their ligands. Handb Exp Pharmacol 2008:1–20. [DOI] [PubMed] [Google Scholar]
- 37.Kumar H, Kawai T, Akira S. 2011. Pathogen recognition by the innate immune system. Int Rev Immunol 30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 38.Korn ED, Dearborn DG, Wright PL. 1974. Lipophosphonoglycan of the plasma membrane of Acanthamoeba castellanii. Isolation from whole amoebae and identification of the water-soluble products of acid hydrolysis. J Biol Chem 249:3335–3341. [PubMed] [Google Scholar]
- 39.Karaś MA, Russa R. 2013. New long chain bases in lipophosphonoglycan of Acanthamoeba castellanii. Lipids 48:639–650. doi: 10.1007/s11745-013-3794-2. [DOI] [PubMed] [Google Scholar]
- 40.Pearlman E, Sun Y, Roy S, Karmakar M, Hise AG, Szczotka-Flynn L, Ghannoum M, Chinnery HR, McMenamin PG, Rietsch A. 2013. Host defense at the ocular surface. Int Rev Immunol 32:4–18. doi: 10.3109/08830185.2012.749400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E. 2010. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol 185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattana A, Tozzi MG, Costa M, Delogu G, Fiori PL, Cappuccinelli P. 2001. By releasing ADP, Acanthamoeba castellanii causes an increase in the cytosolic free calcium concentration and apoptosis in wish cells. Infect Immun 69:4134–4140. doi: 10.1128/IAI.69.6.4134-4140.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattana A, Alberti L, Delogu G, Fiori PL, Cappuccinelli P. 2009. In vitro activity of Acanthamoeba castellanii on human platelets and erythrocytes. Infect Immun 77:733–738. doi: 10.1128/IAI.00202-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriquez FL, Campbell SJ, Sundararaj BK, Cano A, Muench SP, Roberts CW. 2015. The Acanthamoeba shikimate pathway has a unique molecular arrangement and is essential for aromatic amino acid biosynthesis. Protist 166:93–105. doi: 10.1016/j.protis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Menzies FM, Henriquez FL, Alexander J, Roberts CW. 2010. Sequential expression of macrophage anti-microbial/inflammatory and wound healing markers following innate, alternative and classical activation. Clin Exp Immunol 160:369–379. doi: 10.1111/j.1365-2249.2009.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.