Abstract
The change in cell surface properties in the presence of electric currents is of critical concern when the potential to manipulate bacterial movement with electric fields is evaluated. In this study, the effects of different direct electric currents on the cell surface properties involved in bacterial adhesion were investigated by using a mixed phenol-degrading bacterial culture in the exponential growth phase. The traits investigated were surface hydrophobicity (measured by adherence to n-octane), net surface electrostatic charge (determined by measurement of the zeta potential), and the cell surface shape and polymers (determined by scanning electron microscope analysis). The results showed that a lower current (less than 20 mA) induced no significant changes in the surface properties of phenol-degrading bacteria, that an electric current of 20 mA could increase the surface hydrophobicity and flatten the cell shape, and that a higher current (40 mA) could increase the surface extracellular substances and the net negative surface electrostatic charge. The results also revealed that the electric current effects on cell hydrophobicity varied with the suspending medium. We suggest that an electric current greater than 20 mA is not suitable for use in manipulation of the movement of the phenol-degrading bacteria, although such a current might favor the electrophoretic movement of the bacterial species.
Cell surface properties are recognized as the key factors that influence bacterial adhesion to surfaces. Among the critical surface properties are surface hydrophobicity, extracellular polymers, and surface electrostatic charge (1, 9, 18). Cell hydrophobicity is generally explained as a “dislike of microbial surfaces for water” (18). Hydrophobic interactions define the strong attraction between hydrophobic molecules and surfaces in water. In biological systems hydrophobic interactions are the strongest long-range noncovalent interactions and are considered a determining factor in microbial adhesion to surfaces (9, 19). Extracellular substances are critical for bacterium-surface interactions and cell hydrophobicity. Extracellular polysaccharides are the important extracellular substances. Their existence, quality, and composition may all play important roles. It is known that proteins and amino acids are the hydrophobic components of the extracellular polysaccharides, while the polysaccharides are the more hydrophilic components (8, 18, 19). The net surface electrostatic charge, typically measured by the zeta potential on the cell surface, determines the electrostatic interaction between bacterial cells and surfaces. The following three ionized groups have been considered to determine the surface electrical properties: phosphate groups involved in (lipo)teichoic acids, carboxylate, and protonated amino groups of proteins (3).
It has been shown that direct electric current can be used to manipulate bacterial detachment and movement from surfaces. Electric manipulation of bacteria is possible since bacterial cells are generally negatively charged, which dictates their electrophoretic movement in direct current (DC) fields (6). It was demonstrated previously that an electric current of 800 μA could induce detachment of oral bacterial strains from a conditioning film (17). An electric current of more than 40 mA was used to transport a Pseudomonas strain in a bioelectrokinetic remediation test (12).
The DC effects on bacterial cells have been studied for several decades, and studies have focused mainly on the viability, metabolism, and transport of the cells. In particular, viability studies have concentrated on the use of pulsed high voltage for inactivation (7, 10). However, there has been little research on the effects of DC on the cell surface properties involved in bacterial attachment and movement. When bacterial species are exposed to an electric current or induced field, environmental stresses on the bacterial cells are generated. While bacteria respond to environmental stresses physiologically, surface properties and even cell shape change (18, 19). However, it is not clear how the cell surface properties change during exposure to electric currents. Furthermore, external currents also affect bacterial activity and growth and even destroy bacterial cells (7). Only a specific range (a so-called window) of electric current or field strength can be used for bacterial manipulation (2, 14). However, the DC window that contributes to bacterial detachment and movement is not well understood.
This study was conducted to determine the effects of DC on cell surface properties of phenol-degrading bacteria as a prelude to combining in situ bioremediation and electrokinetics in a soil environment contaminated with phenol. Electrokinetics is the application of a weak DC or potential to soil and aquifers. The traits investigated here were surface hydrophobicity and net surface electrostatic charge. This analysis was important in helping to explore the way to use electrokinetics to drive bacterial cells in porous soil environments and to understand how the bacterial cells attach or detach on soil subsurfaces. The extracellular substances and cell shape were also examined due to their substantial influence on bacterial mobility in the subsurface (18, 21). Our interest in these traits was derived from the potential for using DC to transport and mix bacterial inoculants for bioaugmentation (4, 7, 11, 12, 15).
MATERIALS AND METHODS
Bacteria.
A mixed culture of phenol-degrading bacteria was used in this study. The bacterial species were isolated by enrichment techniques from a soil contaminated with petrochemicals by using basic mineral medium with phenol as the sole carbon source (designated MP medium here). MP medium (full strength) contained (per liter of deionized water) 3.0 g of K2HPO4, 1.5 g of KH2PO4, 1.25 g of (NH4)2SO4, 10 mg of NaCl, 100 mg of MgSO4, 1.0 mg of FeSO4 · 7H2O, and 500 mg of phenol. The pH was adjusted to 7.0. All of the chemicals used were analytical grade.
The bacterial cells were grown in MP medium on a shaker at 30°C and 150 rpm, harvested in the exponential growth phase by centrifugation, washed twice with sterilized water, and then resuspended in 0.1× MP medium or sterilized deionized water to obtain bacterial suspensions having a concentration of approximately 1.2 × 109 to 1.5 × 109 cells per ml for the tests. A bacterial culture in the exponential phase was expected to have the greatest capacity to withstand environmental stresses.
Testing system.
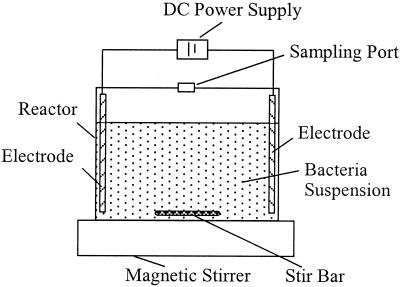
The experimental setup is shown in Fig. 1. It consisted of a reactor, a couple of electrodes, a power supply, and a magnetic stirrer. The reactor was constructed by using a 250-ml glass beaker with a Perspex glass cover; a round hole (diameter, 1.5 cm) in the middle of the cover was used for sampling. During the test period, the hole was covered with a 0.45-μm-pore-size membrane. In all tests with DC, column-shaped graphite electrodes were utilized to ensure that the anode and cathode were inert and unreactive and to minimize the direct contact between the bacteria and the electrodes. The graphite electrodes had a purity of 99.9%, a density of approximately 1.75 to 1.80 g per cm3, and a rigidity of 45; they were 0.5 cm in diameter and 10 cm long. The electrodes were inserted with a 5.0-cm space between them through a rubber bung and were attached to a DC power supply. The power supply (WYK-603; Yangzou Dongfang) could provide a constant DC for the test. A magnetic stirrer was used to ensure that the bacterial suspension was homogeneous during the test. Except for the power supply, the whole setup was placed in an incubator (SHH-200C; Chongqing Huamao) which could supply a constant temperature of 30°C for the tests.
FIG. 1.
Schematic diagram of the experimental reactor.
Testing procedure.
Each test was performed by using 175 ml of a bacterial suspension into which electrodes that were about 8 cm long were immerged. A constant electric current of 5, 10, 20, or 40 mA was applied through the bacterial suspension with the electrodes in separate tests. A control test with no current applied was conducted in parallel. The test was performed for 9 to 12 h, and every hour 3.5 ml of the bacterial suspension was obtained from the sampling port for measurement. All tests were performed in triplicate, and the results were expressed as averages.
Analytical methods.
The cell surface hydrophobicity was determined by measuring the bacterial attachment to hydrocarbons, as described by Gannon et al. (9) and modified by Sanin et al. (19). n-Octane was used as the hydrocarbon phase for the test of bacterial attachment to hydrocarbon. The test tubes were acid washed and rinsed prior to use. Three milliliters of the bacterial suspension was transferred to a 10-mm round-bottom test tube. After the initial turbidity (optical density at 600 nm) was determined with a spectrophotometer (VIS-7220; Beijing Ruili), 0.3 ml of n-octane was added. The mixture was vortexed for 2 min and then allowed to settle for 15 min at room temperature. The final optical density of the octane-free bacterial suspension was determined. The results were expressed as percentages calculated by using the following relationship: percent hydrophobicity = 100(1 − final optical density/initial optical density).
The net surface electrostatic charge of the bacterial cells was measured with a zeta potential analyzer (Zeta Plus; Brookhaven Instrument Co.). The instrument recorded the zeta potential at 20°C, but the values were corrected for the experimental temperature (30°C) by use of the fact that the zeta potential changes by approximately 2% for each 1°C (9). A 0.5-ml portion of the bacterial suspension was diluted in 4.5 ml of sterilized water (pH 7.0) to obtain a concentration of about 108 cells per ml before determination of the zeta potential at 20°C. Duplicate assays were performed for each sample.
Scanning electron microscope analysis was performed to observe the induced changes in cell shape and extracellular polymers in the presence of an electric current. Bacterial samples were washed gently with a phosphate buffer solution (pH 7.0) and fixed with 2.5% glutaraldehyde and a 1% osmic acid solution. The specimen was dehydrated by using sequential ethanol concentrations ranging from 30 to 100% in 20 or 15% increments with 20 min of exposure per concentration, and then the ethanol was replaced by acetate isoamyl ester. After dehydration, the specimen was critical point dried with CO2. Finally, the specimen was sputter coated with gold in an ion coater for 2 min at an applied current of 50 mA (IB-3 Ioncoater; Eiko) and then examined with a scanning electron microscope (S-570; Hitachi).
RESULTS
Change in surface hydrophobicity.
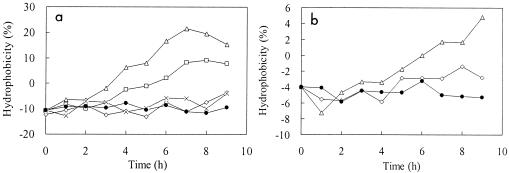
The changes in surface hydrophobicity of phenol-degrading bacteria in the presence of various DCs are shown in Fig. 2. The results showed that the hydrophobicity increased after application of an electric current depending on the magnitude of the electric current applied and the suspending medium during tests.
FIG. 2.
Effect of DC on cell surface hydrophobicity of phenol-degrading bacteria in 0.1× MP medium (a) and deionized water (b). (a) Symbols: •, control; ×, 5 mA; ⋄, 10 mA; □, 20 mA: ▵, 40 mA. (b) Symbols: •, control; ⋄, 10 mA; ▵, 40 mA.
A concentrated bacterial culture in the exponential phase was suspended in 0.1× MP medium and deionized water, and average hydrophobicities of −10.8 and −4.0%, respectively, were observed. The negative hydrophobicities indicate that the surfaces of the phenol-degrading bacteria were hydrophilic at the beginning of test and were more hydrophilic in MP medium than in deionized water.
When electric currents of 5 and 10 mA were applied to the bacterial suspension in 0.1× MP medium, no significant changes in the hydrophobicity were observed compared with the control test in which no electric current was applied, in spite of slight increases with fluctuations. However, when electric currents of 20 and 40 mA were applied, the surface hydrophobicity increased sharply to 7.7 and 15.3%, respectively, and maximum hydrophobicities of 9.0 and 21.5%, respectively, were observed during the test period (9 h). With an electric current of 20 or 40 mA, the negative hydrophobicity changed to positive hydrophobicity after about 5 and 3 h, indicating that the hydrophilic surface changed to a hydrophobic surface. The results suggested that higher electric currents can significantly increase the surface hydrophobicity of bacterial cells and thus might promote bacterial attachment to surfaces.
For the bacterial culture suspended in deionized water, similar increases in surface hydrophobicity were observed when electric currents were applied, but the induced change was not as significant as the change observed for the bacterial suspension in MP medium. In the control test, the bacterial culture in deionized water showed a slight decrease in surface hydrophobicity from −4 to −6%. When an electric current of 10 mA was applied, the hydrophobicity decreased (with fluctuations) to −6% and then slightly increased to −2%. However, when a higher electric current (40 mA) was applied, the surface hydrophobicity of the bacterial cells in deionized water exhibited a sudden sharp drop to about −7% and then a stable increase to about 5% during the 9-h test.
Change in net surface electrostatic charge.
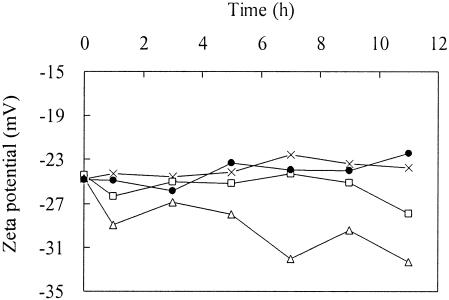
The net surface electrostatic charge was determined in this study by measuring the zeta potential of a bacterial culture. Figure 3 shows the change in zeta potential of the bacterial suspension in deionized water in the presence of different DCs.
FIG. 3.
Effect of DC on the zeta potential of phenol-degrading bacteria suspended in deionized water. Symbols: •, control; ×, 10 mA; □, 20 mA; ▵, 40 mA.
We found that the cell surfaces of phenol-degrading bacteria in deionized water had net negative charges that ranged from −22 to −26 mV when no electric current was applied. When a 10-mA electric current was applied, the surface charge was still in this range, while in the presence of an electric current of 20 mA the net negative surface charge increased slightly from −24 to −28 mV. However, when a higher electric current (40 mA) was applied, the negative surface charge dramatically increased from −25 to about −32 mV during the 11-h test. The results revealed that only a higher electric current can increase the negative surface electrostatic charge of phenol-degrading bacteria.
Change in cell shape and extracellular substances.
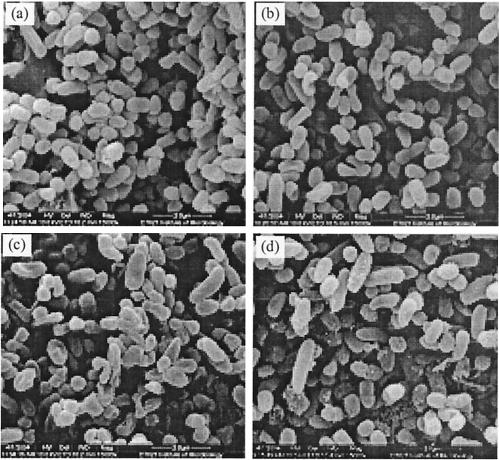
The phenol-degrading bacteria in deionized water were exposed to electric currents for 12 h and then examined by scanning electron microscopy. The changes in bacterial cell shape in the presence of various electric currents are shown in Fig. 4.
FIG. 4.
Electron micrographs of phenol-degrading bacteria not exposed to DC (a) or exposed to a DC of 10 mA (b), 20 mA (c), or 40 mA (d) for 12 h.
The micrographs show that the phenol-degrading bacteria tested in this study were mainly short rods that were 1 by 0.4 μm; a minority of the cells were rods and cocci. When no electric current was applied, the bacteria were the normal shape in spite of starvation due to the absence of nutrients for growth during the test period. When 10 mA of electric current was applied to the bacterial suspension, the majority of the bacteria were the same normal shape as the bacteria in the control test, except that a minority of the coccoid rod-shaped bacteria had concave areas on their surfaces. When 20 mA of electric current was applied, a majority of the bacterial species tested were concave and even flattened, and some cocci or coccoid rod-shaped bacteria, in particular, were roughened due to the presence of exudates on the cell surface. More seriously, when a higher electric current (40 mA) was applied to the bacterial suspension, the majority of the bacteria, including some rod-shaped species, exhibited considerable amounts of exudates on their surfaces during a 12-h period, by which the bacteria were stuck and clumped together. These results revealed that the phenol-degrading bacterial culture could resist an electric current of less than 20 mA when it was suspended in deionized water and that an electric current of more than 20 mA could change the bacterial shapes and even destroy the bacterial cells.
DISCUSSION
Mechanisms affecting DC effects on cell surface properties.
When an electric current is applied to a bacterial suspension with immersed electrodes, electrolysis on the electrodes may generate a variety of chemical oxidants, depending on the presence of oxygen and coexisting ions, such as chloride ions (5, 7, 13). Such oxidants are responsible for most of the inactivation and lethality of the applied direct current. This mechanism might occur with bacteria in MP medium due to the presence of coexisting ions in solution. The oxidative stress and the applied electric current might contribute synergistically to the significant change in hydrophobicity for the suspended bacteria in MP medium.
For the bacterial species in deionized water, bacterial growth and the effects on hydrophobicity could be minimized due to a deficit of carbon and nitrogen nutrients. Moreover, the coexisting ions capable of carrying electric current were eliminated in deionized water, and thus the bacterial species were the only carriers of the electric current applied during the test period. Therefore, any changes in hydrophobicity could be reasonably attributed to the applied electric current.
Electric current may affect the orientation of membrane lipids and consequently cell viability. A high electric current can cause irreversible permeabilization of the cell membrane and can even directly oxidize cellular constituents (7, 16, 20). For the bacterial culture in deionized water, the current effects on cells might have occurred mainly in this way so that only a higher electric current (40 mA) induced a significant change in the surface properties and cell shape. The presence of exudate on cell surfaces indicated that irreversible permeabilization of cellular substances might occur due to the exposure to higher currents. The results of a bioelectrokinetic test also suggested that bacterial inactivation might occur by interaction with the surfaces of the electrodes, resulting in cell wall or membrane degradation through oxidation or reduction (10).
Relationship between a change in cell surface properties and electrokinetic movement.
The impact of electric current on cell surface properties is of critical concern when workers evaluate the potential for injecting and transporting a bacterial culture by using electric fields for the purpose of bioaugmentation. This investigation was designed to evaluate the effect of DC on cell surface properties of bacteria in liquids. It did not focus on bacterial movement through a subsurface, but it might be expected that the induced change in surface properties and cell shape would contribute to the electrokinetic movement of bacterial species in the subsurface (6, 9).
The present study revealed that a weak electric current induced no significant changes in the cell surface properties of phenol-degrading bacteria. However, exposure to DC of more than 20 mA could cause an increase in surface hydrophobicity, the flattening of cells, and the presence of exudate on the cell surface. Such changes could stimulate the attachment of phenol-degrading bacteria to solid surfaces and thus diminish the extent of transport. A soil slurry bioelectrokinetic study supported this conclusion because it demonstrated that bacteria tended to form biofilms on solid surfaces when they were exposed to an electric current of 20 mA with a spacing of 2 cm (10). Formation of a biofilm can protect the cells from the effects of current passing through their walls and membranes and at the same time hinder bacterial movement.
On the other hand, the zeta potential measurements showed that the cell surface was charged more negatively when the cells were exposed a higher DC, as indicated by the sharp increase in the negative zeta potential after exposure to an electric current of 40 mA. This should favor the detachment of bacteria from negatively charged particles of soils and the electrophoretic movement in the subsurface (11, 12, 15). However, considering the harmful effect discussed above, a current of more than 20 mA does not seem to be suitable for practical applications for injection and transport of phenol-degrading bacteria for the purpose of bioaugmentation. In order to determine the specific surface characteristics and the DC range that allow bacteria to be transported through soils and aquifers, additional work to extend these studies to soil matrices is necessary.
Acknowledgments
This work was supported in part by RITE of Japan and in part by the National 863 program of China (grants 2002AA601120 and 2004AA649220).
REFERENCES
- 1.Ahn, I. S., and C. H. Lee. 2003. Kinetic studies of attachment and detachment of microbial cells from soil. Environ. Technol. 24:411-418. [DOI] [PubMed] [Google Scholar]
- 2.Alshawabkeh, A. N., and K. Maillacheruvu. 2001. Electrochemical and biogeochemical interactions under DC electric fields, p. 73-90. In J. A. Smith and S. E. Burns (ed.), Physicochemical groundwater remediation. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 3.Boonaert, C. J. P., and P. G. Rouxhet. 2000. Surface of lactic acid bacteria: relationships between chemical composition and physicochemical properties. Appl. Environ. Microbiol. 66:2548-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilingar, G. V., and W. W. Loo. 1997. Electrobioremediation of soils contaminated with hydrocarbons and metals: progress report. Energy Sources 19:129-146. [Google Scholar]
- 5.Davis, C. P., M. E. Shirtliff, N. M. Trieff, S. L. Hoskins, and M. M. Warren. 1994. Quantification, qualification, and microbial killing efficiencies of antimicrobial chlorine-based substances produced by iontophoresis. Antimicrob. Agents Chemother. 38:2768-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFlaun, M. F., and C. W. Condee. 1997. Electrokinetic transport of bacteria. J. Hazard. Mater. 55:263-277. [Google Scholar]
- 7.Dreesa, K. P., M. Abbaszadegan, and R. M. Maiera. 2003. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res. 37:2291-2300. [DOI] [PubMed] [Google Scholar]
- 8.Dufrene, Y. F., and P. G. Rouxhet. 1996. Surface composition, surface properties, and adhesiveness of Azospirillum brasilense—variation during growth. Can. J. Microbiol. 42:548-556. [Google Scholar]
- 9.Gannon, J. T., V. B. Manilal, and M. Alexander. 1991. Relationship between cell surface properties and transport of bacteria through soil. Appl. Environ. Microbiol. 57:190-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackman, S. A., G. Maini, A. K. Sharman, and C. J. Knowles. 1999. The effects of direct electric current on the viability and metabolism of acidophilic bacteria. Enzyme Microb. Technol. 24:316-324. [Google Scholar]
- 11.Jackman, S. A., G. Maini, A. K. Sharman, G. Sunderland, and C. J. Knowles. 2001. Electrokinetic movement and biodegradation of 2,4-dichlorephenoxyacetic acid in silt soil. Biotechnol. Bioeng. 74:40-48. [DOI] [PubMed] [Google Scholar]
- 12.Lee, H. S., and K. Lee. 2001. Bioremediation of diesel-contaminated soil by bacterial cells transported by electrokinetics. J. Microbiol. Biotechnol. 11:1038-1045. [Google Scholar]
- 13.Liu, W. K., M. R. W. Brown, and T. S. J. Elliot. 1997. Mechanisms of the bactericidal activity of low amperage electric current (DC). J. Antimicrob. Chemother. 39:687-695. [DOI] [PubMed] [Google Scholar]
- 14.Maillacheruvu, K., and A. N. Alshawabkeh. 2000. Anaerobic microbial activity under electric fields, p. 69-79. In W. D. Tedder and F. G. Pohland (ed.), Emerging technologies in hazardous waste management, vol. 8. Kluwer Academic/Plenum Publishers, New York, N.Y. [Google Scholar]
- 15.Marks, R. E., Y. B. Acar, R. J. Gale, and O. A. Elif. 2000. In situ remediation of contaminated soils by bioelectrokinetic remediation and other competitive technologies, p. 579-605. In D. L. Wise, D. J. Trantolo, and E. J. Cichon (ed.), Bioremediation of contaminated soils. Marcel Dekker Inc., New York, N.Y.
- 16.Matsunaga, T., S. Nakasono, T. Takamuku, J. G. Burgess, N. Nakamura, and K. Sode. 1992. Disinfection of drinking water by using a novel electrochemical reactor employing carbon-cloth electrodes. Appl. Environ. Microbiol. 58:686-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poortinga, A. T., J. Smit, H. C. van der Mei, and H. J. Busscher. 2001. Electric field induced desorption of bacteria from a conditioning film covered substratum. Biotechnol. Bioeng. 76:395-399. [DOI] [PubMed] [Google Scholar]
- 18.Sanin, S. L. 2003. Effect of starvation on resuscitation and the surface characteristics of bacteria. J. Environ. Sci. Health A 38:1517-1528. [DOI] [PubMed] [Google Scholar]
- 19.Sanin, S. L., F. D. Sanin, and J. D. Bryers. 2003. Effect of starvation on the adhesive properties of xenobiotic degrading bacteria. Process Biochem. 38:909-914. [Google Scholar]
- 20.Weaver, J. C., and Y. A. Chizmadzhev. 1996. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 41:135-160. [Google Scholar]
- 21.Weiss, T. H., A. L. Mills, G. M. Hornberger, and J. S. Herman. 1995. Effect of bacterial cell shape on transport of bacteria in porous media. Environ. Sci. Technol. 29:1737-1740. [DOI] [PubMed] [Google Scholar]