Abstract
A native composite transposon was isolated from Corynebacterium glutamicum ATCC 14751. This transposon comprises two functional copies of a corynebacterial IS31831-like insertion sequence organized as converging terminal inverted repeats. This novel 20.3-kb element, Tn14751, carries 17.4 kb of C. glutamicum chromosomal DNA containing various genes, including genes involved in purine biosynthesis but not genes related to bacterial warfare, such as genes encoding mediators of antibiotic resistance or extracellular toxins. A derivative of this element carrying a kanamycin resistance cassette, minicomposite Tn14751, transposed into the genome of C. glutamicum at an efficiency of 1.8 × 102 transformants per μg of DNA. Random insertion of the Tn14751 derivative carrying the kanamycin resistance cassette into the chromosome was verified by Southern hybridization. This work paves the way for realization of the concept of minimum genome factories in the search for metabolic engineering via genome-scale directed evolution through a combination of random and directed approaches.
Transposons and insertion sequences are mobile genetic elements that are present in virtually every living organism (2), although Bacillus subtilis strain 168 is a notable exception (19). A specific enzyme, transposase, which generates cuts at the inverted repeats that constitute the boundaries of the mobile element, mediates transposition. In contrast to the small cryptic insertion sequences (which are less than 2.5 kb long), transposons are complex elements that carry additional genes, including genes conferring antibiotic resistance. This property has enabled isolation of transposons from several pathogenic organisms (16, 24). Composite transposons, such as Tn10, comprise two identical insertion sequences as converging terminal inverted repeats, each of which is able to promote transposition events (16). Transposition activity is modulated by several host factors that are generally specific for each element or family of elements (23). Insertion sequences can be used to construct artificial transposons and artificial composite transposons (10, 33). However, isolation of these sequences is relatively tedious due to their cryptic nature. Strategies that rely on a positive selection scheme (e.g., strategies based on expression of a lethal gene) have been successful in circumventing this technical hurdle. For instance, the insertion sequence IS31831 originating from Corynebacterium glutamicum (35) was cloned by taking advantage of the properties of the B. subtilis sacB gene product, the enzyme levan sucrase (7), whose expression is lethal to coryneform bacteria growing on medium containing 10% sucrose (14).
IS31831 is a 1,453-bp insertion sequence with 24-bp imperfect indirect terminal inverted repeats. It belongs to the ISL3 family of insertion sequences (23). Like all members of this family, IS31831 generates 8-bp direct repeats upon insertion. It exhibits no obvious target sequence specificity, even though data are consistent with a preference for AT-rich regions. Chimeric derivatives of this insertion sequence have been used to mutagenize the genome of C. glutamicum, an important amino acid producer (18), with an efficiency of approximately 4 × 104 mutants per μg of DNA (33).
Several other insertion sequences have been identified in corynebacteria. Tn5564 is a Corynebacterium striatum transposon that confers chloramphenicol resistance (32). Transposition of this transposon in the C. glutamicum genome was performed by conjugation from an Escherichia coli strain to obtain a frequency of approximately 3 × 10−8. The transposon was preferentially inserted into target sites containing the palindromic tetranucleotide CTAG (32). The insertion sequence ISCg2 shows preferential integration into target sequences located adjacent to genes involved in aspartate and glutamate metabolism (27). IS13869 is an insertion element that closely resembles IS31831 (79% amino acid identity) (6), and it is detectable by hybridization when IS31831 DNA is used as a probe (6, 35). IS1206 (3) is not related to IS31831 and belongs to the IS3 family of insertion sequences. This family of insertion sequences is characterized by two consecutive and partially overlapping open reading frames (ORFs) in relative translational reading frames 0 and −1 (23). IS1206 may thus be governed by complex mechanisms that could hinder its use for practical applications.
The availability of the complete sequence of C. glutamicum enables manipulation of this organism at the chromosome level rather than at the gene level. While gene disruption and replacement have been performed in this organism by using a variety of methods (20, 30, 34), there is still a need for molecular tools for chromosome manipulation. Transposons are particularly versatile molecular biology tools and have been used, for example, to deliver rare restriction sites (21).
We propose constructing new transposition vectors for bacterial genome technology aimed at deletion of large DNA fragments or integration of such fragments into bacterial genomes as a means to develop improved production strains. Our objectives for improved production organisms include minimum genome factories (MGFs). MGFs can be defined as recombinant strains whose metabolism has been engineered to accumulate desired products. This typically involves an exhaustive metabolic engineering effort achieved by reducing the gene pool of an organism to its optimal minimum subset, as defined by the targeted application. MGFs can be created either by a targeted approach via homologous recombination or by a random transposon-mediated directed-evolution approach or by a combination of both of these approaches. The intrinsic characteristics of an ideal transposon-based tool for MGF generation can be defined as follows. Homologous native mobile elements should be absent from the strains of interest. The transposon should exhibit no stringent target specificity. Several unrelated elements that can exist simultaneously should be available and should be detectable by probes that do not cross-hybridize. In addition, these elements should be suitable for moving large to very large DNA fragments (10 to >100 kb).
In an effort to isolate novel transposons and insertion sequences that address these different needs, we surveyed several species of coryneform bacteria and isolated a native 20.3-kb composite transposon, Tn14751, which carries approximately 17.4 kb of C. glutamicum chromosomal DNA. We report in this paper the intrinsic characteristics of this novel element. To our knowledge, this is the first example of a native transposable element that confers no antibiotic resistance but carries a large chromosomal DNA fragment.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′ [traD36 proAB+ lacIqlacZΔM15] | Takara |
| JM110 | dam dcm supE44 hsdR17 thi leu rpsL lacY galK galT ara tonA thr tsx Δ(lac-proAB)/F′ [traD36 proAB+ lacIqlacZΔM15] | 29 |
| C. glutamicum strainsa | ||
| R | Wild type | 17 |
| ATCC 14751 | Origin of Tn14751 | ATCC |
| CGR732-1 to CGR732-9 | Minicomposite Tn14751 mutants | This study |
| Plasmids | ||
| pUC119 | Apr; α-lac multicloning site, M13 ori | Takara |
| pHSG398 | Cmr; α-lac multicloning site, M13 ori | Takara |
| pUC4K | Apr Kmr; source of Kmr cartridge | Pharmacia |
| pMV5 | Spr; transposon screening vector including sacR and sacB genes; complete sequence determined in this study | 35 |
| pCRA730 | Spr; pMV5 with 20.3-kb Tn14751 | This study |
| pCRA731 | Cmr; pHSG398 with a 20.3-kb HpaI-HapI DNA fragment containing the entire Tn14751 transposon | This study |
| pCRA732 | Cmr Kmr; pHSG398 with IS14751L, Kmr, and IS14751R (minicomposite Tn14751) | This study |
All other C. glutamicum strains were obtained from the American Type Culture Collection (ATCC).
Chemicals.
All chemicals were the highest purity available and were purchased from Wako Pure Chemical Industries (Osaka, Japan) or Sigma (St. Louis, Mo.).
Culture conditions.
For genetic manipulation, E. coli strains were grown at 37°C in Luria-Bertani medium (29), and C. glutamicum strains were grown at 33°C in A medium (13) with 4% glucose. When appropriate, media were supplemented with antibiotics. The final antibiotic concentrations for E. coli were 50 μg of ampicillin ml−1, 50 μg of kanamycin ml−1, 200 μg of spectinomycin ml−1, and 50 μg of chloramphenicol ml−1; for C. glutamicum, the final antibiotic concentrations were 50 μg of kanamycin ml−1, 200 μg of spectinomycin ml−1, and 5 μg of chloramphenicol ml−1.
DNA techniques.
Chromosomal DNA was isolated with a Genomic Prep kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). Plasmid DNA was isolated by the alkaline lysis procedure (29). Restriction endonucleases, the Klenow fragment, and T4 DNA ligase were obtained from Takara (Kyoto, Japan) and were used according to the manufacturer's instructions. When required, restriction fragments were isolated from agarose gels with a Prep-a-Gene matrix (Bio-Rad, Richmond, Calif.) used according to the manufacturer's instructions. All PCR were carried out with an Applied Biosystems GeneAmp System 9700 as recommended by the manufacturer by using the following amplification protocol: 30 cycles of denaturation at 95°C for 1 min, annealing at 54°C for 1 min, and extension at 72°C for 1 min.
Bacterial cell transformation.
Corynebacteria were transformed by electroporation (36) by using plasmid DNA purified from E. coli JM110. E. coli strains were transformed by the CaCl2 method (29).
Detection of transposition events.
Clones bearing a disrupted copy of the B. subtilis sacB gene were selected as described by Vertès et al. (35) on MMSS minimum medium, which contained (per liter) 7 g of (NH4)2SO4, 2 g of urea, 0.5 g of K2HPO4, 0.5 g of KH2PO4, 0.5 g of MgSO4, 6 mg of FeSO4 · 7H2O, 6 mg of MnSO4 · 6H2O, 200 μg of biotin, 200 μg of thiamine-HCl, 100 g of sucrose, and 200 μg of spectinomycin.
DNA sequencing.
We generated a library of the plasmid carrying the sacB gene interrupted by the composite transposon Tn14751 by sonication with a Misonics sonicator (Misonics, Farmingdale, N.Y.) on power setting 1. The Eppendorf tube containing the DNA sample was placed in an ice bath, and eight cycles of sonication for 1 s interrupted by 1-s intervals were performed. The resulting random fragments were separated according to size on a 1% agarose gel, and the fraction corresponding to the 2- to 3-kb pool was subsequently purified from the gel. The fragments were blunted by treatment with the Klenow fragment and ligated to SmaI-digested pUC119 plasmid DNA. The ligation mixture was used to transform E. coli JM109, and recombinants were selected on isopropyl-β-d-thiogalactopyranoside (IPTG)-supplemented plates (29). For sequencing purposes, clones were grown overnight in 96-well microtiter plates in 1 ml of 2× Luria-Bertani medium by using a TAITEC Bioshaker. The corresponding plasmids were isolated in 96-well plates by using a Millipore Montage Plasmid Miniprep96 kit and a Cosmotec HT Station 500 simple 96-well pipetting robot (Cosmotec, Tokyo, Japan). The plasmid library generated in this way was sequenced at both ends by using M13 universal forward and reverse primers (29) and cycle sequencing by the BigDye method of ABI Biosystems Inc. with an ABI 3700 CE sequencer. The Applied Biosystems GeneAmp System 9700 was used for PCR as described above. Prior to sequencing, the PCR products were purified by treatment with exonuclease by using ExoSAP-IT (U.S. Biochemicals, Cleveland, Ohio).
DNA sequences were analyzed as follows. The raw chromatogram files (.abi files) were collected on a personal computer running Windows 2000 (Microsoft Corp.). The chromatogram files were subsequently transferred to a SunBlade 1000 computer (Sun) with one 900-MHz 64-bit UltraSparc-III CPU and 2 GB of memory. The Pregap4 program of the Staden package (4, 31) was used for clipping vector sequences, as well as for quality clipping and contamination screening after base calling by Phred (8, 9). BLASTX searches were carried out with the Sun computer by using the stand-alone BLAST program (1) of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and matrix BLOSUM62 (11).
Sequencing of IS14751L and IS14751R.
The random sequencing of the 20.3-kb mobile element Tn14751 described above revealed the presence of two copies of IS31831-like elements (IS14751L and IS14751R) at both ends as inverted repeats. To clarify the difference between the nucleotide sequences of IS14751L and IS14751R, we individually cloned two DNA fragments, as follows: an 8.3-kb HpaI-BglII DNA fragment containing IS14751L was subcloned into the SmaI-BamHI sites of pUC119, and a 1.9-kb HpaI-ScaI DNA fragment containing IS14751R was subcloned into the SmaI site of pUC119. Both inserts on the plasmids were sequenced by primer walking methods.
Phylogenetic analysis.
The amino acid sequences encoded by transposase genes of IS14751 and reference insertion elements were aligned by using the CLUSTALW program (http://www.ddbj.nig.ac.jp/E-mail/clustalw-j.html). A phylogenetic tree was constructed by the neighbor-joining method by using the TreeView program (version 1.6.6) and Knuc values (15).
Dot blot hybridization and Southern hybridization.
One microgram of chromosomal DNA from various corynebacteria strains was denatured at 94°C for 10 min and spotted onto nylon membranes. After the membranes were baked at 80°C for 2 h, they were prehybridized for 2 h, hybridized overnight at 60°C, and then washed at high stringency as described by Sambrook and Russell (29). Southern hybridization was carried out as described elsewhere (29). Hybridization probes were labeled by using the CDP-Star detection system (Amersham Biosciences). A 1.5-kb DNA fragment containing either IS14751L or IS14751R (which were identical) in Tn14751 (fragment III), amplified by PCR performed with primers 1 and 2 (Table 2) and with pCRA730 as the template, was used for dot blot hybridization and Southern hybridization. The same DNA probe was also adapted for use in the transposition experiments with the C. glutamicum chromosome. DNA fragment I (0.4 kb; left part of Kmr), amplified with primers 7 and 8 (Table 2) and with pUC4K as the template, and DNA fragment II (0.4 kb; right part of Kmr), amplified with primers 9 and 10 (Table 2) and with pUC4K as the template, were also utilized for transposition into the C. glutamicum chromosome. Hybridization signals were detected with a Fujifilm LAS-1000 image analyzer system (Fuji).
TABLE 2.
Oligonucleotides used in this study
| Target | Primer | Sequence (5′-3′)a | Overhanging restriction site |
|---|---|---|---|
| IS14751L or IS14751R (fragment III)b | 1 | GGCCCTTCCGGTTTTGGGGTACAT | |
| 2 | GGCTCTTCCTGTTTTAGAGTGCAT | ||
| IS14751L, IS14751R, and pHSG398 | 3 | AGTCAGATCTAAGTGGAGCACCTAGATCGC | BglII |
| 4 | AGTCAGATCTAGTCACGCACATCTTCTGCA | BglII | |
| Kmr gene | 5 | AGTCAGATCTTGTGTCTCAAAATCTCTGA | BglII |
| 6 | AGTCAGATCTCTGAGGTCTGCCTCGTGAA | BglII | |
| Left part of Kmr gene (fragment I)b | 7 | ATGAGCCATATTCAACGGGA | |
| 8 | GGACAATTACAAACAGGAAT | ||
| Right part of Kmr gene (fragment II)b | 9 | CGTATTTCGTCTCGCTCAGG | |
| 10 | TTAGAAAAACTCATCGAGCA |
The restriction site overhangs used in the cloning procedure are underlined.
See Fig. 8.
Nucleotide sequence accession numbers.
The DDBJ/EMBL/GenBank accession numbers for the sequences described in this paper are AB183144 (plasmid pMV5), AB183145 (20.3-kb composite transposon Tn14751), and AB183146 (plasmid pCRA732 containing minicomposite Tn14751).
RESULTS
Isolation of transposable element.
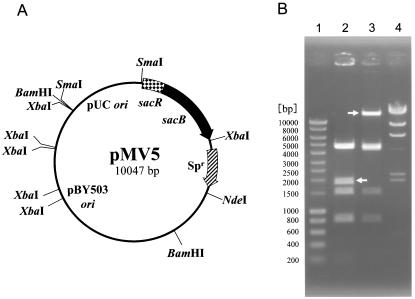
C. glutamicum ATCC 14751 cells were transformed with plasmid pMV5 (Fig. 1A), which harbors the B. subtilis sacB gene (35). Transformants harboring this plasmid were subsequently grown overnight in A medium supplemented with spectinomycin, and 100 μl of this culture was used to seed MMSS medium plates. Several spectinomycin- and sucrose-resistant colonies were present after 2 days of incubation at 33°C. Mutants that gained sucrose resistance via events not related to transposition were identified by restriction digestion and agarose gel electrophoresis of plasmid DNA. These mutants were not analyzed further. Restriction analysis of the plasmid extracted from mutant strain CGR730, designated pCRA730, revealed that the size of the approximately 20-kb SmaI-XbaI band containing the sacB gene from plasmid pMV5 changed (Fig. 1B). Inactivation of the sacB gene was the result of insertion of a 20.3-kb DNA fragment. Plasmid pCRA730 was used for further characterization.
FIG. 1.
Transposition of Tn14751 into the B. subtilis sacB gene. (A) Physical and genetic map of pMV5. pUC ori, replication origin of pUC19; pBY503 ori, replication origin of pBY503; Spr, spectinomycin resistance gene. The arrows indicate the direction of transcription. XbaI, SmaI, BamHI, and NdeI sites are indicated. (B) Lane 1, Smart Ladder (Nippon Gene-Wako, Tokyo, Japan) (the size of each band is indicated on the left); lane 2, pMV5 digested with SmaI-XbaI; lane 3, pCRA730 digested with SmaI-XbaI; lane 4, λ HindIII molecular weight marker. The arrows indicate the position of the 1.8-kb fragment in SmaI-XbaI-digested pMV5 for the band corresponding to the sacB gene (lane 2) and the position of the 20.3-kb fragment in SmaI-XbaI-digested pCRA730 for the band corresponding to the Tn14751 inserted into the sacB gene (lane 3).
Sequence analysis of Tn14751.
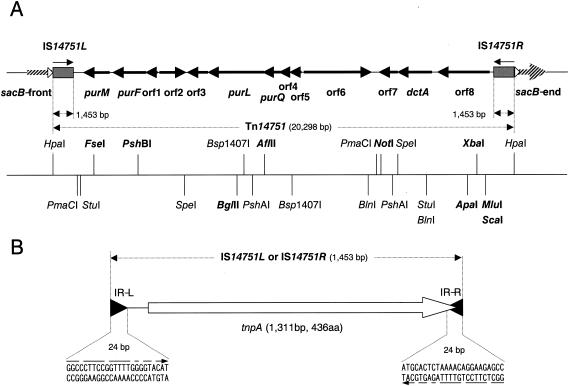
The complete nucleotide sequence of both strands of the 20.3-kb mobile DNA fragment in plasmid pCRA730 was determined. A restriction cleavage map of this fragment is shown in Fig. 2. This fragment was inserted at positions 589 to 697 into the sacB gene (position 1 is the A of ATG) in plasmid pCRA730. The 20.3-kb DNA fragment comprised identical 1,453-bp inverted repeats at each end and a 17,392-bp fragment between these elements (Fig. 2A).
FIG. 2.
Genetic and physical maps of the composite transposon Tn14751. (A) The deduced amino acid sequences encoded by the 13 open reading frames in Tn14751 were identified. The copies of IS31831 elements constitute inverted repeats (represented by gray boxes). Eight-base-pair direct repeats are indicated by open arrowheads. The arrows indicate the directions of transcription. The physical map is below the genetic map. The sacB gene is indicated by a cross-hatched arrow. Unique restriction sites are indicated by boldface type. (B) IS14751L and IS14751R, which are identical, contain the tpnA gene. The solid arrowheads indicate the 24-bp imperfect inverted repeats (IR-L and IR-R). The nucleotide sequences of IR-L and IR-R are indicated below the arrowheads. Nucleotides that were identical in two sequences are indicated by converging arrows. aa, amino acids.
Computer analysis of the inverted repeats indicated the presence of one potential ORF. This ORF consisted of 1,311 nucleotides corresponding to 436 amino acids with a predicted molecular weight of 49,629 (Fig. 2B). The deduced amino acid sequence encoded by the ORF showed high sequence similarity (99.5%) to the sequence encoded by the transposase gene (tnpA) of IS31831 (35), which belongs to the ISL3 family. Each 1,453-bp inverted repeat had a 24-bp imperfect inverted repeat (5-bp mismatches) at both ends (IR-L and IR-R) (Fig. 2B), and the 3′ end of the tnpA gene and IR-R had an 11-bp overlap. The overall level of DNA sequence similarity between the inverted repeats and IS31831 was 99.4%. These data suggested that the 1,453-bp inverted repeats were IS31831-like elements and that the 20.3-kb mobile element, designated Tn14751, was a composite transposon which comprised two copies of IS31831-like elements as inverted repeats. The insertion elements at the ends of Tn14751 were designated IS14751L and IS14751R (Fig. 2A).
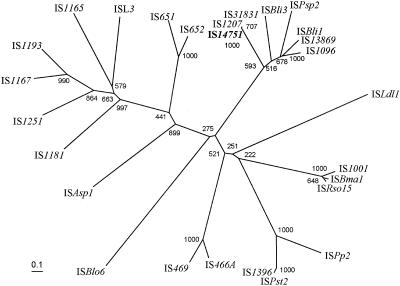
To confirm the phylogenetic position of the transposase of IS14751 (IS14751L or IS14751R), the amino acid sequence of the IS14751 transposase was compared to the sequences of known transposases which belong to the ISL3 family (Fig. 3). The IS14751 transposase formed a tight cluster with the transposases of IS1207 from C. glutamicum Bl15, IS31831 from C. glutamicum ATCC 31831, ISBli3 from Brevibacterium linens, ISPsp2 from Pseudomonas sp. strain EST1001(pEST1226), ISBli1 from B. linens, IS13869 from Brevibacterium lactofermentum ATCC 13869, and IS1096 from Mycobacterium smegmatis ATCC 607. Except for Pseudomonas sp. strain EST1001 harboring plasmid pEST1226, which contains the transposase gene of ISPsp2, all these strains containing insertion elements are closely related (Corynebactrium, Brevibacterium, and Mycobacterium species).
FIG. 3.
Unrooted distance matrix tree showing the phylogenetic relationships of known insertion elements belonging to the ISL3 family based on the corresponding transposases. The topology of the phylogenetic tree was evaluated by performing a bootstrap analysis with 1,000 replicates. The numbers at the nodes are bootstrap values based on 1,000 replicates. Scale bar = 0.1 Knuc. The accession numbers or source of information for the sequences used are as follows: IS1207, accession no. X96962; IS31831, accession no. D17429; ISBli3, http://www-is.biotoul.fr/is.html; ISPsp2, accession no. M57500; ISBli1, accession no. AF052055; IS13869, accession no. Z66534; IS1096, accession no. M76495; ISLdl1, accession no. AJ302652; IS1001, accession no. X66858; ISBma1, accession no. AF285635; ISRso15, accession no. NC_003295; ISPp2, accession no. U25434; ISPst2, accession no. AJ012352; IS1396, accession no. AF027768; IS466A, accession no. AB032065; IS469, accession no. AB032065; ISBlo6, accession no. NC_004307; ISAsp1, accession no. U13767; IS1181, accession no. L14544; IS1251, accession no. L34675; IS1167, accession no. M36180; IS1193, accession no. Y13713; IS1165, accession no. X62617; ISL3, accession no. X79114; IS651, accession no. NC_002570; and IS652, accession no. NC_002570.
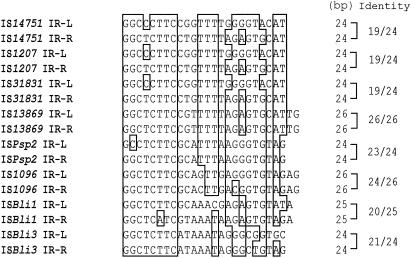
A comparison of the inverted repeat (IR-L and IR-R) sequences of eight insertion elements which formed a tight cluster as determined by phylogenetic analysis (Fig. 3) revealed a high level of conserved sequences, especially the first 8 bp at the 5′ end (Fig. 4).
FIG. 4.
Comparison of the inverted repeat sequences of insertion elements IS14751, IS1207, IS31831, IS13869, ISPsp2, IS1096, ISBli1, and ISBli3. Nucleotides that were identical in at least 9 of 16 sequences are enclosed in boxes. IR-L and IR-R indicate the inverted repeats at the 5′ end upstream and the 3′ end downstream of the tnpA gene, respectively. The length of each inverted repeat sequence and the identity ratio (number of identical nucleotides/length of inverted repeat) for IR-L and IR-R of each insertion element are indicated on the right.
The overall G+C content of Tn14751 excluding the two insertion elements (IS14751L and IS14751R) was calculated to be 55.3%. The two insertion elements bracket a large piece of chromosomal DNA containing the following 13 open reading frames: purM (encoding 5′-phosphoribosyl-5-aminoimidazole synthase), purF (encoding amidophosphoribosyl transferase), ORFs encoding three hypothetical proteins (orf1, orf2, and orf3), purL (encoding 5′-phosphoribosyl-formylglycinamidine synthase II), purQ (encoding 5′-phosphoribosyl-formylglycinamidine synthase I), ORFs encoding four hypothetical proteins (orf4 to orf7), dctA (encoding aerobic C4-dicarboxylate transporter), and an ORF encoding one hypothetical protein (Fig. 2A). The similarities between these proteins and previously identified proteins are shown in Table 3. All of the genes are present in the same order in the genomes of C. glutamicum R (unpublished data) and C. glutamicum ATCC 13032 (accession number NC_003450) (12), but they are not flanked by two insertion sequences organized in indirect repeats. The gene cluster resembles a similar gene cluster in Corynebacterium efficiens with lower sequence similarity than the similarity in C. glutamicum strains, except for the dctA gene, which is located at a different locus on the chromosome. On the other hand, analysis of the Corynebacterium diphtheriae genome showed that several genes in the cluster are absent and that the remaining genes are scattered on the chromosome. The differences in gene distribution among the strains mentioned above corresponded to the differences in phylogenetic classification determined by 16S rRNA gene analysis for corynebacteria (25).
TABLE 3.
C. glutamicum chromosomal genes encoded by Tn14751a
| Genea | Direction of transcriptionb | Coordinatesc |
C. glutamicum R homologous gene
|
C. glutamicum ATCC 13032 homologous gene
|
C. efficiens homologous gene
|
C. diphtheriae homologous gene
|
Other homologous gene
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gened | % Homologye | Gened | % Homologye | Gened | % Homologye | Gened | % Homologye | Speciesf | Gene or protein | Accession no. | % Homologye | |||
| purM | − | 1480-404 | 2486 | 100 | 2494 | 99 | 2475 (purM) | 89 | 1919 (purM, purG) | 85 | Mt | purM | NP_335258.1 | 71 |
| purF | − | 3153-1606 | 2487 | 99 | 2495 | 99 | 2476 (purF) | 87 | 1920 | 72 | Mt | purF | NP_335257.1 | 68 |
| orf1 | − | 3583-3206 | 2488 | 97 | 2496 | 99 | 2477 | 70 | 1921 | 64 | Mt | Hypothetical protein Rv0807 | NP_335256.1 | 58 |
| orf2 | + | 3621-4640 | 2489 | 97 | 2497 | 99 | 2478 | 83 | So | Cytosolic long-chain acyl-coenzyme A thioester Hydrolase family protein | AAN55797.1 | 46 | ||
| orf3 | − | 5449-4685 | 2490 | 95 | 2498 | 99 | 2479 | 78 | 1922 | 55 | Mt | Hypothetical protein Rv1085c | NP_335559.1 | 38 |
| purL | − | 7898-5550 | 2491 | 99 | 2499 | 99 | 2480 (purL) | 86 | 2130 | 23 | Mt | purL | NP_854484.1 | 65 |
| purQ | − | 8523-7852 | 2492 | 100 | 2500 | 99 | 2481 (purQ) | 86 | Mt | purQ | NP_335241.1 | 68 | ||
| orf4 | − | 8765-8520 | 2493 | 98 | 2501 | 98 | 2482 | 93 | Mt | Phosphoribosylformylglycinamidine synthase, PurS component | NP_335240.1 | 73 | ||
| orf5 | − | 9332-8853 | 2494 | 98 | 2502 | 99 | 2483 | 74 | 1925 | 54 | Sc | Putative glutathione peroxidase | CAB88451.1 | 54 |
| orf6 | + | 9520-12324 | 2495 | 97 | 2503 | 93 | 2484 | 69 | Pa | Hypothetical protein | E83157 | 32 | ||
| orf7 | − | 13408-12719 | 2496 | 98 | 2505 | 99 | 2485 | 77 | Mt | Hypothetical protein | AAK45050.1 | 40 | ||
| dctA | − | 14793-13453 | 2497 | 99 | 2506 | 99 | 2864 | 21 | 1992 | 28 | Mt | dctA | NP_337003.1 | 68 |
| orf8 | − | 17240-15120 | 2498 | 99 | 2507 | 99 | 2486 | 77 | 1926 | 74 | Mb | Probale protease II PtrB (oligo- pepetidase B) | CAD93666.1 | 53 |
The open reading frames in Tn14751 were identified by BLASTX searches.
The direction of transcription is indicated by a plus sign (forward strand) or a minus sign (reverse strand).
The coordinate origin is the base immediately adjacent to the last base of IS14751L.
The genes of C. glutamicum R (unpublished data), C. glutamicum ATCC 13032 (accession no. NC_003450) (12), C. efficiens (accession no. NC_004369) (26), and C. diphtheriae (accession no. NC_002935) (5) are numbered according to the previously described annotations.
Levels of amino acid sequence homology.
Mt, Mycobacterium tuberculosis; So, Shewanella oneidensis; Sc, Streptomyces coelicolor; Pa, Pseudomonas aeruginosa; Mb, Mycobacterium bovis.
Distribution of IS14751 in various corynebacteria.
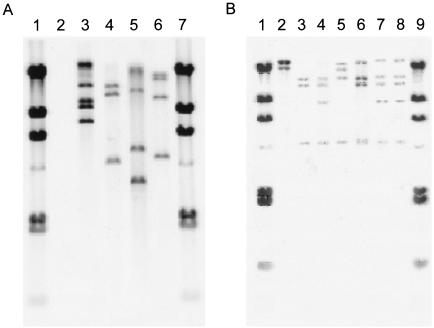
To investigate the presence of IS14751 in various corynebacteria, dot blot hybridization of chromosomal DNA was performed by using a 1.5-kb DNA fragment containing IS14751 (see Materials and Methods) as a probe. The results are shown in Fig. 5. C. glutamicum ATCC 13032, ATCC 13869, and ATCC 31831, which are frequently used for studies, showed strong signals equivalent to those of several strains, such as C. glutamicum ATCC 13745, ATCC 14996, ATCC 15025, ATCC 19052, ATCC 19053, ATCC 19055, and ATCC 19058. The copy number of IS14751 in the strains mentioned above was verified by Southern hybridization by using the same DNA fragment containing IS14751 in Tn14751 (Fig. 6). These strains contained at least three to five copies of IS14751 or homologous insertion sequences (e.g., IS31831).
FIG. 5.
Dot blot analysis of chromosomal DNA from various corynebacterial strains hybridized with IS14751 as the probe. The numbers above the signals indicate the C. glutamicum strains. R is C. glutamicum R, while all other strains are American Type Culture Collection strains.
FIG. 6.
Southern blot of chromosomal DNA from various corynebacterial strains hybridized with IS14751 as the probe. (A) Lanes 1 and 7, λ HindIII molecular weight marker; lanes 2 to 6, SmaI-digested chromosomal DNA of C. glutamicum R, ATCC 14751, ATCC 13032, ATCC 13869, and ATCC 31831, respectively. (B) Lanes 1 and 9, λ HindIII molecular weight marker; lanes 2 to 8, SmaI-digested chromosomal DNA of C. glutamicum ATCC 13745, ATCC 14996, ATCC 19055, ATCC 19058, ATCC 15025, ATCC 19052, and ATCC 19053, respectively.
In contrast, the laboratory strain C. glutamicum R (17), as well as C. glutamicum ATCC 13058, ATCC 13761, ATCC 13826, ATCC 14020, ATCC 14306, ATCC 14752, ATCC 14999, ATCC 15455, and ATCC 15990, appeared to be devoid of IS14751 or homologous insertion sequences. The lack of signals was also confirmed by Southern hybridization (Fig. 6A and data not shown). Therefore, in C. glutamicum R and the other nine strains mentioned above the native IS14751 homologous elements are not on the chromosome, suggesting that Tn14751 or IS31831 is an ideal transposon-based tool for these strains.
Transposition of Tn14751 derivatives into C. glutamicum.
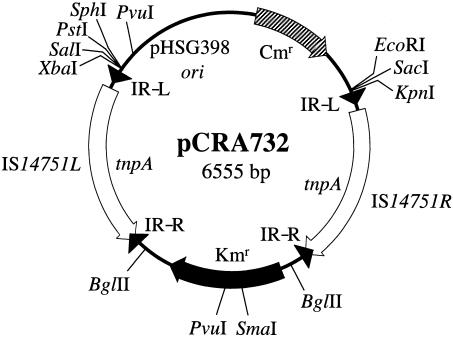
To clarify the transposition efficiency of Tn14751, we constructed an artificial minicomposite Tn14751 transposon. The 17.4-kb corynebacterial chromosome portion was omitted from Tn14751 in order to avoid a background for transposition efficiency caused by homologous recombination. A 20.3-kb HpaI-HpaI DNA fragment containing the entire Tn14751 transposon (Fig. 2) was recovered from pCRA730 and was ligated to the SmaI site of pHSG398, resulting in plasmid pCRA731. Inverse PCR was performed by using primers 3 and 4 (Table 2) and pCR731 as the template for amplifying a 5.1-kb DNA fragment containing IS14751L, pHSG398, and IS14751R. The amplified DNA fragment was digested with BglII and ligated to a BglII-digested 1.2-kb kanamycin cassette, which was amplified by PCR by using primers 5 and 6 (Table 2) and pUC4K as the template. The resultant plasmid, pCRA732 (Fig. 7) containing a minicomposite Tn14751 transposon, was used for further study.
FIG. 7.
Physical and genetic map of pCRA732. Kmr, kanamycin resistance gene; Cmr, chloramphenicol resistance gene; pHSG398 ori, origin of replication from pHSG398; tnpA, transposase gene from IS14751L and IS14751R. The solid arrowheads indicate the positions of IR-L and IR-R of IS14751. The arrows indicate the direction of transcription.
Plasmid pCRA732, which did not replicate in corynebacteria, was electroporated into C. glutamicum R as described previously (34) to transpose the minicomposite Tn14751 transposon into the chromosome. All colonies grown on A medium containing kanamycin which were tested had chloramphenicol sensitivity, suggesting that the minicomposite Tn14751 transposon was transposed into the chromosome but not into the pHSG398 vector part containing the chloramphenicol resistance gene. The transposition efficiency was 1.8 × 102 mutants per μg of DNA.
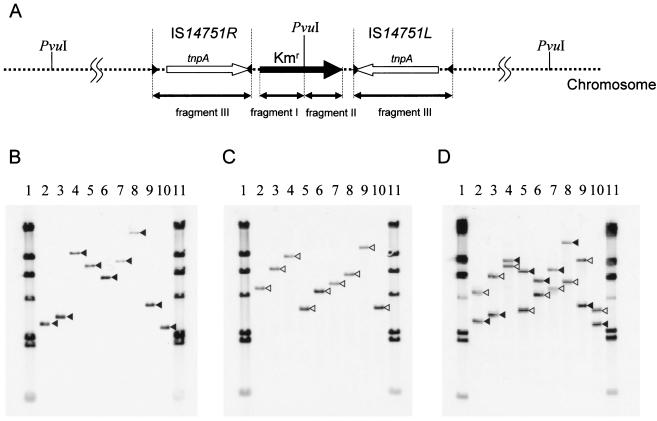
In order to verify that the derivative of Tn14751 transposed randomly, genomic Southern hybridization of nine randomly selected minicomposite Tn14751 integrants (CGR732-1 to CGR732-9) was conducted (Fig. 8). Three different kinds of probes (fragments I, II, and III) were used in Southern hybridization to determine whether the whole minicomposite Tn14751 transposon or either one of the two IS14751 elements at both ends of Tn14751 transposed into chromosomal DNA in these integrants (Fig. 8A). Chromosomal DNA was digested with PvuI, whose unique recognition site was located only at the center of the kanamycin resistance gene and not in IS14751. When the left side of a fragment of the kanamycin resistance gene (fragment I) was used as the probe, hybridization signals were detected at different sizes, indicating a variety of insertion mutations (Fig. 8B). Although utilization of the right side of the fragment of the kanamycin resistance gene (fragment II) as the probe also resulted in different sizes of hybridization signals, the hybridization patterns obtained with fragments I and II as the probes did not overlap (Fig. 8B and C). Southern hybridization with fragment III (IS14751) as the probe revealed two bands in each lane, and the hybridization pattern was a composite of the two hybridization patterns described above (Fig. 8D), suggesting that the whole minicomposite Tn14751 transposon was transposed into the C. glutamicum chromosomal DNA. No multiple insertions were observed.
FIG. 8.
Transposition of minicomposite Tn14751 into C. glutamicum. (A) Schematic physical map of the minicomposite Tn14751 transposon in the chromosome of C. glutamicum mutants. The positions of Southern hybridization probes (fragments I, II, and III) are indicated by bidirectional arrows below the map. PvuI sites are indicated above the map. The tnpA genes and inverted repeat sequences of IS14751L and IS14751R are indicated by open arrows and solid arrowheads, respectively. The kanamycin resistance gene is represented by a solid arrow. (B to D) Southern hybridization of PvuI-digested chromosomal DNA from nine minicomposite Tn14751 integrated C. glutamicum mutants with fragment I (B), fragment II (C), and fragment III (D) as the probes. Lanes 1 and 11, λ HindIII molecular weight marker; lanes 2 to 10, nine minicomposite Tn14751 integrated C. glutamicum mutants (CGR732-1 to CGR732-9). (B) The solid arrowheads indicate the migration positions of hybridization signals when fragment I was the probe. (C) The open arrowheads indicate the migration positions of hybridization signals when fragment II was the probe. (D) Southern hybridization with fragment III as the probe resulted in two bands in each lane (lanes 2 to 10). The migration positions of signals corresponding to signals shown in panel B are indicated by solid arrowheads, and the migration positions of signals corresponding to signals shown in panel C are indicated by open arrowheads.
Target sequence of Tn14751 insertions.
The site of insertion of Tn14751 into plasmid pCRA730 was determined by sequencing (Fig. 9). Insertion of Tn14751 into strain CGR730 resulted in an 8-bp duplication (GTTAACGT) (direct repeats), as observed upon transposition of the insertion sequence IS31831. To confirm the conservation of direct repeats accompanied by transposition of Tn14751 derivatives, the insertion sites of the minicomposite Tn14751 transposon in the chromosomes of six C. glutamicum mutants, which were randomly selected from minicomposite Tn14751 mutants, were also analyzed (Fig. 9). All of the direct repeats created by CGR732-1, -2, -4, -5, -8, and -9 (Fig. 8) were 8 bp long and AT rich in the middle portion, but they were different, suggesting that Tn14751 and its derivatives randomly inserted at several different loci in the C. glutamicum chromosome. Furthermore, it was reported previously that IS13869, IS1096, and ISBli1 (http://www-is.biotoul.fr/is.html), which form a tight phylogenetic cluster (Fig. 3) with IS14751 and IS31831, generated direct repeats at the insertion site by transposition, whose properties (different 8-bp sequences and AT rich in the middle portion) were similar to those observed with IS14751 and IS31831.
FIG. 9.
Direct repeats with transposition of Tn14751 and its derivative on plasmids or chromosomal DNA in C. glutamicum. The 8-bp direct repeats created by transposition of Tn14751 and six minicomposite Tn14751 mutants (CGR732-1, -2, -4, -5, -8, and -9) are shown. Direct repeats with transposition of IS31831 in C. glutamicum (p150B, p70D, pCG1) from the study of Vertès et al. (33) are also indicated.
By chance, the 8-bp duplication included an HpaI site (GTTAAC), which made Tn14751 available on a 20.3-kb HpaI cassette. We therefore used this construct for subcloning, as described above (Fig. 2).
DISCUSSION
In an effort to develop molecular biology tools for bacterial genome technology to realize the concept of MGFs, we isolated a novel transposon that is capable of translocating large pieces of chromosomal DNA. This transposon, from C. glutamicum ATCC 14751, was designated Tn14751. It is 20.3 kb long and carries two copies of IS31831-like elements (IS14751) organized in inverted repeats flanking an approximately 17.4-kb chromosomal DNA fragment. The G+C content of this chromosomal DNA fragment was determined to be 55.3%. This value is in agreement with the G+C contents of nonmedical strains of the genus Corynebacterium (22), including C. glutamicum R (unpublished data) and C. glutamicum ATCC 13032 (accession number NC_003450), whose G+C contents were calculated to be 54.1 and 53.8%, respectively. The amino acid sequences deduced from the open reading frames which are present in Tn14751 exhibit a high degree of similarity with amino acid sequences encoded by genes from C. glutamicum R and ATCC 13032 (Table 3). These observations corroborate the view that the DNA fragment carried by transposon Tn14751 originates from the Corynebacterium chromosome and does not result from a horizontal gene transfer event.
To our knowledge, transposons carrying large chromosomal DNA fragments have not been isolated frequently. Genes involved in microbial warfare, such as antibiotic resistance genes or biosynthetic genes for extracellular toxins, have been encountered frequently in mobile genetic elements, whereas housekeeping genes, like those of Tn14751, have not. However, transposons containing a more limited number of chromosomal genes have been reported, as exemplified by an IS10 derivative carrying sequences from the E. coli gal operon flanked by two IS10 in a direct repeat structure (28). Transposon Tn14751 probably formed by insertion of a copy of an IS31831-like element (IS14751) in an inverted repeat fashion 17.4 kb from an initial copy of IS14751, thus generating a composite transposon. The fact that these two copies of IS14751 function as a composite transposon, and not as individual insertion sequences, is remarkable. Individual sequencing of these two insertion elements confirmed that they encode a functional transposase and are flanked by functional inverted repeats and that they are thus likely to be capable of individual transposition.
This property of IS14751, as well as IS31831, makes it a very useful tool for engineering corynebacteria. On the other hand, its role in the evolution of the genus may have been particularly important, both at the level of intracellular genome rearrangement and at the level of horizontal gene transfer. Furthermore, it is of fundamental and biotechnological interest to verify whether such transposons are formed as a means for the cell to increase the dosage of particular genes (for instance, under conditions of stress, such as the presence of a toxic or bacteriostatic compound). It is worth noting that the mechanism of transposition of IS14751, as well as IS31831, is still unclear. It would also be useful to determine whether IS14751 and IS31831 transpose via a cut-and-paste conservative mechanism or via a replicative mechanism that facilitates gene duplication.
Acknowledgments
We thank R. H. Doi (University of California, Davis) for critical reading of the manuscript. K. Ninomiya is acknowledged for technical support.
This study was carried out as part of the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers of the Ministry of Economy, Trade & Industry and was entrusted by New Energy and Industrial Technology Development Organization.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg, D. E., and M. M. Howe (ed.). 1989. Mobile DNA. American Society for Microbiology Press, Washington, D.C.
- 3.Bonamy, C., J. Labarre, O. Reyes, and G. Leblon. 1994. Identification of IS1206, a Corynebacterium glutamicum IS3-related insertion sequence, and phylogenetic analysis. Mol. Microbiol. 14:571-581. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield, J. K., K. Smith, and R. Staden. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerdeno-Tarraga, A. M., A. Efstratiou, L. G. Dover, M. T. Holden, M. Pallen, S. D. Bentley, G. S. Besra, C. Churcher, K. D. James, A. De Zoysa, T. Chillingworth, A. Cronin, L. Dowd, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Moule, M. A. Quail, E. Rabbinowitsch, K. M. Rutherford, N. R. Thomson, L. Unwin, S. Whitehead, B. G. Barrell, and J. Parkhill. 2003. The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res. 31:6516-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia, A., A. Pisabarro, J. M. Castro, and J. F. Martin. 1996. Cloning and characterization of an IS-like element present in the genome of Brevibacterium lactofermentum ATCC 13869. Gene 170:91-94. [DOI] [PubMed] [Google Scholar]
- 7.Dedonder, R. 1966. Levan sucrase from Bacillus subtilis. Methods Enzymol. 8:500-505. [Google Scholar]
- 8.Ewing, B., and P. Green. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8:186-194. [PubMed] [Google Scholar]
- 9.Ewing, B., L. Hillier, M. C. Wendl, and P. Green. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 8:175-185. [DOI] [PubMed] [Google Scholar]
- 10.Guilhot, C., I. Otal, I. Van Rompaey, C. Martin, and B. Gicquel. 1994. Efficient transposition in mycobacteria: construction of Mycobacterium smegmatis insertional mutant libraries. J. Bacteriol. 176:535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henikoff, S., and J. G. Henikoff. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 89:10915-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda, M., and S. Nakagawa. 2003. The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 62:99-109. [DOI] [PubMed] [Google Scholar]
- 13.Inui, M., S. Murakami, S. Okino, H. Kawaguchi, A. A. Vertès, and H. Yukawa. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen-deprivation conditions. J. Mol. Microbiol Biotechnol. 7:182-196. [DOI] [PubMed] [Google Scholar]
- 14.Jager, W., A. Schafer, A. Puhler, G. Labes, and W. Wohlleben. 1992. Expression of the Bacillus subtilis sacB gene leads to sucrose sensitivity in the gram-positive bacterium Corynebacterium glutamicum but not in Streptomyces lividans. J. Bacteriol. 174:5462-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 16.Kleckner, N. 1989. Transposon Tn10, p. 227-268. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology Press, Washington, D.C.
- 17.Kotrba, P., M. Inui, and H. Yukawa. 2001. The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem. Biophys. Res. Commun. 289:1307-1313. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai, H. 2000. Microbial production of amino acids in Japan. Adv. Biochem. Eng. Biotechnol. 69:71-85. [DOI] [PubMed] [Google Scholar]
- 19.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 20.Labarre, J., O. Reyes, A. Guyonvarch, and G. Leblon. 1993. Gene replacement, integration, and amplification at the gdhA locus of Corynebacterium glutamicum. J. Bacteriol. 175:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard, C., O. Zekri, and J. Mahillon. 1998. Integrated physical and genetic mapping of Bacillus cereus and other gram-positive bacteria based on IS231A transposition vectors. Infect. Immun. 66:2163-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebl, W. 1992. The genus Corynebacterium, nonmedical, p. 1157-1171. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed. Springer-Verlag, Heidelberg, Germany.
- 23.Mahillon, J., and M. Chandler. 1998. Insertion sequences. Microbiol. Mol. Biol. Rev. 62:725-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy, E. 1989. Transposable elements in gram-positive bacteria, p. 269-288. In D. E. Berg and M. M. Howe (ed.), Mobile DNA. American Society for Microbiology Press, Washington, D.C.
- 25.Nakamura, Y., Y. Nishio, K. Ikeo, and T. Gojobori. 2003. The genome stability in Corynebacterium species due to lack of the recombinational repair system. Gene 317:149-155. [DOI] [PubMed] [Google Scholar]
- 26.Nishio, Y., Y. Nakamura, Y. Kawarabayasi, Y. Usuda, E. Kimura, S. Sugimoto, K. Matsui, A. Yamagishi, H. Kikuchi, K. Ikeo, and T. Gojobori. 2003. Comparative complete genome sequence analysis of the amino acid replacements responsible for the thermostability of Corynebacterium efficiens. Genome Res. 13:1572-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quast, K., B. Bathe, A. Puhler, and J. Kalinowski. 1999. The Corynebacterium glutamicum insertion sequence ISCg2 prefers conserved target sequences located adjacent to genes involved in aspartate and glutamate metabolism. Mol. Gen. Genet. 262:568-578. [DOI] [PubMed] [Google Scholar]
- 28.Raleigh, E. A., and N. Kleckner. 1984. Multiple IS10 rearrangements in Escherichia coli. J. Mol. Biol. 173:437-461. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schwarzer, A., and A. Puhler. 1991. Manipulation of Corynebacterium glutamicum by gene disruption and replacement. Bio/Technology 9:84-87. [DOI] [PubMed] [Google Scholar]
- 31.Staden, R. 1996. The Staden sequence analysis package. Mol. Biotechnol. 5:233-241. [DOI] [PubMed] [Google Scholar]
- 32.Tauch, A., Z. Zheng, A. Puhler, and J. Kalinowski. 1998. Corynebacterium striatum chloramphenicol resistance transposon Tn5564: genetic organization and transposition in Corynebacterium glutamicum. Plasmid 40:126-139. [DOI] [PubMed] [Google Scholar]
- 33. Vertès, A. A., Y. Asai, M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1994. Transposon mutagenesis of coryneform bacteria. Mol. Gen. Genet. 245:397-405. [DOI] [PubMed] [Google Scholar]
- 34. Vertès, A. A., K. Hatakeyama, M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1993. Replacement recombination in coryneform bacteria: high efficiency integration requirement for non-methylated plasmid DNA. Biosci. Biotechnol. Biochem. 57:2036-2038. [Google Scholar]
- 35. Vertès, A. A., M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1994. Isolation and characterization of IS31831, a transposable element from Corynebacterium glutamicum. Mol. Microbiol. 11:739-746. [DOI] [PubMed] [Google Scholar]
- 36. Vertès, A. A., M. Inui, M. Kobayashi, Y. Kurusu, and H. Yukawa. 1993. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res. Microbiol. 144:181-185. [DOI] [PubMed] [Google Scholar]