ABSTRACT
Pertussis (whooping cough), caused by Bordetella pertussis, is resurging in the United States and worldwide. Adenylate cyclase toxin (ACT) is a critical factor in establishing infection with B. pertussis and acts by specifically inhibiting the response of myeloid leukocytes to the pathogen. We report here that serum components, as discovered during growth in fetal bovine serum (FBS), elicit a robust increase in the amount of ACT, and ≥90% of this ACT is localized to the supernatant, unlike growth without FBS, in which ≥90% is associated with the bacterium. We have found that albumin, in the presence of physiological concentrations of calcium, acts specifically to enhance the amount of ACT and its localization to the supernatant. Respiratory secretions, which contain albumin, promote an increase in amount and localization of active ACT that is comparable to that elicited by serum and albumin. The response to albumin is not mediated through regulation of ACT at the transcriptional level or activation of the Bvg two-component system. As further illustration of the specificity of this phenomenon, serum collected from mice that lack albumin does not stimulate an increase in ACT. These data, demonstrating that albumin and calcium act synergistically in the host environment to increase production and release of ACT, strongly suggest that this phenomenon reflects a novel host-pathogen interaction that is central to infection with B. pertussis and other Bordetella species.
KEYWORDS: Bordetella pertussis, RTX toxins, adenylate cyclase toxin, albumin, calcium
INTRODUCTION
Pertussis (whooping cough) is a respiratory illness caused by Bordetella pertussis that can be life-threatening, especially in infants. In 2012, the number of cases of whooping cough in the United States was the highest since 1960 despite high vaccine coverage (1). Limited duration of protection by acellular pertussis vaccines is a major factor in the resurgence of pertussis (2), and one approach to this problem is reformulation of the acellular vaccines to include additional B. pertussis antigens (3). Adenylate cyclase toxin (ACT) is a critical factor in establishing infection with B. pertussis and a documented protective antigen (4–6). In light of these features, this toxin is a leading candidate for inclusion in new acellular pertussis vaccines (7, 8).
ACT is a single polypeptide of 1,706 amino acid residues that constitute a protein of ∼200 kDa (9–12). ACT belongs to the RTX (repeats-in-toxin) family of proteins and has two activities (13). The toxin function involves insertion of the adenylate cyclase (AC) catalytic domain into the cytoplasm of host cells, activation by the host protein calmodulin, and conversion of intracellular ATP into cyclic AMP (cAMP), resulting in dysregulation of signaling processes and depletion of ATP in the intoxicated cell (14, 15). At higher concentrations, ACT undergoes oligomerization to form pores in the target cell membrane (15–17); these pores are responsible for hemolysis during B. pertussis growth on Bordet-Gengou agar (18). The repeat regions in the RTX domain bind calcium, and calcium binding induces conformational changes that are critical for efficient secretion and toxin activity (19–24). ACT uses the β2 integrin, CD11b/CD18, as its receptor (25, 26), suggesting that a primary role for ACT in the pathogenesis of pertussis is inhibition of the functions of CD11b/CD18-expressing myeloid leukocytes (including neutrophils, monocytes, macrophages, and dendritic cells), which are involved in the clearance of B. pertussis (26–29).
ACT is encoded within the cya operon, which includes the structural gene cyaA, as well as cyaB, cyaD, and cyaE encoding the type 1 secretion system (T1SS) by which it is secreted, the acyl transferase gene cyaC, which is responsible for posttranslational acylation, and cyaX, of unknown function but annotated as a LysR family transcriptional regulator (13, 30, 31). Expression of cyaA, as well as other B. pertussis factors critical to establishing infection, is controlled by the Bvg two-component regulatory system, which has been described as the master transcriptional regulator of virulence in Bordetella species (32–34). To initiate transcription of the Bvg regulon, the sensor kinase BvgS phosphorylates the response regulator BvgA, which binds to promoter elements upstream of target genes (35–38). The default operation of this regulatory system appears to be in the “on” position, since the only signals that have been identified are those that decrease expression of target genes: for example magnesium sulfate (MgSO4) or a shift in temperature from 37°C to 25°C (39). A Bvg-activated state, promoting transcription of cyaA, is required and sufficient for infection (40).
During in vitro growth in Stainer-Scholte medium (SSM), ACT is primarily associated with the bacterial cell surface (12, 41–43); however, we showed previously that it is the released, not surface-associated, ACT that is responsible for increasing cAMP and causing cytotoxicity (44). This is consistent with the study of nasal washes from humans with pertussis and from infected baboons showing that virtually all of the ACT is in the released form in vivo (45). These observations together support the concept that released ACT is the active and most relevant molecule during infection and suggest that the in vitro conditions currently in use to study ACT and other components of pertussis pathogenesis are not representative of the environment within the host. While seeking to understand the differences between culture conditions in vitro and the environment within the host respiratory tract, we noted that ACT is also primarily in the supernatant when B. pertussis is studied in vitro with eukaryotic cells (45). Under these conditions, B. pertussis is exposed to tissue culture medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). Since serum components are present in respiratory secretions, this led us to hypothesize that one or more molecules in serum promote ACT release into the culture medium. The testing of this hypothesis yielded the data presented herein.
RESULTS
Serum increases the amount of ACT and shifts localization to the supernatant.
We tested our hypothesis that serum promotes an increase in extracellular ACT by measuring the amount of ACT in cultures of wild-type (WT) B. pertussis strain UT25 grown in Stainer-Scholte medium (SSM) with and without 10% FBS for 8 h. The amount and relative distribution of ACT were determined by enzyme activity using a cell-free assay measuring conversion of [32P]ATP to [32P]cAMP (10, 46) (Fig. 1A), and relative differences in protein amount were confirmed by Western blotting with a polyclonal rabbit anti-ACT antibody recognizing the full-length, 200-kDa protein (Fig. 1B). We have shown previously that this adenylate cyclase (AC) assay is highly sensitive and quantitative, with a linear range of 0.064 to 80 ng/ml (45). When necessary, samples were diluted to be within this range of toxin concentrations. In addition, we confirmed that the presence of FBS had no effect on enzymatic activity of purified ACT (data not shown). As seen previously, ≥90% of the total ACT is associated with the bacterium during growth in SSM (41); however, during growth in the presence of FBS, 90% of this ACT was located in the supernatant (Fig. 1A), similar to published observations about the distribution of ACT in vivo (45). In addition, there was an unexpected 12.6-fold increase in the amount of ACT during growth with FBS relative to absence of serum (Fig. 1A), resulting in concentrations equivalent to 2 μg/ml ACT in the supernatant at 8 h. These data support our hypothesis that serum components change the distribution of ACT and also reveal that they stimulate an increase in the amount of ACT.
FIG 1.
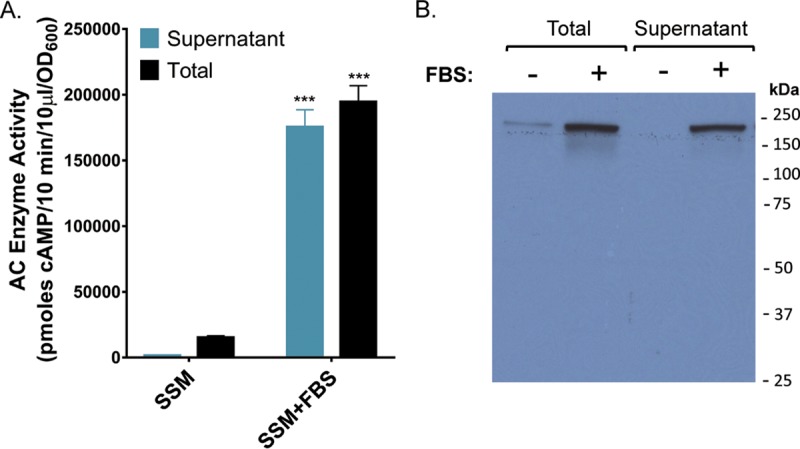
Serum elicits a massive increase in the amounts of total and extracellular B. pertussis ACT. B. pertussis UT25 was grown in SSM ± 10% FBS for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. (A) AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. Data represent the mean ± standard deviation (SD) from 9 independent experiments. A Student's t test was used to determine statistical significance. ***, P ≤ 0.0001. (B) Western blot analysis using a rabbit polyclonal anti-ACT antibody detecting the 200-kDa ACT protein.
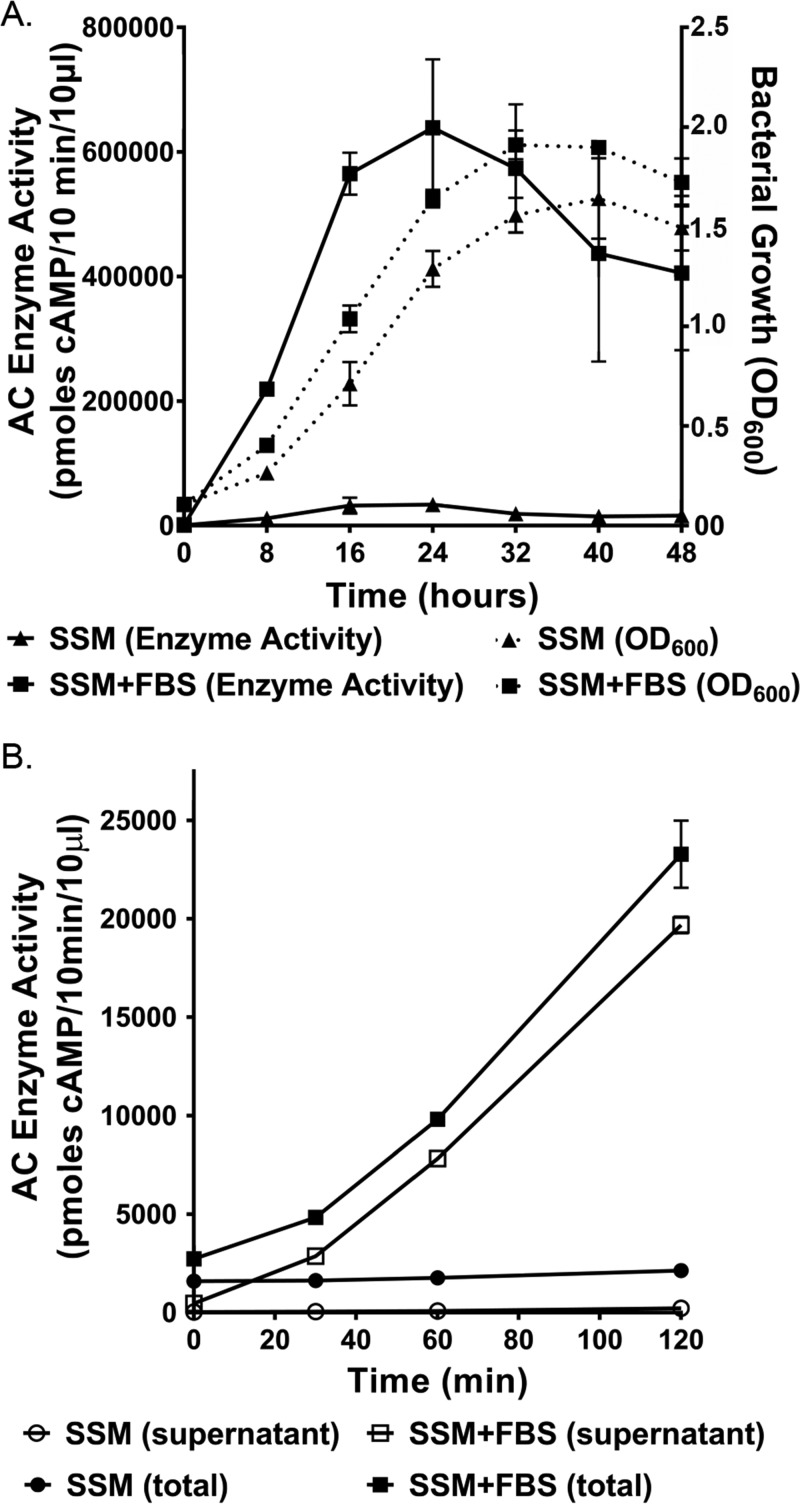
To understand whether there are differences in growth ± FBS that could account for ACT amount and localization, we grew B. pertussis UT25 in SSM ± 10% FBS and measured bacterial growth by optical density at 600 nm (OD600) every 8 h for 2 days. The bacterial densities were significantly higher between 8 to 32 h when B. pertussis was grown in SSM plus FBS versus SSM alone (Fig. 2A). Because of this difference in growth, quantities of ACT measured by enzyme activity presented herein, except for Fig. 2, are normalized to bacterial density (OD600). Importantly, the quantity of ACT peaked at 24 h in the presence or absence of FBS, but that amount was nearly 30-fold greater at this time point for B. pertussis grown with serum versus without (Fig. 2A). The response to serum is rapid, including even modest increases at the zero time point (Fig. 2B), most likely due to the ∼10-min sample processing time. By 30 min, there is a 3-fold increase in the amount of toxin, and 59% of the total ACT is in the supernatant. By 120 min, there is a 10.9-fold increase in the amount of ACT during growth in SSM plus FBS versus SSM alone, and 85% of the total ACT is in the supernatant. These early increases occur with minimal changes in bacterial density. Together, these results show that the response to serum is rapid, robust, and peaks near the end of the logarithmic phase of B. pertussis growth.
FIG 2.
The response to serum is rapid and peaks at 24 h of growth. B. pertussis UT25 was grown in SSM ± 10% FBS, and samples were taken at the indicated time points. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods. (A) The data represent the mean ± SD from 3 independent experiments. Comparisons between enzyme activity ± FBS at all time points are statistically significant as determined by an unpaired t test (P ≤ 0.05). Comparisons between bacterial density ± FBS were statistically significant (P ≤ 0.05) at 8, 16, 24, and 32 h. (B) Data represent the mean ± SD from 2 independent experiments done in duplicate.
We tested multiple wild-type and mutant strains of B. pertussis, B. parapertussis, and B. bronchiseptica to determine if the response to serum is conserved. All strains were grown in SSM or SSM plus 10% FBS for 8 h. ACT was detected by measuring AC enzyme activity, and the percentage of activity in the supernatant and fold increase in total ACT were determined (Table 1). Seven strains of B. pertussis (all strains tested except a Bvg− strain, discussed further below) responded to serum by increasing the amount of ACT and releasing the majority of the ACT to the supernatant. B. pertussis strain UT25 makes approximately 10× more ACT than the B. pertussis reference strain Tohama, and the elevated level of ACT produced by UT25 is more comparable to the level of ACT produced by recent clinical isolates tested in our laboratory (data not shown). For this reason, B. pertussis UT25 was used for the majority of the experiments presented herein. As shown previously (43), B. pertussis strains deficient in the adhesin filamentous hemagglutinin (FHA [encoded by fhaB]) have a higher percentage of ACT present in the supernatant during growth in SSM (Table 1). We found, however, that B. pertussis BP353, a BP338 derivative with a Tn5 insertion in fhaB, still responded to FBS: the amount of ACT increased 9.2-fold, and 100% of ACT was present in the supernatant. B. parapertussis CN8234 responded to serum comparably to B. pertussis, with a 7.2-fold increase in total ACT and a shift from 16% to 88% in the supernatant (Table 1). Strains of B. bronchiseptica have a higher percentage of ACT localized to the supernatant during growth in SSM compared to strains of B. pertussis, but both the total amount (3.1-fold or 5.1-fold depending on the strain) and percentage of ACT in the supernatant are enhanced by serum. As might be expected, basal levels of ACT in the supernatant in the absence of FBS are higher in the B. bronchiseptica fhaB deletion strain RBX9 than in its wild-type parent strain, RB50, but the levels of total and supernatant-localized ACT were further enhanced during growth in the presence of serum, consistent with data from the B. pertussis BP353 strain. These findings demonstrate that the response to serum is conserved among B. pertussis, B. parapertussis, and B. bronchiseptica and also indicate that strains secreting higher levels of ACT under basal conditions still respond to serum by further increasing the amount and proportion of ACT in the supernatant.
TABLE 1.
Response to serum is conserved among the B. pertussis, B. parapertussis, and B. bronchiseptica strains tested in this study
| Strain | Description (reference) | % AC enzyme activity in supernatant |
Fold increase in AC enzyme activity | |
|---|---|---|---|---|
| SSM | SSM + FBS | |||
| B. pertussis | ||||
| UT25 | Clinical isolate (73) | 10 | 90 | 12.6 |
| Tohama | WHO reference strain (83) | 13 | 99 | 5.2 |
| BP338 | Laboratory strain (84) | 8 | 100 | 12.6 |
| BP347 | BP338 bvgA::Tn5 (84) | NDa | ND | ND |
| BP353 | BP338 fhaB::Tn5 (84) | 34 | 100 | 9.2 |
| BPSM | Laboratory strain (85) | 23 | 90 | 10.2 |
| V252 | Clinical isolate | 14 | 92 | 12.8 |
| D420 | Clinical isolate (86) | 9 | 100 | 5.5 |
| B. parapertussis | ||||
| CN8234 | Clinical isolate (87) | 16 | 88 | 7.2 |
| B. bronchiseptica | ||||
| RB50 | Laboratory strain (40) | 61 | 99 | 3.1 |
| RBX9 | RB50 ΔfhaB (88) | 88 | 99 | 3.8 |
| 1289 | Hypervirulent isolate (89) | 64 | 95 | 5.2 |
ND, none detected.
Albumin and calcium act synergistically to increase the amount and distribution of ACT to the supernatant.
To determine which components in serum promote the observed changes in the amount and distribution of ACT, we performed preliminary fractionation of serum using spin columns with different size exclusions (≤10, ≤50, and ≥50 kDa). B. pertussis UT25 was then cultured in SSM with the individual serum fractions for 8 h as indicated in Table 2. ACT was detected by measuring AC enzyme activity, and the percentage of activity in the supernatant and fold increase in total ACT were determined. The responses to the ≤10- and ≤50-kDa fractions, as reflected by percentage of ACT in the supernatant and fold increases in ACT, were comparable, suggesting that active component or components in these two fractions are less than 10 kDa in size. There was partial activity in each of the three fractions, and the majority of the response to serum was recapitulated by recombining the ≤50- and ≥50-kDa fractions (Table 2). These experiments suggested that the combined activity of at least two factors in serum was responsible for the observed effects on ACT amount and localization.
TABLE 2.
ACT production during growth of B. pertussis UT25 with crude fractions of FBS
| Growth condition | % AC enzyme activity in supernatant | Fold increase in AC enzyme activity |
|---|---|---|
| SSM | 6.0 ± 0.7 | |
| SSM + 10% FBS | 79.6 ± 2.2 | 10.7 ± 0.3 |
| SSM + ≤10-kDa fraction | 40.4 ± 2.4 | 1.3 ± 0.1 |
| SSM + ≤50-kDa fraction | 43.6 ± 1.6 | 1.8 ± 0.1 |
| SSM + ≥50-kDa fraction | 62.8 ± 0.3 | 3.6 ± 0.1 |
| SSM + ≤50-kDa fraction + ≥50-kDa fraction | 85.1 ± 0.9 | 8.3 ± 0.0 |
A recent publication by Bumba et al. elucidated the requirement for physiological concentrations of calcium (2 mM, equivalent to the concentration in human respiratory tract) to enhance secretion of ACT (24, 47). SSM contains 0.136 mM calcium, which is not sufficient for proper folding of ACT and thus results in less efficient secretion and accumulation of unfolded, secreted ACT on the bacterial surface (24). Bumba et al. showed that growth of B. pertussis Tohama in SSM (with 1 g/liter heptakis and 1 g/liter Casamino Acids) in the presence of 2 mM calcium allows for proper folding of ACT and increased efficiency of secretion by greater than 20-fold, with ∼95% of total ACT localized to the supernatant. There was, however, minimal effect on the total amount of ACT produced (24). Because of their findings, we hypothesized that calcium is the active molecule in the ≤10-kDa fraction. Under our growth conditions, there is an increase in the proportion of ACT released to the supernatant when B. pertussis is grown in SSM with 2 mM calcium compared to SSM alone (26.3% ±7.6% and 11.4% ±2.9%, respectively) but no increase in the total amount of ACT (Fig. 3).
FIG 3.
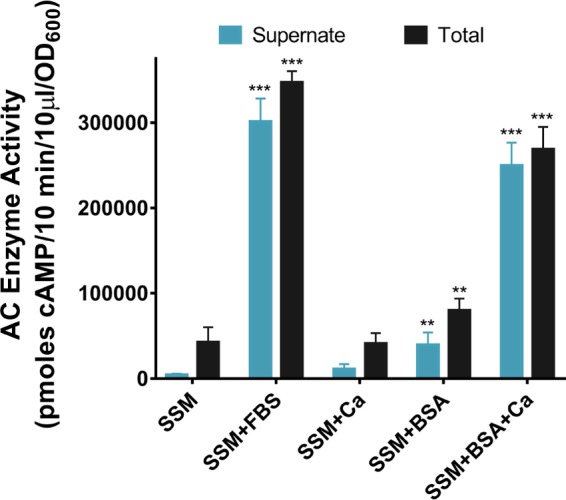
Albumin and calcium act synergistically to increase ACT production and secretion. B. pertussis UT25 was grown in SSM ± 2 mg/ml BSA, 2 mM CaCl2, and/or 10% FBS (as indicated) for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. Data represent the mean ± SD from 3 independent experiments done in duplicate. Statistical significance was assessed using a 2-way analysis of variance (ANOVA). **, P ≤ 0.01, and ***, P ≤ 0.001, compared to growth in SSM.
Recapitulation of the response to FBS by the ≤50- and ≥50-kDa fractions suggested a combined effect of at least two components, and we tested whether calcium and another molecule, >50 kDa, together affect the amount and distribution of ACT. Albumin has a molecular mass of 66 kDa and is the most abundant protein in serum, accounting for about 60% of the total serum protein, yielding concentrations ranging between 20 and 36 mg/ml in FBS and 35 and 52 mg/ml in human serum (HS) (48). Albumin is also present in respiratory secretions, and its abundance increases during inflammation (49–51). In addition, previous work by Bellalou et al. showed that growth of B. pertussis with albumin-supplemented SSM resulted in a high level of ACT in the supernatant (52). On the basis of this information, we asked whether albumin could be the active component in the ≥50-kDa fraction. When B. pertussis UT25 was grown with SSM plus 2 mg/ml bovine serum albumin (BSA) for 8 h, there was a 1.9-fold increase in the amount of ACT, and 50% of the total ACT was detected in the supernatant (Fig. 3). This suggested that BSA, or perhaps protein in general, shifts localization of ACT to the supernatant but not to the magnitude detected with FBS. Next, we tested BSA in combination with 2 mM calcium, and the results were striking: BSA and calcium together promoted a 6.3-fold increase in ACT production, and 93% of the ACT was localized to the supernatant (Fig. 3). We also tested whether contamination of purified albumin with calcium may contribute to the effect on ACT. We grew B. pertussis UT25 with BSA or EGTA-treated BSA, both in the absence of supplemented calcium, and compared the responses in total and released ACT. We found that there was no significant difference between BSA or EGTA-treated BSA (Fig. 4). These findings are consistent with our preliminary fractionation data implicating two separate components and suggest synergistic roles for calcium and albumin to enhance the amount of ACT and change its localization during growth with FBS.
FIG 4.
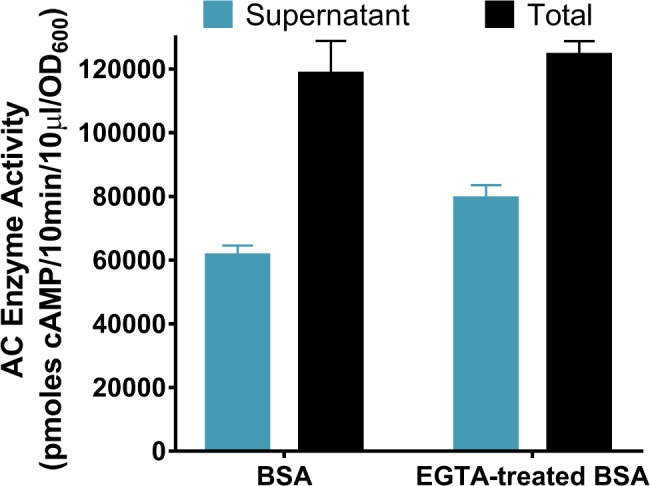
Albumin and calcium-stripped albumin have equivalent effects on ACT amount and release. B. pertussis UT25 was grown in SSM ± 2 mg/ml BSA or EGTA-treated BSA for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. Data represent the mean ± SD from 3 independent experiments done in duplicate. Statistical significance was assessed using a 2-way ANOVA, and comparisons between SSM plus BSA and SSM plus EGTA-treated BSA were not significantly different.
To determine what concentration of calcium is required for the full response to albumin, we grew B. pertussis UT25 in SSM plus 2 mg/ml BSA with various concentrations of calcium between 0.136 mM (present in SSM) and 2 mM. We identified that 0.5 mM calcium was sufficient to promote the full increase in amount of ACT and release by albumin (Fig. 5). Of note, SSM supplemented with 10% FBS contains about 0.47 mM calcium, which is close to our determined concentration of calcium required for the maximal effect of albumin. Because calcium is necessary for the response to BSA and the physiological concentration of calcium in the human respiratory tract is ∼2 mM, the remaining experiments in this article were performed in the presence of 2 mM calcium.
FIG 5.
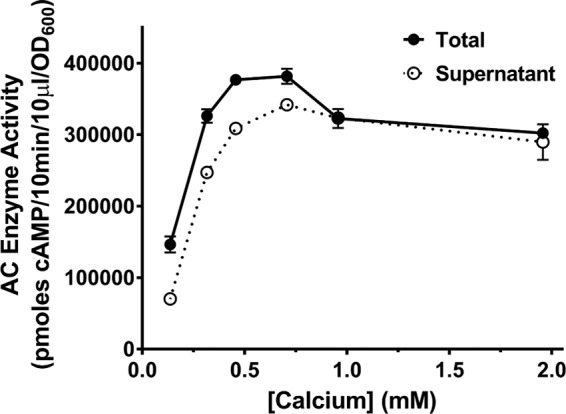
The maximum effect on ACT amount and release requires a minimum of 0.5 mM calcium. B. pertussis UT25 was grown in SSM with 2 mg/ml BSA with the indicated concentrations of total calcium for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. The data presented are the mean ± SD from a single experiment done in duplicate but representative of 4 similar experiments.
Albumin is required for the increased amount and altered distribution of ACT during growth in the presence of mouse serum.
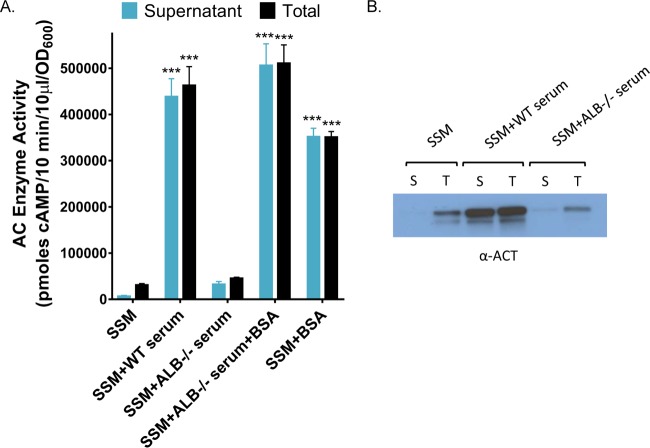
We next asked if albumin is both necessary and sufficient (the sole protein in serum required) for the effects on the amount of ACT. Roopenian et al. have developed an albumin-deficient (analbuminemic) mouse strain to study the metabolism of human albumin and the pharmacokinetics of albumin-conjugated drugs (53). Serendipitously, this animal provides a unique resource with which to probe the specificity of albumin in our observed effects on ACT. Similar to humans with a genetic deficiency in albumin, analbuminemic mice increase concentrations of other serum proteins to compensate for the loss of albumin (53). We utilized serum from these mice to investigate the role of albumin in alteration of the amount and distribution of ACT, adjusted to ensure comparable protein concentrations. Consistent with published data on this mouse strain (53), we determined that total protein in the analbuminemic (ALB−/−) mouse serum was 68% of that in serum from the wild-type C57BL/6 mouse and confirmed that the analbuminemic mouse serum lacked albumin, as demonstrated by SDS-PAGE and Coomassie straining (Fig. 6). Because of the difference in protein concentrations, we grew B. pertussis UT25 in the presence of 2 mM calcium and 6.8% wild-type mouse serum or 10% analbuminemic mouse serum, yielding equivalent total protein concentrations. The wild-type mouse serum enhanced the amount of ACT by 15.7-fold, and 90% of ACT was in the supernatant compared to 21% in SSM. We found that ACT levels in the total and supernatant fractions were not significantly different during growth with the analbuminemic mouse serum compared to growth in SSM alone (Fig. 7A and B). Since the amounts of total protein were equivalent under both conditions, these data indicate that the effects are specific to albumin and not simply a consequence of elevated protein concentrations during growth with serum or purified albumin. Further, the response was restored (16.4-fold increase and 99% of total ACT in the supernatant) when 2 mg/ml BSA, a concentration equivalent to amount of albumin present in wild-type mouse serum, was added to the analbuminemic serum (Fig. 7A). Together, these data strongly support the concept that albumin, in the presence of calcium, is responsible for the increase in the amount of ACT and shift to localization in the supernatant elicited by mouse serum, highlighting the key roles of these two molecules in regulating the availability of this critical bacterial virulence factor.
FIG 6.
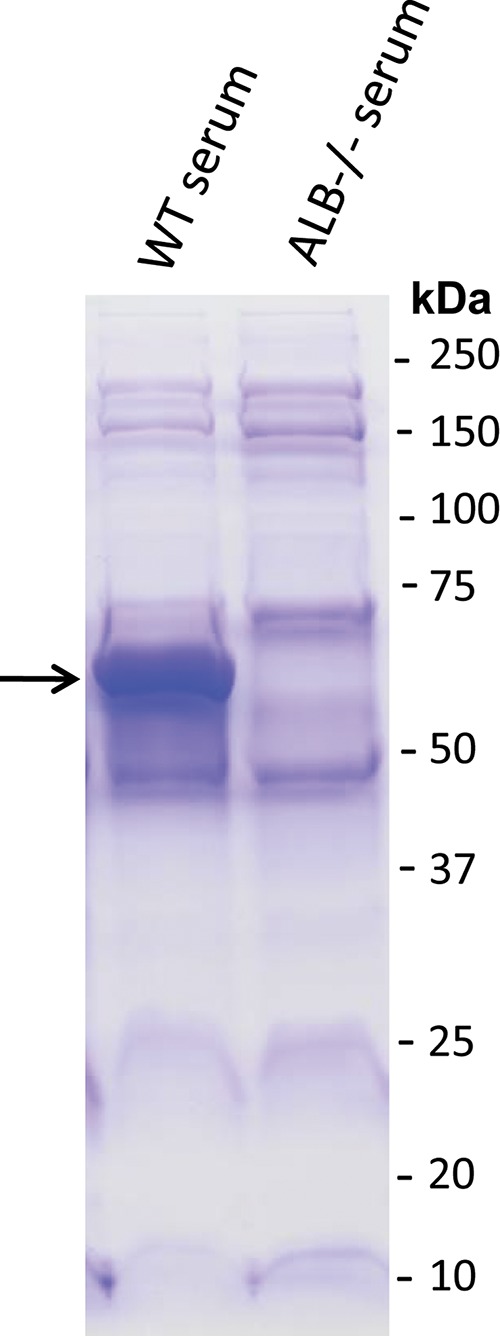
Serum collected from analbuminemic mice lacks albumin. Wild-type (WT) C56BL/6 mouse serum or ALB−/− mouse serum were analyzed by SDS-PAGE and Coomassie staining to detect protein profiles. The band corresponding to albumin in the wild-type sample is indicated with an arrow.
FIG 7.
Albumin is required for an increased amount of ACT during growth in the presence of mouse serum. (A) B. pertussis UT25 was grown in SSM ± wild-type C56BL/6 mouse serum or ALB−/− mouse serum ± 2 mg/ml BSA, all with 2 mM CaCl2, for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. Data represent the mean ± SD from 3 independent experiments done in duplicate. Statistical significance was assessed using a 2-way ANOVA. ***, P ≤ 0.001 compared to growth in SSM. (B) Western blot analysis using a rabbit polyclonal anti-ACT antibody detecting the 200-kDa ACT protein. Lanes: S, supernatant; T, total.
HSA, either purified or present in HS, increases the amount and release of ACT.
Because B. pertussis is a human pathogen, we next assessed whether albumin in human samples can influence the amount and localization of ACT. Human serum (HS) samples were combined from a pool of healthy donors. The concentration of albumin in the pool was determined by the Clinical Chemistry Lab at the University of Virginia to be 46 mg/ml. B. pertussis UT25 was grown for 8 h in SSM with HS, ranging from 0.05% to 2%, or the equivalent concentration of human serum albumin (HSA), ranging from 0.0225 to 0.92 mg/ml, all in the presence of 2 mM calcium. As shown in Fig. 8, there was an albumin-concentration-dependent response of B. pertussis UT25 to heat-inactivated HS or HSA. In the presence of 2 mM calcium, ≥85% of the total ACT was found in the supernatant at all concentrations of HSA and HS tested, suggesting that human albumin at very low concentrations (≤0.0225 mg/ml) is able to shift the predominant distribution of ACT to the supernatant (data not shown). In the presence of calcium, the quantity of ACT plateaued at albumin concentrations of ≥0.23 mg/ml albumin (Fig. 8). These data suggest that albumin, in the presence of calcium, is the critical component in human serum to increase the amount of ACT and alter its distribution.
FIG 8.
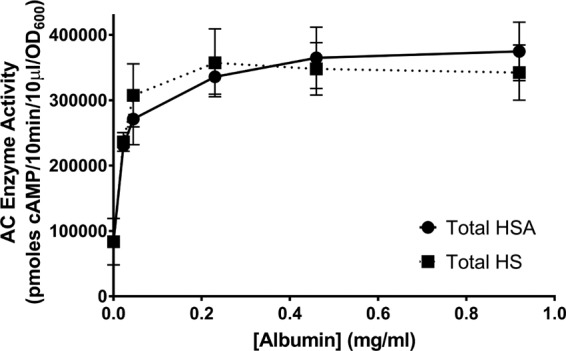
Human serum and human serum albumin increase the amount of ACT and shift localization of ACT to the supernatant. B. pertussis UT25 was grown in SSM ± HS or HSA (as indicated), all with 2 mM CaCl2, for 8 h. The total fraction includes both culture supernatant and bacterial cells. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. The data represent the mean ± SD from 2 independent experiments done in duplicate.
Role of Bvg two-component system in control of ACT expression in response to albumin.
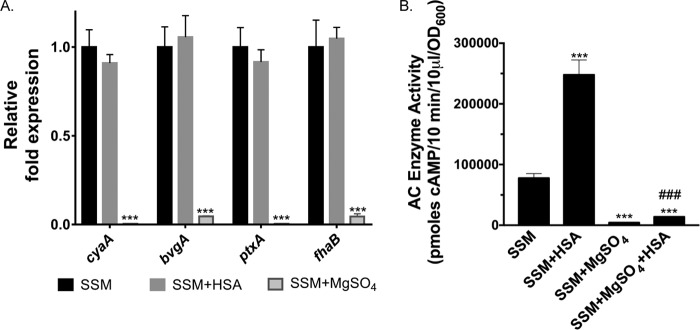
As with other virulence factors, ACT expression in Bordetella species is transcriptionally controlled by the Bvg two-component system. For that reason, we tested whether the increased amount of ACT detected during growth with albumin was due to higher levels of Bvg activation and enhanced transcription of cyaA. We performed quantitative reverse transcription-PCR (qRT-PCR) analyses on bvgA and cyaA as well as fhaB and ptxA, two other genes within the bvg regulon, which encode filamentous hemagglutinin and pertussis toxin, respectively. Expression of none of these genes was significantly different ± HSA in the presence of calcium (Fig. 9A), indicating that increased ACT during growth in the presence of albumin is not due to further activation of Bvg and that cyaA is not being regulated at the transcriptional level ± albumin. The Bvg+ state is, however, required for expression of ACT; when B. pertussis is modulated to Bvg−, either genetically by a transposon insertion in bvgA (Table 1) or chemically with 40 mM MgSO4 (Fig. 9B), virtually no ACT is detected either with or without HSA or FBS. There was a small but significant increase in the amount of ACT during growth with MgSO4 and HSA, when added simultaneously, compared to with MgSO4 alone (Fig. 9B); possible explanations include that the response to albumin is faster than modulation with MgSO4 or that albumin acts through an additional mechanism that is not dependent on Bvg activation and cyaA transcription. In summary, these data suggest that regulation of ACT production by albumin is downstream of transcriptional regulation of cyaA by the Bvg system, potentially through a previously uncharacterized posttranscriptional regulatory process.
FIG 9.
Regulation of cyaA during growth ± HSA is not occurring at the level of transcription through the Bvg two-component system. (A) RNA was isolated from B. pertussis UT25 grown in SSM ± 2 mg/ml HSA and/or 40 mM MgSO4 with 2 mM calcium for 4 h. Expression of target genes was determined using the relative quantification method. Statistical significance was analyzed by an unpaired t test. Data represent the mean ± SD from 3 independent experiments. ***, P ≤ 0.001 compared to growth in SSM. (B) B. pertussis UT25 was grown in SSM with calcium ± 2 mg/ml HSA and/or 40 mM MgSO4 for 4 h. AC enzyme activity was measured in the total fraction as described in Materials and Methods and normalized by OD600. Data represent the mean ± SD from 3 independent experiments done in duplicate. ***, P ≤ 0.001 compared to growth in SSM; ###, P ≤ 0.001 compared to growth in SSM plus MgSO4.
Since we found that more ACT is being released in the presence of albumin and calcium, we next tested whether HSA increases transcription of the genes within the cya operon: the T1SS (cyaBDE), cyaC (the acyltransferase responsible for posttranslational acylation of cyaA), and cyaX (a putative transcriptional regulator of unknown function) (30, 31). The T1SS is comprised of three proteins: an ATP-binding inner membrane protein (CyaB), an outer membrane protein (CyaE), and a membrane-fusion protein spanning the periplasm (CyaD) to connect CyaB and CyaE (30). As determined by qRT-PCR, the expression of none of these genes was significantly altered ± HSA (Fig. 10), but as anticipated, expression of all genes was significantly reduced during growth with 40 mM MgSO4. These data indicate that HSA is also not acting transcriptionally to regulate genes contained within the cya operon that are involved in secretion or activation of ACT.
FIG 10.
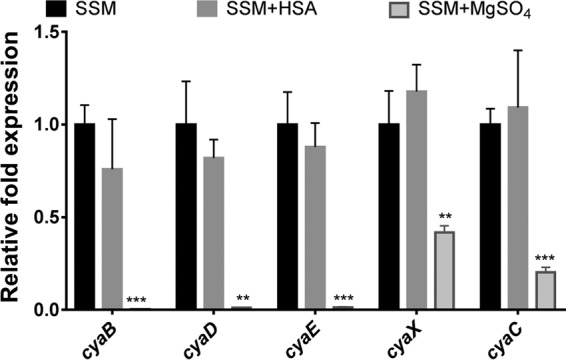
Transcription of genes encoded in the cya operon is not altered ± HSA. (A) RNA was isolated from B. pertussis UT25 grown in SSM ± 2 mg/ml HSA and/or 40 mM MgSO4 with 2 mM calcium for 4 h. Expression of target genes was determined using the relative quantification method. Statistical significance was analyzed by an unpaired t test. Data represent the mean ± SD from 3 independent experiments. **, P ≤ 0.01, and ***, P ≤ 0.001, compared to growth in SSM.
Human respiratory secretions contain albumin and stimulate increased amounts of extracellular ACT.
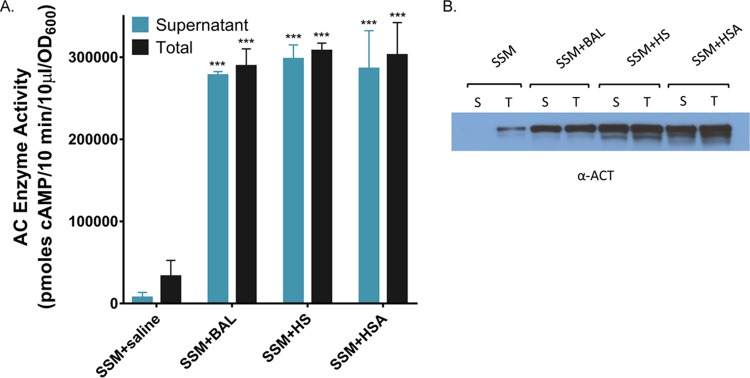
To address the role of albumin during human infection with B. pertussis, we tested human respiratory secretions for the ability to affect the amount and distribution of ACT. Respiratory secretions contain serum components, notably albumin, and the concentrations of these components increases during inflammation (49–51). Bronchoalveolar lavage (BAL) was performed on 7 patients with interstitial lung disease or bronchiectasis, and the resulting samples were pooled and concentrated as described. The amount of albumin present in the pooled BAL fluid sample was determined to be approximately 2.0 mg/ml by SDS-PAGE using purified HSA as a standard over a range of concentrations (not shown) and confirmed by Western blotting analyses with an anti-BSA antibody to be comparable to the amount of albumin present in 5% HS (2.3 mg/ml) (Fig. 11). Since endogenous calcium is diluted during sample acquisition with saline washes, the BAL pool was tested in the presence of calcium at a physiological concentration (2 mM). As shown in Fig. 12A and B, growth of B. pertussis UT25 in the BAL specimen elicits an 8.7-fold increase in the amount of ACT and a shift in distribution to the supernatant (96%) compared to SSM alone (22%). Similar concentrations of HS and purified HSA elicited 9.3- and 9.1-fold increases in the amount of ACT and 96% or 97% of ACT in the supernatant, respectively (Fig. 12A and B).
FIG 11.
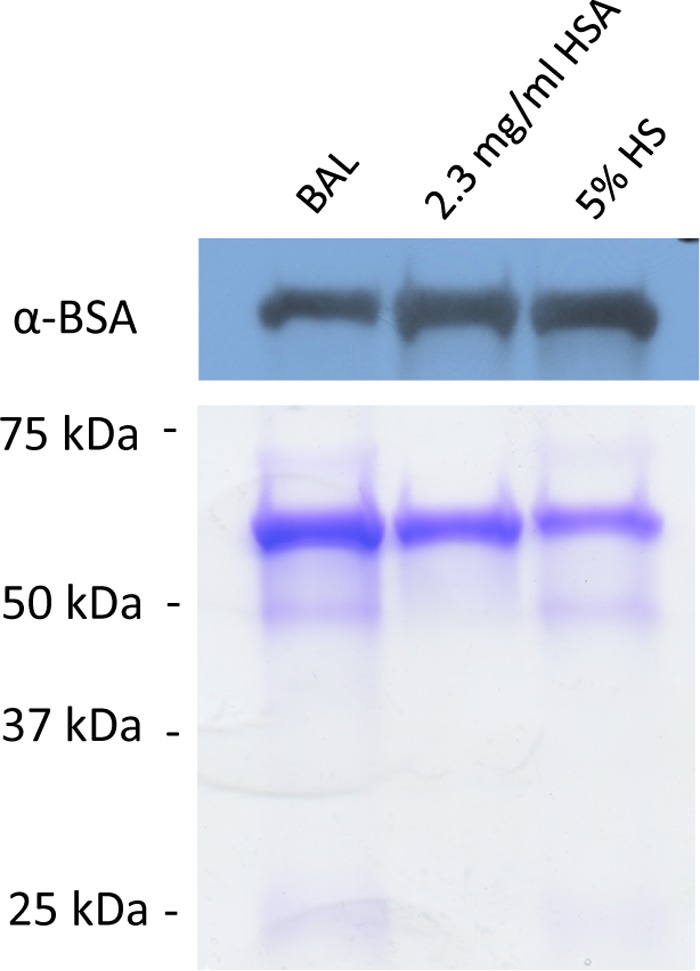
Respiratory secretions, isolated by bronchoalveolar lavage (BAL), contain albumin. SSM plus BAL fluid, SSM plus 2.3 mg/ml HSA, and SSM plus 5% HS samples were analyzed by Western blotting with an anti-BSA antibody as well as SDS-PAGE and Coomassie staining to detect protein profiles and confirm the presence of albumin. Molecular masses of known components of serum and respiratory secretions are as follows: albumin, 66 kDa; gammaglobulin heavy chains, 55 to 60 kDa; and gammaglobulin light chains, 25 to 28 kDa.
FIG 12.
Respiratory secretions increase the total and extracellular amounts of ACT. (A) B. pertussis UT25 was grown in SSM ± saline, BAL fluid, 2 mg/ml HSA, or 5% HS, all with 2 mM CaCl2, for 8 h. The total fraction includes both culture supernatant and bacterial cells. The supernatant was collected after centrifugation. AC enzyme activity was measured as described in Materials and Methods and normalized by OD600. Data represent the mean ± SD from 2 independent experiments done in duplicate. Statistical significance was assessed using a 2-way ANOVA. ***, P ≤ 0.001 compared to growth in SSM. (B) Western blot analysis using a rabbit polyclonal anti-ACT antibody detecting the 200-kDa ACT protein. Lanes: S, supernatant; T, total.
To determine if the increased ACT obtained during growth in the BAL fluid is comparable in toxin activity, we incubated the culture supernatant from B. pertussis UT25 grown in SSM plus BAL fluid for 8 h with J774 cells and measured cytotoxicity. ACT produced in the presence of human respiratory secretions elicited concentration-dependent cytotoxicity of J774 cells and was equivalent to that of recombinant ACT (rACT) at concentrations with equal enzyme activities (Fig. 13). These results confirm that albumin is present in human respiratory secretions, which B. pertussis encounters during infection of the human respiratory tract, and that these secretions promote an increase in the amount of functional ACT that is almost entirely present in the supernatant. We believe albumin, in the presence of calcium, acts as a critical factor in the host environment to increase active, newly secreted ACT, which is essential for establishment of B. pertussis infection.
FIG 13.
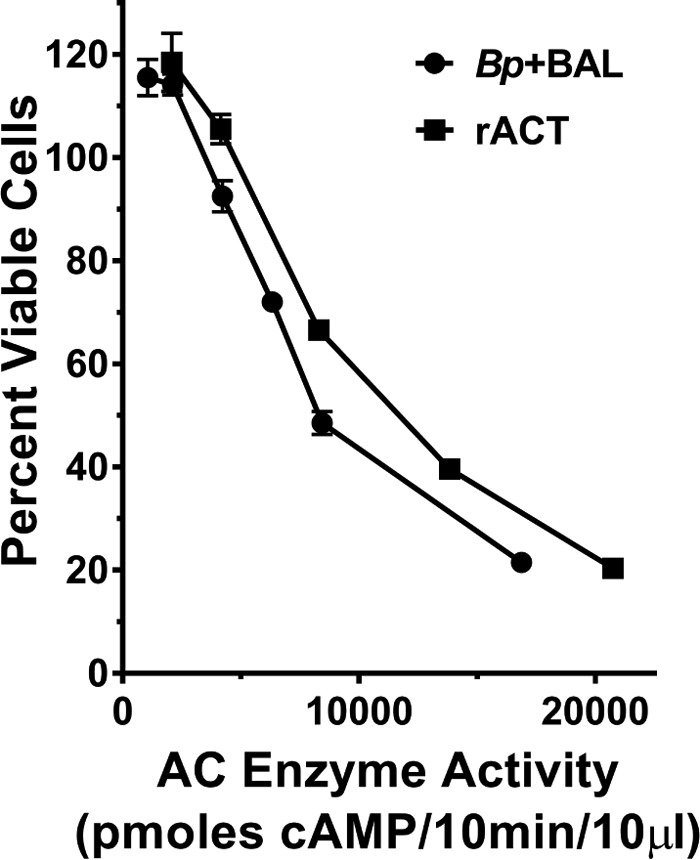
Extracellular ACT produced during growth with respiratory secretions is a functional toxin. J774 cells were incubated with the supernatant from growth of B. pertussis UT25 in SSM plus BAL fluid or rACT (normalized to equivalent enzyme activity) for 3 h at 37°C. The number of viable cells was calculated using the CCK8 assay (see Materials and Methods). The data represent the mean ± SD from a single experiment done in triplicate, which is representative of three additional experiments.
DISCUSSION
During quantification of ACT in samples from B. pertussis-infected humans and baboons, we observed that ACT localization is different than during in vitro growth of B. pertussis (43, 45). While working to make in vitro conditions more reflective of the environment within the host respiratory tract, we found that serum components, specifically albumin and calcium, stimulate a robust increase in ACT and change its distribution. It had previously been reported that B. pertussis secreted high levels of ACT during growth in SSM with albumin (52). We show that the amount of ACT is increased even further in the presence of albumin and calcium together. Since calcium alone does not increase the amount of ACT but does affect release under our growth conditions, we postulate that calcium enhances the effect of albumin by aiding in secretion of ACT (24). Both albumin and calcium are present in the human respiratory tract, and we show that respiratory secretions stimulate ACT production and release (Fig. 12), leading us to hypothesize that what we describe here represents the magnitude and localization of ACT during B. pertussis-host interactions. Furthermore, these results indicate that current conditions established for in vitro growth in SSM are not representative of bacterial growth or virulence factor expression within the host and may stimulate a shift within the Bordetella research community to define new culture conditions that more accurately replicate the host environment.
Albumin comprises approximately 60% of the total serum protein in healthy human adults and performs many functions, importantly maintaining oncotic pressure and binding fatty acids, ions, amino acids, and drugs (54–56). The nononcotic properties of albumin include transport of metabolites and drugs, free radical scavenging, and modulation of the inflammatory response (54). Our data indicate that albumin is responsible for a massive increase in ACT. We have not yet determined what properties of albumin are required and the mechanism involved. Hypotheses include direct protein-protein interactions between albumin and ACT or albumin and a bacterial protein receptor/signaling molecule. It is also feasible, although we believe unlikely, that the effects are not from albumin itself but from a molecule that albumin delivers to the bacterial cell. Because of our data, this molecule would need to be present in the highly purified albumin used in this study. Regardless of the mechanism, the concept that a host protein, present at the site of infection, is acting specifically to elicit a significant enhancement in the amount of toxin represents an additional level of regulation of a well-characterized, critical virulence factor in response to the host environment.
When the Bvg two-component regulatory system was discovered more than 30 years ago, it was termed the master regulator of virulence, at least for the Bordetella species in which it was studied (32–34). We now know that there are other pathways by which virulence is controlled in this genus (57–61), but how these pathways relate to one another and to Bvg is still to be fully determined. The Bvg system controls production of many bacterial proteins—importantly ACT and other virulence factors—and expression is downregulated in response to sulfate, nicotinic acid, and a shift to lower temperature (25°C). Except for studies showing that Bvg activation is sufficient for infection and that the Bvg− phase is not required for infection (40, 62, 63), there is no direct linkage between what is known about Bvg regulation in vitro and the behavior of B. pertussis in vivo. It is still not known whether Bvg modulation occurs in vivo, and if modulation occurs, what signals within the host are responsible. Our data suggest that the response to albumin operates downstream from Bvg, and we postulate that it may represent a novel mechanism for fine-tuning expression of virulence traits at the posttranscriptional level. We show here that human respiratory secretions elicit an increased amount of released ACT (Fig. 12), highlighting that this regulatory mechanism likely is activated within the host environment and may be a critical determinant in controlling the amount and localization of ACT during infection. This work represents the first steps to understand a previously unrecognized regulatory process involving communication between the host and pathogen through which albumin affects production of ACT.
Previous studies have identified that albumin influences the growth and virulence of microorganisms (64–67). Specifically, albumin has been shown to bind to the bacterial surface of group B streptococci and inactivate the antibacterial peptide CXCL9, increase expression of virulence genes in Pseudomonas aeruginosa, and specifically induce natural competence in Acinetobacter baumannii (65–67). Albumin and serum also affect other RTX toxins. Albumin enhances the activity of the leukotoxin produced by Mannheimia haemolytica (formerly Pasteurella haemolytica), through disruption of toxin aggregates (68, 69). This mechanism is not, however, consistent with our observations about ACT; mainly, we do not see a functional difference between rACT and ACT secreted in the presence of albumin (Fig. 13), and we see a corresponding increase in ACT amount and enzyme activity (Fig. 1). ACT aggregation would decrease the functional activity but would not impact enzyme activity. Additionally, serum and albumin promote the release of leukotoxin from Actinobacillus actinomycetemcomitans from the cell surface, although the association of this leukotoxin with the membrane appears to be different from that of ACT with B. pertussis (70). These observations about the conservation of effects of albumin on pathogenic bacteria and RTX family toxins are intriguing; our data add to the growing body of literature on the importance of the host molecule albumin in infection.
Although ACT is established as a critical virulence factor and protective antigen, it was not considered for inclusion in the acellular pertussis vaccines because its purification and characterization were not adequately defined when those products were being developed. Serious evaluation of ACT as a vaccine antigen has been stimulated more recently by the limited duration of protection by current acellular pertussis vaccines and resultant increase in reported pertussis cases. Because only 10% of the total ACT is in the supernatant during growth in SSM, previous studies of ACT have been limited to use of recombinant ACT (from Escherichia coli) or surface-associated ACT (extracted from B. pertussis with urea and refolded in the presence of calcium). We now recognize that standard conditions for in vitro culture lack sufficient calcium for folding and efficient secretion of ACT and are not reflective of what B. pertussis encounters within the host (24, 47). Furthermore, previous work has suggested that there may be structural or functional differences between secreted and surface-associated ACT (20, 24, 44, 58). We show here that albumin, in the presence of physiological concentrations of calcium, stimulates a massive increase of secreted ACT. It is possible that previous observations about the amount and localization of ACT have been influenced by growth conditions in vitro that are not equivalent to those in the environment within the host during infection, as has been shown for the effect of physiological concentrations of calcium on ACT folding, secretion, and purification (20–24, 71, 72), and we now add that albumin, in combination with calcium, has an even more robust effect on the amount and secretion of ACT. Growth under these conditions produces large quantities of secreted ACT, which were not possible to obtain previously but will now be available for characterization as a candidate vaccine antigen.
In summary, we identified that albumin and calcium stimulate a robust increase in the amount of ACT produced by B. pertussis, that the localization of ACT shifts from being primarily on the surface of the bacterium to the majority of the toxin being in the supernatant, and that this toxin is functionally equivalent to purified ACT in its ability to intoxicate cells. These findings make a significant contribution to our knowledge of B. pertussis adenylate cyclase biology by revealing conditions to greatly enhance production of secreted ACT, the most relevant molecule to consider as a candidate vaccine antigen, as well as conditions that better replicate ACT production and localization within the respiratory tract. Future studies are planned to evaluate the role of albumin in B. pertussis infection utilizing albumin-deficient mice and to elucidate the molecular mechanism of how albumin influences ACT production. Since serum components comprise almost half of the protein in respiratory secretions and albumin is responsible for almost two-thirds of these serum-derived proteins (54, 55), we speculate that the response of B. pertussis to albumin represents a basic mechanism by which B. pertussis recognizes that it has entered a host and is critical to establishing infection.
MATERIALS AND METHODS
Growth of B. pertussis, B. parapertussis, and B. bronchiseptica.
For the majority of the experiments presented here, B. pertussis clinical isolate UT25 (73) was used. B. pertussis, B. parapertussis, and B. bronchiseptica strains (listed in Table 1) were plated on Bordet-Gengou agar (Gibco) containing 15% defibrinated sheep blood (Cocalico) and incubated for 48 to 72 h at 37°C. Bacteria were transferred to modified synthetic Stainer-Scholte liquid medium (SSM) (74, 75), grown for 20 to 24 h at 35.5°C with shaking, and then diluted to an OD600 of 0.08 and grown for another 20 to 24 h. On the day of the experiment, bacteria were diluted to an OD600 of 0.1 and grown for 8 h, unless otherwise stated. Bacteria were cultured as indicated in the presence or absence of fetal bovine serum (FBS [Gibco]), mouse serum, human serum (from a pool of healthy human donors as approved by the Institutional Review Board at the University of Virginia), bovine serum albumin (Sigma A3059; further purified fraction V, fatty acid depleted, essentially gamma globulin free, ∼99% pure), or human serum albumin (Sigma A3782; fatty acid free, globulin free, ≥99% pure). All serum was heat inactivated for 30 min at 56°C before addition to bacterial cultures. At indicated time points, 1 ml of bacterial culture was removed (total), an equal volume of culture was spun at 15,000 rpm for 10 min, and the supernatant was reserved. Samples were stored at −80°C until tested.
Adenylate cyclase enzymatic activity.
B. pertussis organisms were grown as indicated in the figure legends, and the total and supernatant fractions were obtained as described. Adenylate cyclase enzymatic activity was measured by the conversion of [32P]ATP to [32P]cAMP as described previously (10). Briefly, each assay tube contained 60 mM Tricine, 10 mM MgCl2, and 2 mM ATP with 3 × 105 cpm of [α-32P]ATP and 1 μM calmodulin at pH 8.0. The reaction was carried out at 30°C for 10 min and terminated by the addition of 100 μl of a solution containing 1% SDS, 20 mM ATP, and 6.24 mM cAMP with 2.0 × 104 cpm of [3H]cAMP. Cyclic AMP was quantified by the double-column method of Salomon et al. (46) and reported as picomoles of cAMP per 10 min per 10 μl. All measurements were taken on the linear part of a curve comparing the ACT amount and enzyme activity, generated using known concentrations of recombinant ACT, providing reproducible quantification of the amount of ACT present in the sample (45). Data are also presented as milliunits per milliliter, where 1 unit of AC corresponds to 1μmol of cAMP formed in 1 min at pH 8.0 at 30°C (76) in Fig. S1 in the supplemental material.
Western blot analysis.
B. pertussis cells were grown as indicated in the figure legends, and the total and supernatant fractions were obtained as described. Samples were normalized according to the optical density at 600 nm, boiled in reducing sample buffer (Thermo Scientific) for 5 min, and loaded on a 10% polyacrylamide gel for electrophoresis. Gels were then electroblotted at 20 V onto polyvinylidene difluoride (PVDF) membranes overnight at 4°C. The membrane was blocked with 5% nonfat dry milk in PBS plus 0.1% Tween 20 at pH 7.4 (PBS-T) for 1 h and then incubated with primary antibodies (polyclonal rabbit anti-ACT antibody, which recognizes all major domains of the toxin molecule [77], or anti-BSA [EMD Millipore Corporation]) at 1:10,000 for 2 h. The membrane was extensively washed in PBS-T and probed with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (Cell Signaling Technologies) at a 1:15,000 dilution. ECL enhanced chemiluminescence (Amersham) was used to detect HRP-labeled secondary antibodies.
Fractionation of FBS.
Spin columns with 10-kDa or 50-kDa size exclusions (Amicon) were used to fractionate FBS according to the manufacturer's instructions. Thirteen milliliters of FBS was concentrated into 1 ml with a 50-kDa-molecular-mass-cutoff column, generating the ≥50-kDa and ≤50-kDa fractions. Four milliliters of flowthrough was added to a spin column with a 10-kDa-molecular-mass-cutoff and centrifuged until almost the entire volume was collected as flowthrough to generate the ≤10-kDa fraction. Fractions were added to bacterial cultures at concentrations as equivalent as possible to 10% FBS.
EGTA treatment of BSA.
Three milliliters of BSA at 50 mg/ml was dialyzed against 1 liter PBS (pH 7.5) plus 2 mM EGTA 4 times for a total of 4 liters, using a Slide-A-Lyzer dialysis cassette (Thermo Scientific). This material was then dialyzed versus PBS (pH 7.5), and the protein was measured before its use in the indicated experiments.
Mouse serum.
Mice were euthanized and blood collected through ventricular puncture. Serum was isolated from whole blood using a BD Microtainer serum separator following the manufacturer's instructions. All mice were treated in accordance with the guidelines of the Animal Care and Use Committee at The Jackson Laboratory. Total protein was quantified using the Pierce bicinchoninic acid (BCA) protein assay (Thermo Scientific) to be 65 mg/ml for the wild-type serum and 44 mg/ml for the analbuminemic serum, and the concentration of albumin in 10% wild-type mouse serum was determined to be 2 mg/ml by SDS-PAGE with a concentration range (0.25 to 4 mg/ml) of purified BSA.
RNA extraction and qRT-PCR.
B. pertussis was grown as indicated. Bacterial cells from three biological replicate cultures were pelleted and treated with RNAprotect (Qiagen). RNA was extracted using the RNeasy kit (Qiagen) following the manufacturer's instructions. The primers used in real-time qPCR assays were validated before use, and their sequences are listed in Table 3. Reaction mixtures were prepared as previously described (78). qRT-PCR was performed using a one-step reaction in an ABI 7500-Fast sequence detection system (Applied Biosystems). All data were normalized to the levels of rpoB and analyzed using the comparative cycle threshold (CT) method (79). The relative quantification method was used to determine the expression level of target genes in various growth conditions. Statistical significance was determined by an unpaired t test, and a P value of ≤0.05 was considered significant.
TABLE 3.
Primers used in this study
| Namea | Sequence | Reference |
|---|---|---|
| rpoB Bp F | GCTGGGACCCGAGGAAAT | 57 |
| rpoB Bp R | CGCCAATGTAGACGATGCC | 57 |
| cyaA Bp F | CGAGGCGGTCAAGGTGAT | 57 |
| cyaA Bp R | GCGGAAGTTGGACAGATGC | 57 |
| bvgA Bp F | AGGTCATCAATGCCGCCA | 57 |
| bvgA Bp R | GCAGGACGGTCAGTTCGC | 57 |
| fhaB Bp F | CAAGGGCGGCAAGGTGA | 57 |
| fhaB Bp R | ACAGGATGGCGAACAGGCT | 57 |
| ptxA Bp F | CCAGAACGGATTCACGGC | 57 |
| ptxA Bp R | CTGCTGCTGGTGGAGACGA | 57 |
| cyaBRTF | TCATGCTGGCTCGCTATCAC | This study |
| cyaBRTR | TCGCTACAGAATGCCTGCTC | This study |
| cyaDRTF | AGCAAGGACATCGGCTTTGT | This study |
| cyaDRTR | TTCGAGCGTTCCGTACTTCG | This study |
| cyaERTF | CGCCCTATTATCCCAGCGTC | This study |
| cyaERTR | TACCGCCATCACATTGTTGC | This study |
| cyaXRTF | CCGATGTCTTGCGCCTGTAT | This study |
| cyaXRTR | GCGCATACGACACATAGGGA | This study |
| cyaCRTF | ATGAACTCTCCCATGCACCG | This study |
| cyaCRTR | TATGCAACCGGCACGTCATT | This study |
F, forward; R, reverse.
BAL.
Bronchoalveolar lavage (BAL) was performed for clinical indications on 7 patients with interstitial lung disease or bronchiectasis using published protocols (80). Three 50-ml aliquots of saline were sequentially perfused and then aspirated in the right middle lobe or lingula. A 10-ml aliquot of the sample that would have otherwise been discarded was used for this study via a protocol that was classified as exempt by the Institutional Review Board at the University of Virginia. Samples were centrifuged at 3,000 rpm to remove cells, and the supernatants were pooled and concentrated 10-fold using Amicon Centricon spin columns with a 10-kDa molecular mass cutoff. The concentrated BAL fluid was filter sterilized before addition to bacterial cultures.
Culture of J774.1 cells.
Cells from the J774.1 (here J774) murine macrophage cell line were maintained at 37°C in Dulbecco's modified Eagle's medium with high glucose (Gibco) plus 10% heat-inactivated FBS in 5% CO2.
Cytotoxicity assay.
ACT causes cytotoxicity of J774 cells (81, 82). J774 cells (30,000 in 90 μl) were seeded in each well of a 96-well plate and allowed to attach overnight at 37°C in 5% CO2. Samples were added, and cells were incubated at 37°C for 3 h. The number of viable cells was determined using the CCK8 assay (Dijindo Molecular Technologies, Gaithersburg, MD), which measures the reduction of WST-8, a water-soluble tetrazolium salt, by dehydrogenases in viable cells. The percentage of viable cells was determined by the following equation: [(experimental – blank)/(control cells – blank)] × 100. A blank is a well containing medium and CCK8 reagent, but no cells. Control cells are J774 cells that are not treated.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH/NIAID grant AI18000 (E.L.H.), and L.A.G. is recipient of a Hartwell Foundation Postdoctoral Fellowship.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00198-17.
REFERENCES
- 1.Warfel JM, Edwards KM. 2015. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol 35:48–54. doi: 10.1016/j.coi.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Cherry JD. 2013. Pertussis: challenges today and for the future. PLoS Pathog 9:e1003418. doi: 10.1371/journal.ppat.1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meade BD, Plotkin SA, Locht C. 2014. Possible options for new pertussis vaccines. J Infect Dis 209(Suppl 1):S24–S27. doi: 10.1093/infdis/jit531. [DOI] [PubMed] [Google Scholar]
- 4.Weiss AA, Goodwin MS. 1989. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun 57:3757–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodwin MS, Weiss AA. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun 58:3445–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guiso N, Szatanik M, Rocancourt M. 1991. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb Pathog 11:423–431. doi: 10.1016/0882-4010(91)90038-C. [DOI] [PubMed] [Google Scholar]
- 7.Sebo P, Osicka R, Masin J. 2014. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines 13:1215–1227. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin SA. 2014. Pertussis: pertussis control strategies and the options for improving current vaccines. Expert Rev Vaccines 13:1071–1072. doi: 10.1586/14760584.2014.944166. [DOI] [PubMed] [Google Scholar]
- 9.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. 1988. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol 2:19–30. doi: 10.1111/j.1365-2958.1988.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Hewlett EL, Gordon VM, McCaffery JD, Sutherland WM, Gray MC. 1989. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem 264:19379–19384. [PubMed] [Google Scholar]
- 11.Rogel A, Schultz JE, Brownlie RM, Coote JG, Parton R, Hanski E. 1989. Bordetella pertussis adenylate cyclase: purification and characterization of the toxic form of the enzyme. EMBO J 8:2755–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellalou J, Ladant D, Sakamoto H. 1990. Synthesis and secretion of Bordetella pertussis adenylate cyclase as a 200-kilodalton protein. Infect Immun 58:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser P, Danchin A, Ladant D, Barzu O, Ullmann A. 1988. Bordetella pertussis adenylate cyclase: the gene and the protein. Tokai J Exp Clin Med 13(Suppl):239–252. [PubMed] [Google Scholar]
- 14.Hanski E. 1989. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci 14:459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- 15.Gray M, Szabo G, Otero AS, Gray L, Hewlett E. 1998. Distinct mechanisms for K+ efflux, intoxication, and hemolysis by Bordetella pertussis AC toxin. J Biol Chem 273:18260–18267. doi: 10.1074/jbc.273.29.18260. [DOI] [PubMed] [Google Scholar]
- 16.Basler M, Masin J, Osicka R, Sebo P. 2006. Pore-forming and enzymatic activities of Bordetella pertussis adenylate cyclase toxin synergize in promoting lysis of monocytes. Infect Immun 74:2207–2214. doi: 10.1128/IAI.74.4.2207-2214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vojtova-Vodolanova J, Basler M, Osicka R, Knapp O, Maier E, Cerny J, Benada O, Benz R, Sebo P. 2009. Oligomerization is involved in pore formation by Bordetella adenylate cyclase toxin. FASEB J 23:2831–2843. doi: 10.1096/fj.09-131250. [DOI] [PubMed] [Google Scholar]
- 18.Ehrmann IE, Weiss AA, Goodwin MS, Gray MC, Barry E, Hewlett EL. 1992. Enzymatic activity of adenylate cyclase toxin from Bordetella pertussis is not required for hemolysis. FEBS Lett 304:51–56. doi: 10.1016/0014-5793(92)80587-7. [DOI] [PubMed] [Google Scholar]
- 19.Hewlett EL, Gray L, Allietta M, Ehrmann I, Gordon VM, Gray MC. 1991. Adenylate cyclase toxin from Bordetella pertussis. Conformational change associated with toxin activity. J Biol Chem 266:17503–17508. [PubMed] [Google Scholar]
- 20.Rose T, Sebo P, Bellalou J, Ladant D. 1995. Interaction of calcium with Bordetella pertussis adenylate cyclase toxin. Characterization of multiple calcium-binding sites and calcium-induced conformational changes. J Biol Chem 270:26370–26376. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes CR, Gray MC, Watson JM, Muratore TL, Kim SB, Hewlett EL, Grisham CM. 2001. Structural consequences of divalent metal binding by the adenylyl cyclase toxin of Bordetella pertussis. Arch Biochem Biophys 395:169–176. doi: 10.1006/abbi.2001.2553. [DOI] [PubMed] [Google Scholar]
- 22.Chenal A, Guijarro JI, Raynal B, Delepierre M, Ladant D. 2009. RTX calcium binding motifs are intrinsically disordered in the absence of calcium: implication for protein secretion. J Biol Chem 284:1781–1789. doi: 10.1074/jbc.M807312200. [DOI] [PubMed] [Google Scholar]
- 23.Chenal A, Karst JC, Sotomayor Perez AC, Wozniak AK, Baron B, England P, Ladant D. 2010. Calcium-induced folding and stabilization of the intrinsically disordered RTX domain of the CyaA toxin. Biophys J 99:3744–3753. doi: 10.1016/j.bpj.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bumba L, Masin J, Macek P, Wald T, Motlova L, Bibova I, Klimova N, Bednarova L, Veverka V, Kachala M, Svergun DI, Barinka C, Sebo P. 2016. Calcium-driven folding of RTX domain β-rolls ratchets translocation of RTX proteins through type I secretion ducts. Mol Cell 62:47–62. doi: 10.1016/j.molcel.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eby JC, Gray MC, Mangan AR, Donato GM, Hewlett EL. 2012. Role of CD11b/CD18 in the process of intoxication by the adenylate cyclase toxin of Bordetella pertussis. Infect Immun 80:850–859. doi: 10.1128/IAI.05979-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbonetti NH. 2010. Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol 5:455–469. doi: 10.2217/fmb.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eby JC, Hoffman CL, Gonyar LA, Hewlett EL. 2015. Review of the neutrophil response to Bordetella pertussis infection. Pathog Dis 73:ftv081. doi: 10.1093/femspd/ftv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas S, Holland IB, Schmitt L. 2014. The type 1 secretion pathway—the hemolysin system and beyond. Biochim Biophys Acta 1843:1629–1641. doi: 10.1016/j.bbamcr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Betsou F, Sebo P, Guiso N. 1993. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun 61:3583–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arico B, Miller JF, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. 1989. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A 86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter PA, Jones AM. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol 11:367–373. doi: 10.1016/S0966-842X(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 34.Melvin JA, Scheller EV, Miller JF, Cotter PA. 2014. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12:274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boucher PE, Menozzi FD, Locht C. 1994. The modular architecture of bacterial response regulators. Insights into the activation mechanism of the BvgA transactivator of Bordetella pertussis. J Mol Biol 241:363–377. [DOI] [PubMed] [Google Scholar]
- 36.Boucher PE, Stibitz S. 1995. Synergistic binding of RNA polymerase and BvgA phosphate to the pertussis toxin promoter of Bordetella pertussis. J Bacteriol 177:6486–6491. doi: 10.1128/jb.177.22.6486-6491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steffen P, Goyard S, Ullmann A. 1996. Phosphorylated BvgA is sufficient for transcriptional activation of virulence-regulated genes in Bordetella pertussis. EMBO J 15:102–109. [PMC free article] [PubMed] [Google Scholar]
- 38.Boucher PE, Murakami K, Ishihama A, Stibitz S. 1997. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J Bacteriol 179:1755–1763. doi: 10.1128/jb.179.5.1755-1763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melton AR, Weiss AA. 1989. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol 171:6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cotter PA, Miller JF. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun 62:3381–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hewlett EL, Urban MA, Manclark CR, Wolff J. 1976. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A 73:1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Confer DL, Eaton JW. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 43.Zaretzky FR, Gray MC, Hewlett EL. 2002. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Mol Microbiol 45:1589–1598. doi: 10.1046/j.1365-2958.2002.03107.x. [DOI] [PubMed] [Google Scholar]
- 44.Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. 2004. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol 53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 45.Eby JC, Gray MC, Warfel JM, Paddock CD, Jones TF, Day SR, Bowden J, Poulter MD, Donato GM, Merkel TJ, Hewlett EL. 2013. Quantification of the adenylate cyclase toxin of Bordetella pertussis in vitro and during respiratory infection. Infect Immun 81:1390–1398. doi: 10.1128/IAI.00110-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salomon Y, Londos C, Rodbell M. 1974. A highly sensitive adenylate cyclase assay. Anal Biochem 58:541–548. doi: 10.1016/0003-2697(74)90222-X. [DOI] [PubMed] [Google Scholar]
- 47.Potter JL, Matthews LW, Spector S, Lemm J. 1967. Studies on pulmonary secretions. II. Osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis 96:83–87. [DOI] [PubMed] [Google Scholar]
- 48.Peters T. 1996. All about albumin: biochemistry, genetics, and medical applications. Academic Press, San Francisco, CA. [Google Scholar]
- 49.Masson PL, Heremans JF, Prignot J. 1965. Studies on the proteins of human bronchial secretions. Biochim Biophys Acta 111:466–478. doi: 10.1016/0304-4165(65)90056-5. [DOI] [PubMed] [Google Scholar]
- 50.Yeager H., Jr 1971. Tracheobronchial secretions. Am J Med 50:493–509. doi: 10.1016/0002-9343(71)90338-X. [DOI] [PubMed] [Google Scholar]
- 51.Nicholson JP, Wolmarans MR, Park GR. 2000. The role of albumin in critical illness. Br J Anaesth 85:599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 52.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. 1990. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun 58:3242–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roopenian DC, Low BE, Christianson GJ, Proetzel G, Sproule TJ, Wiles MV. 2015. Albumin-deficient mouse models for studying metabolism of human albumin and pharmacokinetics of albumin-based drugs. MAbs 7:344–351. doi: 10.1080/19420862.2015.1008345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans TW. 2002. Review article: albumin as a drug—biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther 16(Suppl 5):S6–S11. doi: 10.1046/j.1365-2036.16.s5.2.x. [DOI] [PubMed] [Google Scholar]
- 55.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. 2005. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 57:787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 56.Merlot AM, Kalinowski DS, Richardson DR. 2014. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol 5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bibova I, Skopova K, Masin J, Cerny O, Hot D, Sebo P, Vecerek B. 2013. The RNA chaperone Hfq is required for virulence of Bordetella pertussis. Infect Immun 81:4081–4090. doi: 10.1128/IAI.00345-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanawa T, Yonezawa H, Kawakami H, Kamiya S, Armstrong SK. 2013. Role of Bordetella pertussis RseA in the cell envelope stress response and adenylate cyclase toxin release. Pathog Dis 69:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahuja U, Shokeen B, Cheng N, Cho Y, Blum C, Coppola G, Miller JF. 2016. Differential regulation of type III secretion and virulence genes in Bordetella pertussis and Bordetella bronchiseptica by a secreted anti-sigma factor. Proc Natl Acad Sci U S A 113:2341–2348. doi: 10.1073/pnas.1600320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbier M, Boehm D, Sen-Kilic E, Bonnin C, Pinheiro T, Hoffman C, Gray M, Hewlett E, Damron FH. 2016. Modulation of pertussis and adenylate cyclase toxins by sigma factor RpoE in Bordetella pertussis. Infect Immun 85:e00565-16. doi: 10.1128/IAI.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coutte L, Huot L, Antoine R, Slupek S, Merkel TJ, Chen Q, Stibitz S, Hot D, Locht C. 2016. The multifaceted RisA regulon of Bordetella pertussis. Sci Rep 6:32774. doi: 10.1038/srep32774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vergara-Irigaray N, Chavarri-Martinez A, Rodriguez-Cuesta J, Miller JF, Cotter PA, Martinez de Tejada G. 2005. Evaluation of the role of the Bvg intermediate phase in Bordetella pertussis during experimental respiratory infection. Infect Immun 73:748–760. doi: 10.1128/IAI.73.2.748-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez de Tejada G, Cotter PA, Heininger U, Camilli A, Akerley BJ, Mekalanos JJ, Miller JF. 1998. Neither the Bvg− phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect Immun 66:2762–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Chateau M, Holst E, Bjorck L. 1996. Protein PAB, an albumin-binding bacterial surface protein promoting growth and virulence. J Biol Chem 271:26609–26615. doi: 10.1074/jbc.271.43.26609. [DOI] [PubMed] [Google Scholar]
- 65.Egesten A, Frick IM, Morgelin M, Olin AI, Bjorck L. 2011. Binding of albumin promotes bacterial survival at the epithelial surface. J Biol Chem 286:2469–2476. doi: 10.1074/jbc.M110.148171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kruczek C, Wachtel M, Alabady MS, Payton PR, Colmer-Hamood JA, Hamood AN. 2012. Serum albumin alters the expression of iron-controlled genes in Pseudomonas aeruginosa. Microbiology 158:353–367. doi: 10.1099/mic.0.053371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Traglia GM, Quinn B, Schramm ST, Soler-Bistue A, Ramirez MS. 2016. Serum albumin and Ca2+ are natural competence inducers in the human pathogen Acinetobacter baumannii. Antimicrob Agents Chemother 60:4920–4929. doi: 10.1128/AAC.00529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waurzyniak BJ, Clinkenbeard KD, Confer AW, Srikumaran S. 1994. Enhancement of Pasteurella haemolytica leukotoxic activity by bovine serum albumin. Am J Vet Res 55:1267–1274. [PubMed] [Google Scholar]
- 69.Urban-Chmiel R, Wernicki A, Puchalski A, Mikucki P. 2004. Evaluation of Mannheimia haemolytica leukotoxin prepared in nonsupplemented and BSA or FBS supplemented RPMI 1640 medium. Pol J Vet Sci 7:1–8. [PubMed] [Google Scholar]
- 70.Johansson A, Claesson R, Hanstrom L, Kalfas S. 2003. Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans. Eur J Oral Sci 111:209–215. doi: 10.1034/j.1600-0722.2003.00030.x. [DOI] [PubMed] [Google Scholar]
- 71.Masure HR, Oldenburg DJ, Donovan MG, Shattuck RL, Storm DR. 1988. The interaction of Ca2+ with the calmodulin-sensitive adenylate cyclase from Bordetella pertussis. J Biol Chem 263:6933–6940. [PubMed] [Google Scholar]
- 72.Karst JC, Ntsogo Enguene VY, Cannella SE, Subrini O, Hessel A, Debard S, Ladant D, Chenal A. 2014. Calcium, acylation, and molecular confinement favor folding of Bordetella pertussis adenylate cyclase CyaA toxin into a monomeric and cytotoxic form. J Biol Chem 289:30702–30716. doi: 10.1074/jbc.M114.580852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parker CD, Doyle S, Field LH, Hewlett E. 1980. Variability in derivative strains of Bordetella pertussis. Dev Biol Stand 45:119–127. [PubMed] [Google Scholar]
- 74.Stainer DW, Scholte MJ. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 75.Hewlett E, Wolff J. 1976. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol 127:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ladant D, Brezin C, Alonso JM, Crenon I, Guiso N. 1986. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem 261:16264–16269. [PubMed] [Google Scholar]
- 77.Gray MC, Lee SJ, Gray LS, Zaretzky FR, Otero AS, Szabo G, Hewlett EL. 2001. Translocation-specific conformation of adenylate cyclase toxin from Bordetella pertussis inhibits toxin-mediated hemolysis. J Bacteriol 183:5904–5910. doi: 10.1128/JB.183.20.5904-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walters M, Sperandio V. 2006. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect Immun 74:5445–5455. doi: 10.1128/IAI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 80.Anonymous. 1990. Clinical guidelines and indications for bronchoalveolar lavage (BAL): report of the European Society of Pneumology Task Group on BAL. Eur Respir J 3:937–976. [PubMed] [Google Scholar]
- 81.Hewlett EL, Donato GM, Gray MC. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol Microbiol 59:447–459. [DOI] [PubMed] [Google Scholar]
- 82.Vojtova J, Kamanova J, Sebo P. 2006. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr Opin Microbiol 9:69–75. doi: 10.1016/j.mib.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 83.Sato Y, Arai H. 1972. Leucocytosis-promoting factor of Bordetella pertussis. I. Purification and characterization. Infect Immun 6:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiss AA, Hewlett EL, Myers GA, Falkow S. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun 42:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menozzi FD, Mutombo R, Renauld G, Gantiez C, Hannah JH, Leininger E, Brennan MJ, Locht C. 1994. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun 62:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boinett CJ, Harris SR, Langridge GC, Trainor EA, Merkel TJ, Parkhill J. 2015. Complete genome sequence of Bordetella pertussis D420. Genome Announc 3:e00657-15. doi: 10.1128/genomeA.00657-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McLafferty MA, Harcus DR, Hewlett EL. 1988. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J Gen Microbiol 134:2297–2306. [DOI] [PubMed] [Google Scholar]
- 88.Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun 66:5921–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buboltz AM, Nicholson TL, Weyrich LS, Harvill ET. 2009. Role of the type III secretion system in a hypervirulent lineage of Bordetella bronchiseptica. Infect Immun 77:3969–3977. doi: 10.1128/IAI.01362-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.